Abstract
Background
Viral infections have long been implicated in the development of chronic rhinosinusitis with nasal polyps (CRSwNP). Given widespread exposure to the common cold coronavirus 229E (HCoV229E), we sought to investigate how HCoV-229E is cleared and stimulates interferon pathways in air–liquid interface (ALI) cultures from patients with CRSwNP.
Objective
The objective of this study was to identify whether viral clearance and ISG expression is different in ALI cultures from donors with CRSwNP compared with controls.
Methods
Plaque assays were used to quantify infectious virus released by infected air–liquid interface (ALI) cultures derived from patients with CRSwNP compared to patients without CRS (controls). Additionally, mock and induced levels of Interferon Stimulated Genes (ISGs) mRNA following HCoV-229E infection were quantified by RT-qPCR.
Results
Quantification of infectious virus by plaque assay reveals that CRSwNP ALI cultures were equally susceptible to HCoV-229E infection, and surprisingly viral titers dropped significantly faster than in the control ALI cultures. We further demonstrate that this accelerated viral clearance correlates with increased mRNA expression of at least 4 ISGs following viral infection in the CRSwNP ALIs compared to the control ALIs.
Conclusion
This study paradoxically demonstrates that ALI cultures from patients with CRSwNP are more efficient at clearing the common cold HCoV-229E virus compared to controls. We also demonstrate significantly increased ISG mRNA expression following HCoV-229E infection in CRSwNP. These findings call for further investigation into the effect of unimpaired interferon signaling on the type 2 inflammatory environment in patients with CRSwNP.
Keywords: common cold, 229E, coronavirus, rhinosinusitis, chronic rhinosinusitis, rhinovirus, interferon, type 1 inflammation, nasal polyps, virus
Introduction
Inflammatory diseases of the respiratory system – including chronic rhinosinusitis (CRS) in the upper airway and asthma in the lower airway – are multifactorial diseases involving increased persistent inflammation of the sinonasal or bronchial mucosa. Patients with CRS with nasal polyps (CRSwNP) characterized by a type 2 immune profile often have severe disease, and asthma is often a comorbidity in these patients. Both disease states are characterized by tissue eosinophilia and high local IgE levels. 1 Viral infections of the upper airway in early life have been associated with an increased risk of developing asthma later in life.2,3,4 Higher incidences of viruses have been reported from nasopharyngeal clinical swabs during asthma attacks in adults.5,6 Furthermore, patients with asthma demonstrate increased viral replication and persistent higher viral titers both in vitro and in bronchioalveolar lavage fluid, which correlates with decreased induction of type I and type III interferons. 7 Overall, there is strong evidence supporting the role of viral infections in both the development of asthma and in asthma exacerbations; however, the role of viral infections in the pathogenesis of CRSwNP is much less well studied.
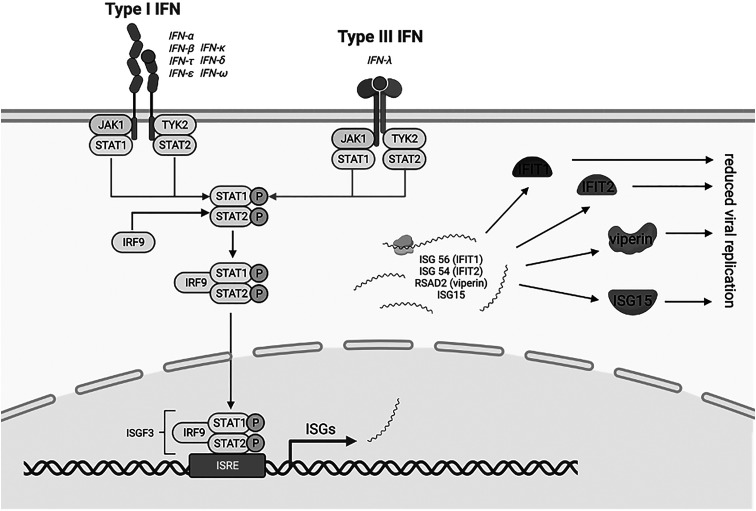
Viral infections are thought to be possible contributors to both the initiation and progression of CRSwNP.8,9,10 Human rhinovirus 16 (HRV16) infection has been shown to result in mucous hypersecretion, alterations to tight junctions, and increased production of remodeling factors in CRSwNP.11,12,13 Additionally, researchers have found that respiratory viruses were more commonly isolated from patients with CRS compared with controls, with common cold coronaviruses being the most commonly isolated.14,15 Mechanistically, this could be due to differences in interferon production, given that interferon in human airway epithelial cells is essential for viral clearance.16,17 In response to viral infection, epithelial cells in the respiratory tract produce antiviral factors that include interferons, which contribute to clearance of the virus (Figure 1).18,19,20,21 Interferon signaling is mediated by many interferon-stimulating genes (ISGs), which inhibit viral replication. 22 There is evidence that baseline interferon levels are decreased in patients with CRSwNP due to the type 2 inflammatory environment.23,24,25 Given the lower basal levels, it was hypothesized that interferon production is also deficient during viral infection in patients with CRSwNP, which would result in prolonged infection and delayed viral clearance, similar to the role that viral infections are thought to play in the pathogenesis of asthma. Furthermore, a possible explanation, at least in part, for the rising incidence of asthma and CRSwNP in industrialized countries in the last few decades is epigenetic changes secondary to environmental exposures.26,27 The role of epigenetics, including DNA methylation and posttranslational histone modifications, has been increasingly investigated in immune mechanisms underlying asthma and CRSwNP, highlighting the importance of investigating epithelial responses to viral infections.
Figure 1.
Interferon and activation of classical JAK-STAT pathways by type I and III interferons. The type I and III IFN receptors are both associated with the Janus activated kinase 1 (JAK1). Activation of JAK1 results in tyrosine phosphorylation of STAT1 (signal transducer and activator of transcription) and STAT2, which leads to the formation of complexes that translocate to the nucleus and bind IFN-stimulated response elements (ISREs) in DNA to initiate gene transcription. Gene transcription then produces mRNA of interferon-stimulating genes (ISGs), including ISG56 (IFIT1), ISG54 (IFIT2), Viperin (gene name RSAD2), and ISG15. While functionally distinct, these ISGs collaborate to reduce viral replication. Created using BioRender (biorender.com; BioRender, Toronto, Ontario, Canada).
The average healthy adult experiences between 1 and 3 common colds per year, which are caused by rhinoviruses (30%–50%) and coronaviruses including strains 229E, OC43, NL63, and HKU1 (5%–30%), as well as respiratory syncytial viruses (RSV) and adenoviruses, among others. 28 One study found that greater than 90% of over 100 patients from seven U.S. cities were seropositive for four coronavirus strains, with 99% having antibodies to human coronavirus 229E. 29 Given the evidence for widespread exposure to coronavirus 229E, we sought to investigate how the common cold coronavirus 229E affects interferon pathways in air–liquid interface (ALI) cultures derived from patients with CRSwNP. This was accomplished by infecting cultures with 229E and measuring infectious virus by plaque assay at different time points following infection. Furthermore, we also evaluated ISG mRNA expression following viral infection in cultures compared with donors with CRSwNP compared with controls.
Materials and Methods
Patient demographics/clinical descriptive data analysis: Patient demographics and comorbid conditions were analyzed in Prism (Graphpad, San Diego, CA). Mean and standard deviation were determined for age and differences between the CRSwNP and control groups were determined by t-test. Differences in sex and race between the CRSwNP and control groups were determined using chi-square test.
Reagents and experimental solutions: Cell culture reagents (DMEM, amino acids, antibiotics, etc) and Dulbecco's PBS reagents were obtained as previously described. 30
Tissue acquisition: Tissue samples were obtained from patients (Table 1) recruited from the Department of Otorhinolaryngology – Head and Neck Surgery, Division of Rhinology, University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center after obtaining IRB approval and written consent from all patients involved. Patients with a history of systemic inheritable disease (eg cystic fibrosis, granulomatosis with polyangiitis, systemic immunodeficiencies) or immunosuppressive medications were excluded from the study. For this study, patients with a history of asthma or allergic rhinitis were also excluded. Epithelial brushings were obtained under direct visualization using at least two cytology brushes rotated against the epithelium of the middle meatus, as identified by the attending otolaryngologist. The cytology brushes were then placed on ice immediately following collection in 15 mL conical tubes containing 10 mL sterile saline and transported to the laboratory.
Generation of pooled primary sinonasal ALI cultures: Sinonasal mucosal specimens were acquired and ALI cultures were established from enzymatically dissociated human sinonasal epithelial cells (HSECs) as previously described.30,31,32 The cells were pooled in a 1:1:1 or 1:1:1:1:1 ratio after basal cell proliferation. The cultures were allowed to differentiate for 3 weeks in differentiation media prior to infection. Confirmatory tests for differentiation were performed on ALI cultures prior to infection, including epithelial morphology assessed via light microscopy to confirm ciliation.
Virus: Human coronavirus 229E was propagated in Huh7 cells, a human immortalized well-differentiated hepatocyte-derived carcinoma cell line. Low MOI (0.01) infections were used to generate the viral stock for all experiments. The viral stock was sequenced and found to be comparable to wild-type reference sequences available on the National Center for Biotechnology Information (NCBI).
Infections and Plaque Assay: All infections were conducted at MOI = 5 plaque forming units (PFU)/cell. Viruses were diluted in serum-free Dulbecco's modified Eagle's medium (DMEM) to achieve a total inoculum volume of 50μL, which was added apically to the nasal ALI cultures. Virus was allowed to adsorb to the nasal ALI cultures for an incubation period of 1 h at 33 °C. 33 The virus was then aspirated and the nasal ALI cultures were washed three times with phosphate-buffered saline (PBS). ALI cultures were then incubated at 33 °C following infection. At the indicated time points in each experiment, 200μL PBS was added to the apical surface of each previously infected transwell, collected and then frozen at −80 °C. For plaque assays, Huh7 cells were plated in 6-well plates. Viral apical washes were diluted in serum-free DMEM and added to plates for adsorption for 1 h at 33 °C. After adsorption, 4 mL liquid overlay (1x sodium pyruvate, 2% FBS, 0.1% agarose) was added to each well. Plates were then incubated at 33 °C. Cells were fixed using 4% paraformaldehyde at 3 days post-infection and virus plaques were visualized by crystal violet staining.
RNA Extraction and Quantitative RT-PCR Analysis: Cells were lysed with RLT Plus buffer (Qiagen RNeasy Plus kit) and RNA was extracted following the Qiagen protocol. RNA was reverse transcribed into complementary DNA (cDNA) using the Applied Biosystems High Capacity Reverse Transcriptase Kit. cDNA was amplified using specific qRT-PCR primers for each target gene using Bio-Rad iQ SYBR Green Supermix and the Thermo Fisher QuantStudio 3 PCR system. Primer sequences are shown in Supplemental Table 1. CT values were calculated using the following formula: CT = CT target gene – CT 18S. Technical triplicates were averaged and changes in mRNA levels were reported as fold changes over sham-treated cultures (50μL DMEM with no virus incubated for 1 h and then washed 3 times with PBS), using the formula .
Data Analyses: Plotting of data and statistical analysis were performed using GraphPad Prism software (GraphPad Software, Inc.). Statistical significance was assessed by comparing one-way ANOVA. Displayed significance is determined by P value, where * = P < 0.05; ** = P < 0.01; *** = P < 0.001; and **** = P < 0.0001; ns = not significant, and, in some figures, ns is not displayed on the graph.
Table 1.
Demographic characteristics of CRSwNP and control patients.
| CRSwNP (n = 8) | Control (n = 8) | p-value | ||
|---|---|---|---|---|
| Sex | Male | 5 | 7 | 0.20 |
| Female | 3 | 1 | ||
| Age | 42.5 (13.8) | 43.5 (15.6) | 0.85 | |
| Race | White | 6 | 5 | 0.67 |
| African American | 2 | 2 | ||
| Hispanic | 0 | 1 |
Results
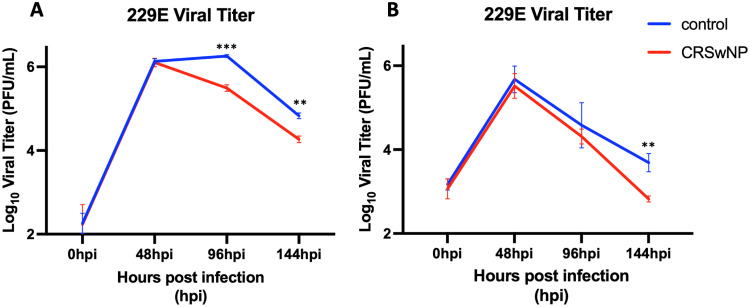
To investigate the effect of the common cold human coronavirus 229E on the upper airway innate immune response, we infected primary human sinonasal ALI cultures with 229E virus and assessed infectious virus at various timepoints following infection by plaque assay. We first generated pooled ALI cultures from three control donors without CRS and three donors with CRSwNP and measured infectious titers at 48-, 96-, and 144-h post infection (hpi). We found that the pooled ALI cultures from the donors with CRSwNP were not only equally susceptible to 229E infection but cleared the virus significantly faster than the non-CRS control ALI cultures at both 96- and 144-hpi (Figure 2A). To confirm this observation, we generated additional pooled ALI cultures from five different control donors without CRS and five different donors with CRSwNP and again measured infectious titers at 48-, 96- and 144-hpi. Again, we found that the pooled ALI cultures from the five donors with CRSwNP cleared the virus faster than the pooled ALI cultures from the five donors without CRS (Figure 2B). These data demonstrate that in an in vitro model, sinonasal epithelial cultures derived from patients with CRSwNP are more efficient at clearing the 229E virus compared to cultures derived from patients without CRS.
Figure 2.
Common cold coronavirus 229E clears faster in ALI cultures from patients with CRSwNP. ALI pooled cultures from 3 control donors without CRS and 3 donors with CRSwNP were infected with 229E (MOI = 5) in triplicate ALI cultures and viral titers were measured at 48-, 96- and 144-hours post infection (hpi) (A). In a second infection, ALI pooled cultures from 5 different control donors without CRS and 5 different donors with CRSwNP were infected with 229E (MOI = 5) again in triplicate ALI cultures (B). * = P < 0.05; ** = P < 0.01; *** = P < 0.001.
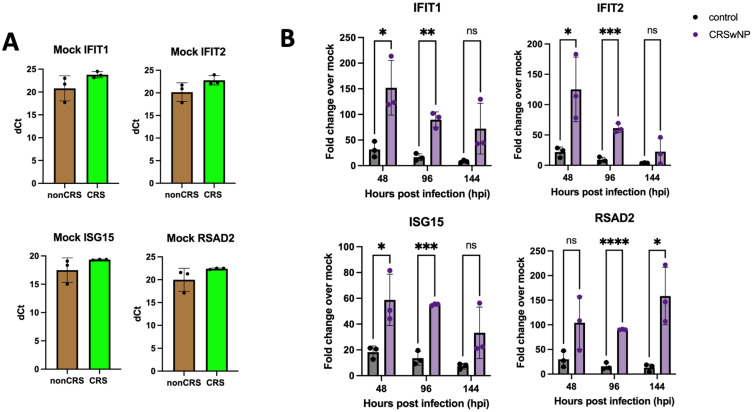
Given that the production of interferon is known to play a crucial role in viral clearance, we next sought to measure ISG responses to a series of canonical antiviral genes in the pooled ALI cultures from patients without CRS compared to the pooled ALI cultures from patients with CRSwNP. First, mock levels of ISG mRNA in uninfected cultures were quantified by RT-qPCR. We found that none of the ISG mRNA under investigation demonstrated differential basal expression between the pooled ALI cultures from donors with or without CRSwNP (Figure 3A). Although not statistically significant, we did find that average ΔCT values were higher for ISGs including IFIT1, IFIT2, RSAD2 (the ISG that encodes the protein viperin), and ISG15 in cultures from patients with CRSwNP, suggesting lower basal levels and consistent with previous reports demonstrating lower levels of interferon and ISGs in cultures from patients with CRSwNP.23,34 However, we wanted to further investigate what happens to ISG levels relative to these respective mock values when the cultures were challenged with a viral infection.
Figure 3.
Common cold coronavirus 229E increases ISG mRNA expression significantly more in ALI cultures from patients with CRSwNP by RT-qPCR. Fold changes were calculated by subtracting the measured fold change of the infected ALIs from the averaged mock values for nonCRS and CRSnp, respectively (A). ALI pooled cultures from 5 control donors without CRS and 5 donors with CRSwNP were infected with 229E (MOI = 5) in triplicate ALI cultures and ISG mRNA fold changes were measured at 48-, 96- and 144-hours post infection (hpi) (B). * = P < 0.05; ** = P < 0.01; *** = P < 0.001; and **** = P < 0.0001; ns = not significant.
We infected the pooled ALI cultures with 229E in triplicate and found that the pooled ALI cultures from donors with CRSwNP exhibited significantly higher ISG mRNA expression across four ISGs as quantified by RT-qPCR (Figure 3B). We found that mRNA expression of IFIT1, IFIT2, and ISG15 peaked at 48-hpi and trended down at later timepoints (96- and 144-hpi), while mRNA expression of RSAD2 (viperin) remained elevated throughout all timepoints measured (48-, 96-, and 144-hpi). This finding suggests that viral replication and clearance have different effects on different ISG mRNA in the context of 229E infection. Overall, our results demonstrate that sinonasal epithelium derived from patients with CRSwNP is paradoxically more efficient at clearing 229E compared to sinonasal epithelium derived from non-CRS control patients. This faster viral clearance correlates with increased ISG mRNA expression.
Discussion
In this study, we demonstrate that ALI cultures derived from patients with CRSwNP are more efficient at clearing the human common cold coronavirus 229E compared to ALI cultures derived from controls. Quantification of infectious virus by plaque assay demonstrates that ALI cultures generated from patients with CRSwNP show decreasing titers faster than in ALI cultures originating from patients without CRS in two separate infections from different donors (n = 8 for each cohort). We also demonstrate significantly increased mRNA copy number in nasal epithelium derived from CRSwNP compared to controls across four ISGs as quantified by RT-qPCR after 229E infection. Taken together, this study supports previous work demonstrating that patients with CRSwNP do not exhibit delayed viral clearance or impaired interferon signaling. This warrants further investigation into how interferon signaling interacts with the type 2 inflammatory environment classically found in patients with CRSwNP.
Type I interferon responses have been linked to type 2 inflammation and antiviral functions in the sinonasal epithelium. Jang et al reported findings suggesting that type I interferon can contribute to eosinophilic CRS and the type 2 inflammatory environment, specifically to the production of IL-5 and IL-13. 35 Additionally, Ko and colleagues demonstrated that HRV16 possibly increases IL-4 production in ALI cultures from some subtypes of patients with CRS. 36 In whole-transcriptome RNA-sequencing studies, type 1 interferon signaling has been noted to be enriched in patients with CRSwNP. 37 Additionally, type III interferon has been found to be a marker in patients with CRSwNP undergoing revision surgery, 38 although subsequent studies have not corroborated this finding. 39 In addition to its role in viral clearance, interferon-γ (IFN-γ) is a type III interferon that has been implicated in fungal eosinophilic rhinosinusitis.40,41 Furthermore, high type III interferon levels have been described in patients with aspirin-exacerbated respiratory disease (AERD), which has also been shown to increase the expression of genes involved in leukotriene synthesis. 42 Overall, our results are consistent with previous findings that interferon signaling is not impaired in patients with CRSwNP unlike in patients with asthma.23,34
Evidence suggests that interferon signaling is not delayed in patients with CRSwNP as it is in patients with asthma.43,44,45 and given that the interferon response may be active to a greater degree in patients with CRSwNP compared with patients with asthma, it is important to investigate interferon signaling in the context of the chronic inflammation in CRSwNP. No other studies to date have looked at other common cold viruses such as human common cold coronaviruses in the context of CRSwNP. This work demonstrates the need for future investigations into how common cold viruses affect interferon signaling and inflammation in the sinonasal epithelium, particularly how they could interact with mediators of type 2 inflammation. Further work into how interferon signaling interacts with the type 2 inflammatory environment in patients with CRSwNP could yield insights into the mechanisms underlying the development of chronic inflammation in these patients.
Conclusion
In summary, this study demonstrates that sinonasal ALI cultures generated from patients with CRSwNP are more efficient at clearing the human common cold coronavirus 229E compared to ALIs derived from non-CRS control patients. We also demonstrate significantly increased mRNA copy number of ISGs as quantified by RT-qPCR in cultures from patients with CRSwNP. A greater understanding of the effects of interferon signaling could reveal important insights into the interplay between interferon signaling and type 2 inflammation in the development of chronic inflammation in patients with CRSwNP.
Supplemental Material
Supplemental material, sj-docx-1-ajr-10.1177_19458924241276274 for Common Cold Coronavirus 229E Induces Higher Interferon Stimulating Gene Responses in Human Nasal Epithelial Cells from Patients with Chronic Rhinosinusitis with Polyposis by Elizabeth A. Sell, B.A. , Li Hui Tan, PhD, David M. Renner, PhD, Jennifer Douglas, M.D., Robert J. Lee, PhD, Michael A. Kohanski, M.D. PhD, John V. Bosso, M.D., David W. Kennedy, M.D., James N. Palmer, M.D., Nithin D. Adappa, M.D., Susan R. Weiss, PhD, and Noam A. Cohen, M.D. PhD in American Journal of Rhinology & Allergy
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health (NIH) R01AI169537, 5TL1TR001880-08, (VA) BX005432. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH nor the VA. We are submitting this paper in connection with the oral presentation, “Common Cold Viruses Activate the Unfolded Protein Response in Chronic Rhinosinusitis,” presented at the 69th annual meeting of the American Rhinologic Society in Nashville, Tennessee, September 29-30, 2023. This study was approved by both the University of Pennsylvania Institutional Review Board (protocol #800614) and the Philadelphia VA Institutional Review Board (protocol #00781). U.S. Department of Veterans Affairs, National Institutes of Health, (grant number BX005432, 5TL1TR001880-08, R01AI169537).
ORCID iDs: Elizabeth A. Sell https://orcid.org/0000-0002-9658-1107
David M. Renner https://orcid.org/0000-0003-0548-3876
Jennifer Douglas https://orcid.org/0000-0002-3634-2067
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Laidlaw TM, Mullol J, Woessner KM, Amin N, Mannent LP. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021 Mar;9(3):1133‐1141. [DOI] [PubMed] [Google Scholar]
- 2.Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015 May 13;17(5):704‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeyama A, Hashimoto K, Sato M, et al. Clinical and epidemiologic factors related to subsequent wheezing after virus-induced lower respiratory tract infections in hospitalized pediatric patients younger than 3 years. Eur J Pediatr. 2014 Jul;173(7):959‐966. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005 Jan 15;171(2):137‐141. [DOI] [PubMed] [Google Scholar]
- 5.Iikura M, Hojo M, Koketsu R, et al. The importance of bacterial and viral infections associated with adult asthma exacerbations in clinical practice. PLoS One. 2015;10(4):e0123584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan F, Zhang N, Gevaert E, Zhang L, Bachert C. Viruses and bacteria in Th2-biased allergic airway disease. Allergy. 2016 Oct;71(10):1381‐1392. [DOI] [PubMed] [Google Scholar]
- 7.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006 Sep;12(9):1023‐1026. [DOI] [PubMed] [Google Scholar]
- 8.Hoggard M, Wagner Mackenzie B, Jain R, Taylor MW, Biswas K, Douglas RG. Chronic rhinosinusitis and the evolving understanding of microbial ecology in chronic inflammatory mucosal disease. Clin Microbiol Rev. 2017 Jan;30(1):321‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha KR, Psaltis AJ, Tan L, Wormald PJ. A sheep model for the study of biofilms in rhinosinusitis. Am J Rhinol. 2007;21(3):339‐345. [DOI] [PubMed] [Google Scholar]
- 10.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009 Jul 1;124(1):37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuta A, Doyle WJ, Gaumond E, et al. Rhinovirus infection induces mucus hypersecretion. Am J Physiol. 1998 Jun;274(6):L1017‐L1023. [DOI] [PubMed] [Google Scholar]
- 12.Yeo NK, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. 2010 Feb;120(2):346‐352. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Kwon HJ, Jang YJ. Rhinovirus upregulates matrix metalloproteinase-2, matrix metalloproteinase-9, and vascular endothelial growth factor expression in nasal polyp fibroblasts. Laryngoscope. 2009;119(9):1834‐1838. [DOI] [PubMed] [Google Scholar]
- 14.Cho GS, Moon BJ, Lee BJ, et al. High rates of detection of respiratory viruses in the nasal washes and Mucosae of patients with chronic rhinosinusitis. J Clin Microbiol. 2013 Mar;51(3):979‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowan NR, Lee S, Sahu N, et al. The role of viruses in the clinical presentation of chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29(6):e197‐e200. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis S, Jouanguy E, Al-Hajjar S, et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003 Mar;33(3):388‐391. [DOI] [PubMed] [Google Scholar]
- 17.Chapgier A, Kong XF, Boisson-Dupuis S, et al. A partial form of recessive STAT1 deficiency in humans. J Clin Invest. 2009 Jun;119(6):1502‐1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005 May;5(5):375‐386. [DOI] [PubMed] [Google Scholar]
- 19.Müller U, Steinhoff U, Reis LF, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994 Jun 24;264(5167):1918‐1921. [DOI] [PubMed] [Google Scholar]
- 20.Samuel CE. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991 Jul;183(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 21.Khaitov MR, Laza-Stanca V, Edwards MR, et al. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009 Mar;64(3):375‐386. [DOI] [PubMed] [Google Scholar]
- 22.Staeheli P. Interferon-induced proteins and the antiviral state. In: Maramorosch K, Murphy FA and Shatkin AJ (eds) Advances in virus research. Academic Press;38;1990:147-200. doi: 10.1016/S0065-3527(08)60862-3 [DOI] [PubMed]
- 23.Hwang JW, Lee KJ, Choi IH, Han HM, Kim TH, Lee SH. Decreased expression of type I (IFN-β) and type III (IFN-λ) interferons and interferon-stimulated genes in patients with chronic rhinosinusitis with and without nasal polyps. J Allergy Clin Immunol. 2019 Dec;144(6):1551‐1565.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014 Mar;133(3):640‐653.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan F, Wang XD, Nauwynck HJ, et al. Th2 biased upper airway inflammation is associated with an impaired response to viral infection with Herpes simplex virus 1. Rhinology. 2016 Jun;54(2):141‐149. [DOI] [PubMed] [Google Scholar]
- 26.Alaskhar Alhamwe B, Khalaila R, Wolf J, et al. Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin Immunol. 2018 May 23;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Zhang N, Perez Novo C, Wang Y, Zhang L. Decreased histone expression in chronic rhinosinusitis with nasal polyps. Asia Pac Allergy. 2024 Jun;14(2):70‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eccles R. Common cold. Front Allergy. 2023;4:1224988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorse GJ, Patel GB, Vitale JN. O’Connor TZ. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin Vaccine Immunol. 2010 Dec;17(12):1875‐1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014 Mar;124(3):1393‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramanathan M, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol. 2007;21(3):373‐377. [DOI] [PubMed] [Google Scholar]
- 32.Lai Y, Chen B, Shi J, Palmer JN, Kennedy DW, Cohen NA. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2011 Dec;128(6):1207‐1215.e1. [DOI] [PubMed] [Google Scholar]
- 33.Otter CJ, Tan FA, Khosla LH, Cohen AS, Weiss NA, R S. Infection of primary nasal epithelial cells differentiates among lethal and seasonal human coronaviruses. Proceedings of the National Academy of Sciences. 2023 Apr 11;120(15):e2218083120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Hu DQ, Xiao Q, et al. Defective STING expression potentiates IL-13 signaling in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2021 May;147(5):1692‐1703. [DOI] [PubMed] [Google Scholar]
- 35.Jang YJ, Lim JY, Kim S, Lee YL, Kweon MN, Kim JH. Enhanced interferon-β response contributes to eosinophilic chronic rhinosinusitis. Front Immunol. 2018 Oct 16;9:2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko YK, Zhang YL, Wee JH, Han DH, Kim HJ, Rhee CS. Human rhinovirus infection enhances the Th2 environment in allergic and non-allergic patients with chronic rhinosinusitis. Clin Exp Otorhinolaryngol. 2021 May;14(2):217‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Zi XX, Tian TF, et al. Whole-transcriptome sequencing reveals heightened inflammation and defective host defence responses in chronic rhinosinusitis with nasal polyps. Eur Respir J. 2019 Nov;54(5):1900732. [DOI] [PubMed] [Google Scholar]
- 38.Ryu G, Kim DK, Dhong HJ, et al. Immunological characteristics in refractory chronic rhinosinusitis with nasal polyps undergoing revision surgeries. Allergy Asthma Immunol Res. 2019 Sep;11(5):664‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28(3):192‐198. [DOI] [PubMed] [Google Scholar]
- 40.Kale P, Rudramurthy SM, Panda NK, Das A, Chakrabarti A. The inflammatory response of eosinophil-related fungal rhinosinusitis varies with inciting fungi. Med Mycol. 2015 May;53(4):387‐395. [DOI] [PubMed] [Google Scholar]
- 41.Yan Y, Zhao Z, Dong G, et al. Using IFN-γ antibodies to identify the pathogens of fungal rhinosinusitis: A novel immunohistochemical approach. Mol Med Rep. 2018 Mar;17(3):3627‐3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent role of IFN-γ in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2013 Oct;132(4):856‐865.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao B, Hu CY, Liu T, Liu Z. Respiratory viral infection in the chronic persistent phase of chronic rhinosinusitis. Laryngoscope. 2014 Apr;124(4):832‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SH, Han MS, Lee TH, et al. Rhinovirus-induced anti-viral interferon secretion is not deficient and not delayed in sinonasal epithelial cells of patients with chronic rhinosinusitis with nasal polyp. Front Immunol. 2022;13:1025796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JH, Kwon HJ, Chung YS, Lee BJ, Jang YJ. Infection rate and virus-induced cytokine secretion in experimental rhinovirus infection in mucosal organ culture: Comparison between specimens from patients with chronic rhinosinusitis with nasal polyps and those from normal subjects. Arch Otolaryngol Head Neck Surg. 2008 Apr 1;134(4):424‐427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ajr-10.1177_19458924241276274 for Common Cold Coronavirus 229E Induces Higher Interferon Stimulating Gene Responses in Human Nasal Epithelial Cells from Patients with Chronic Rhinosinusitis with Polyposis by Elizabeth A. Sell, B.A. , Li Hui Tan, PhD, David M. Renner, PhD, Jennifer Douglas, M.D., Robert J. Lee, PhD, Michael A. Kohanski, M.D. PhD, John V. Bosso, M.D., David W. Kennedy, M.D., James N. Palmer, M.D., Nithin D. Adappa, M.D., Susan R. Weiss, PhD, and Noam A. Cohen, M.D. PhD in American Journal of Rhinology & Allergy