Abstract
Objective
To evaluate the association between smell loss and other aspects of disease, and evaluate dupilumab efficacy in patients with severe chronic rhinosinusitis with nasal polyps (CRSwNP) and moderate or severe smell loss.
Methods
This post-hoc analysis of the SINUS-24/52 studies (NCT02912468/NCT02898454) analyzed nasal polyp score (NPS, 0−8), nasal congestion/obstruction (NC, 0−3), Lund-Mackay CT-scan score (LMK-CT, 0−24), rhinosinusitis severity visual analog scale (RS-VAS, 0-10), and 22-item Sinonasal Outcome Test (SNOT-22, 0−110) according to baseline monthly average patient-reported loss of smell scores (LoS, 0−3) of >1 to 2 (moderate) or >2 to 3 (severe) in patients randomized to dupilumab 300 mg or placebo every 2 weeks.
Results
Of 724 patients randomized, baseline LoS was severe in 601 (83%) and moderate in 106 (15%). At baseline, severe versus moderate LoS was associated with 1-point greater severity of NC (odds ratio [OR] 6.01 [95% confidence interval, (CI) 3.95, 9.15]), 5-point greater severity of LMK-CT (OR 2.19 [1.69, 2.85]), and 8.9-point greater severity of SNOT-22 (OR 1.35 [1.20, 1.49]). At Week 24, least squares mean differences (95% CI) dupilumab versus placebo in change from baseline were: NPS −1.90 (−2.56, −1.25) and −1.95 (−2.20, −1.70) in the moderate and severe baseline LoS subgroups, respectively; NC −.35 (−.64, −.06) and −1.00 (−1.13, −.87); LMK-CT −6.30 (−7.88, −4.72) and −6.22 (−6.82, −5.63); RS-VAS −1.18 (−2.20, −.16) and −3.47 (−3.90, −3.03); and SNOT-22 −7.52 (−14.55, −.48) and −21.72 (−24.63, −18.82); all nominal P < .05 versus placebo. Improvements with dupilumab in NC, RS-VAS, and SNOT-22 were statistically greater in patients with severe versus moderate baseline LoS.
Conclusion
Significant smell impairment in severe CRSwNP is associated with significant disease (NC, RS-VAS, LMK), health-related quality of life impairment (SNOT-22), asthma, and non-steroidal anti-inflammatory drug-exacerbated respiratory disease. Dupilumab significantly improved NPS, NC, LMK-CT, RS-VAS, and SNOT-22 in subjects with moderate and severe baseline smell loss.
Keywords: CRSwNP, olfaction, smell loss, anosmia, HRQoL, biologic, interleukin-4, interleukin-13, interleukin-4 receptor alpha, dupilumab
Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a predominantly type 2-mediated inflammatory disease of the nasal cavity and paranasal sinuses, associated with a high symptom burden and poor health-related quality of life (HRQoL).1,2 Smell loss is a cardinal symptom of CRSwNP and is reported by patients to be one of the most important and troublesome symptoms.3,4 Severity of smell loss is correlated with the presence of coexisting asthma and nonsteroidal antiinflammatory drug-exacerbated respiratory disease (NSAID-ERD), which are common in patients with CRSwNP. 5 The impact of smell loss may be underappreciated by patients but it can have a profound effect on HRQoL, including anxiety and depression.2,6,7 Established medical and surgical treatment options for CRSwNP have different profiles in terms of smell improvement. Corticosteroids are the first-line treatment for CRSwNP and can improve smelling ability in the short term, but may be ineffective in the long term and can have deleterious side effects with systemic use.8,9 Endoscopic sinus surgery can provide long-lasting symptom control but disease recurrence and need for revision surgery are common.10–13
Dupilumab is a fully human VelocImmune®-derived monoclonal antibody that binds to interleukin (IL)-4 receptor α to block the shared receptor component for IL-4 and IL-13, which are key and central drivers of type 2 inflammation in multiple diseases.14–17 In the Phase 3 SINUS-24 and SINUS-52 studies (NCT02912468 and NCT02898454) in patients with uncontrolled CRSwNP, dupilumab significantly improved objective and patient-reported outcomes, including sense of smell, with safety consistent with the known dupilumab safety profile.4,18 However, the efficacy of dupilumab according to severity of baseline smell loss in the SINUS trials has not been established.
Evidence is accumulating that severity of smell loss correlates with severity of other aspects of disease in CRSwNP, but uncertainty remains around the degree of association with different subjective and patient-reported disease measures.19–21 The SINUS trials provide a rich dataset to explore the association between severity of baseline smell loss and other measures of CRSwNP disease. Previous reports from the SINUS trials analyzed smell loss using the University of Pennsylvania Smell Identification Test (UPSIT).4,22 These analyses found correlations between UPSIT and other aspects of CRSwNP, as well as significant improvements in UPSIT with dupilumab treatment versus placebo. However, in real-world practice, most clinicians do not use psychophysical smell tests such as UPSIT. Consequently, here we investigated the simple subjective assessment of smell loss used in the SINUS trials, in which patients rated the severity of their smell loss daily on a scale of 0 to 3 using an eDiary (loss of smell score; LoS), which may mirror everyday clinical practice more closely. The aims of this post-hoc analysis of the SINUS trials were to evaluate the association between baseline smell loss and other aspects of disease severity in patients with severe CRSwNP, and to evaluate the efficacy of dupilumab in subgroups with baseline severe or moderate smell loss using the LoS score.
Methods
SINUS Study Design
SINUS-24 (NCT02912468) and SINUS-52 (NCT02898454) were prospective, randomized, double-blind, placebo-controlled Phase 3 trials of dupilumab in patients with severe CRSwNP. A comprehensive description of the study designs has been published previously. 18 Briefly, patients 18 years of age or older were eligible if they had bilateral nasal polyps (nasal polyp score [NPS] ≥ 5 out of maximum 8 and ≥2 in each nasal cavity) and symptoms of chronic rhinosinusitis despite intranasal corticosteroid therapy, and had received systemic corticosteroids in the preceding 2 years and/or undergone sinonasal surgery. In SINUS-24, patients were randomized 1:1 to subcutaneous (SC) dupilumab 300 mg or placebo once every 2 weeks (q2w) for 24 weeks. In SINUS-52, patients were randomized 1:1:1 to dupilumab 300 mg SC or placebo q2w for 52 weeks, or dupilumab 300 mg SC q2w for 24 weeks, then every 4 weeks for 28 weeks in SINUS-52. In both studies, patients received background mometasone furoate nasal spray from 4 weeks prior to randomization and throughout the study. Both studies met their coprimary endpoints (improvement in NPS and nasal congestion (NC) at 24 weeks) and met all prespecified secondary endpoints. The studies were conducted in accordance with Good Clinical Practice and the principles ordained in the Declaration of Helsinki. The protocols were approved by appropriate ethical review boards, and all patients provided written informed consent.
Assessments
Severity of LoS and NC were recorded daily by patients using an eDiary on a scale of 0 = no symptom, 1 = mild, 2 = moderate, and 3 = severe symptom. NPS, Lund-Mackay CT-scan score (LMK-CT), rhinosinusitis severity visual analog scale (VAS), and the 22-item Sinonasal Outcome Test (SNOT-22) were assessed at clinic visits as described. 18
Post-Hoc Analysis
This post-hoc analysis included patients randomized to dupilumab 300 mg or placebo q2w. Patients were grouped into LoS severity subgroups according to baseline 7-day average LoS scores moderate (>1 to 2) and severe (>2). Odds ratios (ORs) for baseline severe versus moderate LoS were determined for (i) presence/absence of prior sinonasal surgery, each additional surgery, asthma, NSAID-ERD; and (ii) severity of other baseline disease parameters: rhinosinusitis severity VAS >7 cm, SNOT-22 score >50, and clinically meaningful difference in NPS and NC (both 1-point greater score), 23 and SNOT-22 (8.9-point greater score). 24 Least squares mean changes from baseline in NPS, NC, LMK-CT, and SNOT-22 were determined by LoS severity subgroup (moderate or severe) at the first postbaseline assessment common to both studies, at Week 24 (pooled studies), and at Week 52 (SINUS-52). The first postbaseline assessment was Week 4 for NC (average of the first 28 days), Week 8 for NPS and SNOT-22, and Week 24 for LMK-CT.
Statistical Analyses
ORs were derived using logistic regression using SAS with binary logit and Fisher's scoring. Least squares mean change from baseline was analyzed using the same imputed dataset as the primary analysis, with missing values imputed by worst observation carried forward and multiple imputation as described. 10 Each of the imputed complete data was analyzed by fitting an analysis of covariance model with the corresponding baseline value, treatment group, asthma/NSAID-ERD status, prior surgery history, regions, and study as covariates. As this was a post-hoc analysis, all P-values are nominal.
Results
Association Between Moderate Versus Severe Smell Loss and Disease Burden
Of 724 patients randomized, 707 (97.7%) had at least moderate LoS (score >1) at baseline and were included in this analysis (601 [83.0%] with severe LoS [score >2 to 3] and 106 [14.6%] with moderate LoS [score >1 to 2]). Only 11 patients (1.5%) had mild LoS (score >0 to 1) and six (.8%) had no LoS (score 0).
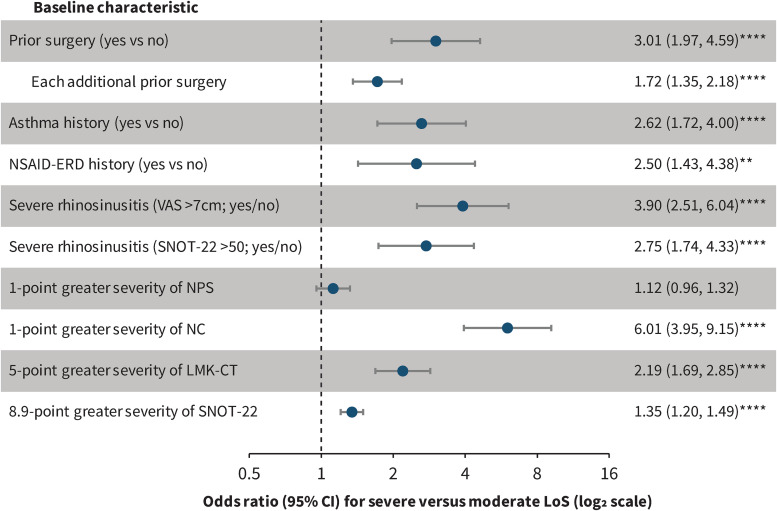
Patients with severe LoS at baseline had significantly higher prevalence of prior nasal polyp surgery, coexisting asthma and NSAID-ERD, and significantly higher baseline NC, LMK-CT, and SNOT-22 scores than patients with moderate LoS (Table 1). Moreover, a significantly greater proportion of patients with severe versus moderate LoS at baseline had severe disease as indicated by rhinosinusitis VAS scores >7 cm and SNOT-22 scores >50. At baseline, severe versus moderate LoS was associated with a 1-point greater severity of NC (OR 6.01 [95% confidence interval, (CI) 3.95, 9.15]; P < .0001), a 5-point greater severity of LMK-CT (OR 2.19 [1.69, 2.85]; P < .0001), and an 8.9-point greater severity of SNOT-22 (OR 1.35 [1.20, 1.49]; P < .0001) (Figure 1). Severe versus moderate LoS at baseline was also associated with significant ORs of having prior surgery, asthma, NSAID-ERD, and severe rhinosinusitis according to VAS > 7 cm or SNOT-22 score > 50 (Figure 1). There was no significant difference in baseline NPS between patients with moderate and severe LoS, and the odds of severe versus moderate baseline LoS did not significantly change with baseline NPS score (OR 1.12 [95% CI 0.96, 1.32]; P = .16).
Table 1.
Demographic and Baseline Disease Characteristics of Patients With Moderate (Score >1 to 2) or Severe (Score >2 to 3) LoS † at Baseline (Pooled SINUS-24/−52 Population).
| Moderate LoS at baseline (N = 106) | Severe LoS at baseline (N = 601) | P value | |
|---|---|---|---|
| Age, years | 52.6 (11.6) | 51.0 (13.0) | .2413 |
| Male, n (%) | 66 (62.3) | 360 (59.9) | .6466 |
| Prior sinonasal surgery, n (%) | 43 (40.6) | 404 (67.2) | <.0001 |
| Asthma, n (%) | 42 (39.6) | 380 (63.2) | <.0001 |
| NSAID-ERD, n (%) | 16 (15.1) | 185 (30.8) | .0010 |
| Severe rhinosinusitis (VAS >7 cm), n (%) | 47 (44.3) | 456 (75.9) | <.0001 |
| Severe rhinosinusitis (SNOT-22 > 50), n (%) | 30 (28.3) | 319 (53.1) | <.0001 |
| NPS (0−8) | 5.8 (1.2) | 6.0 (1.3) ‡ | .1594 |
| NC (0−3) | 1.9 (0.4) | 2.5 (0.6) | <.0001 |
| LMK-CT (0−24) | 16.3 (4.0) § | 18.9 (3.9) || | <.0001 |
| Rhinosinusitis VAS (0−10 cm) | 6.8 (2.2) | 8.1 (1.9) | <.0001 |
| SNOT-22 (0−110) | 40.9 (16.0) ¶ | 53.2 (20.8) †† | <.0001 |
Note: †28-day average.
n = 598.
n = 104.
n = 589.
n = 101.
n = 594.
Data are mean (standard deviation) unless otherwise specified.
P-values are from chi-square test for proportions and from t-test using Satterthwaite approximation for continuous variables (NC and SNOT-22).
LMK-CT, Lund-Mackay CT-scan score; LoS, loss of smell; NC, nasal congestion/obstruction score; NPS, nasal polyp score; NSAID-ERD, nonsteroidal antiinflammatory drug-exacerbated respiratory disease; SNOT-22, 22-item Sinonasal Outcome Test; VAS, visual analog scale.
Figure 1.
Odds ratios for baseline severe versus moderate LoS by other baseline characteristics.
Note: CI, confidence interval; LMK-CT, Lund-Mackay CT-scan score; LoS, loss of smell; NC, nasal congestion/obstruction score; NPS, nasal polyp score; NSAID-ERD, nonsteroidal antiinflammatory drug-exacerbated respiratory disease; SNOT-22, 22-item Sinonasal Outcome Test; VAS, visual analog scale.
**P < .01; ****P < .0001.
Association Between Moderate Versus Severe LoS and Dupilumab Effect on Disease Outcomes
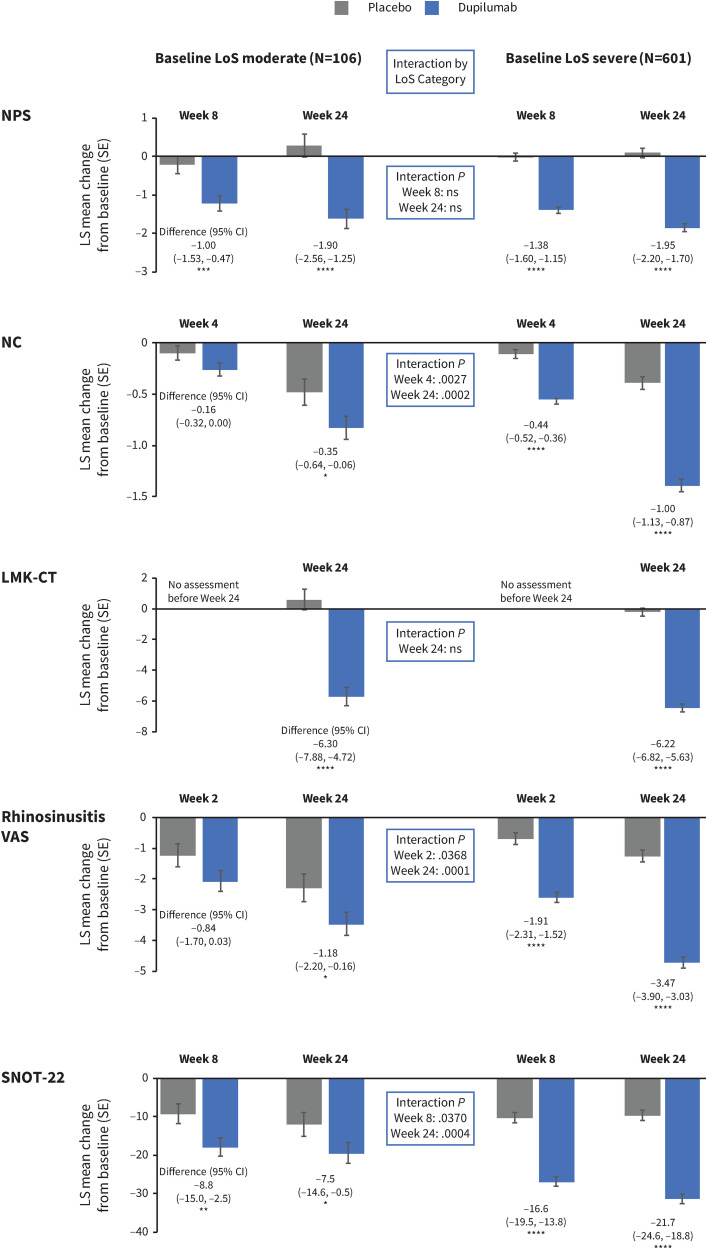
Demographics and baseline characteristics were well balanced between the placebo and dupilumab treatment groups, as shown in Supplemental Table 1. Significant improvements in NPS, NC, LMK-CT, rhinosinusitis VAS, and SNOT-22 scores were observed with dupilumab versus placebo in both the moderate baseline LoS subgroup and the severe baseline LoS subgroups. Improvements at the first postbaseline assessment were sustained or increased at Week 24 in the pooled studies (Figure 2) and at Week 52 in SINUS-52 (Supplemental Figure 1) in both LoS severity subgroups. The magnitude of improvements in NC, rhinosinusitis VAS, and SNOT-22 with dupilumab at Week 24 was statistically greater in patients with severe LoS than in patients with moderate LoS at baseline (P-values for subgroup by treatment interaction .0002, .0001, and .0004, respectively). Improvements in NPS and LMK-CT with dupilumab did not differ significantly between the moderate and severe baseline LoS subgroups (P-values for subgroup by treatment interaction .77 and >.99, respectively).
Figure 2.
Change from baseline after the first post baseline assessment and after 24 weeks in NPS, NC, LMK-CT, rhinosinusitis VAS, and SNOT-22 scores by moderate or severe LoS score at baseline (pooled SINUS 24/−52).
Note: NC values are 28-day average. CI, confidence interval; LMK-CT, Lund-Mackay CT-scan score; LoS, loss of smell; LS, least squares; NC, nasal congestion/obstruction score; NPS, nasal polyp score; ns, not significant; SE, standard error; SNOT-22, 22-item Sinonasal Outcome Test; VAS, visual analog scale.
Nominal P: *P < .05, **P < .01, ***P < .001, ****P < .0001.
Discussion
This post-hoc analysis set out to provide a greater understanding of the impact of LoS in patients with CRSwNP and its association with outcomes with dupilumab treatment. LoS is a prevalent symptom in CRSwNP, as demonstrated by ∼98% of patients in the SINUS study population having at least moderate smell loss despite receiving standard of care (intranasal corticosteroids). This highlights an unmet need and a particularly important one from the patient's perspective, since LoS is cited as one of the most bothersome and often overlooked symptoms of CRSwNP, 25 and one that is associated with reduced HRQoL.4,26 As psychophysical smell tests such as UPSIT may not be administered in real-world clinical practice owing to cost and time constraints, the current analysis focused on the subjective LoS score. With the LoS score, patients rate the severity of their smell loss using a simple scale of no, mild, moderate, or severe symptoms, which may reflect everyday clinical practice more closely than psychophysical tests. Analysis of baseline characteristics by baseline LoS severity indicated that patients with severe LoS at baseline had a higher prevalence of prior nasal polyp surgery, coexisting asthma, and NSAID-ERD and higher baseline NC, LMK-CT, and SNOT-22 scores than patients with moderate LoS. Association of smell loss severity with SNOT-22 is in agreement with the established impact of LoS on HRQoL.2,6,7 Association of smell loss severity with prior surgery may reflect longer-standing and more severe disease compared with patients without surgery, and also illustrates an unmet need following surgery in CRSwNP.
Proposed pathophysiological mechanisms for smell loss in CRSwNP include reduced airflow to the olfactory cleft due to the presence of nasal polyps and/or a sensorineural deficit due to inflammation in the olfactory mucosa. 27 The involvement of a mechanism beyond a simple reduction in airflow is supported by findings that olfactory dysfunction in CRSwNP measured using Sniffin’ Sticks does not depend on nasal obstruction as assessed by either polyp size or airflow limitation.28,29 Our results confirm an absence of association between nasal polyp size and a simple subjective measure of smell loss in a large cohort with uncontrolled CRSwNP, further lending weight to a pathophysiology of smell loss involving sensorineural deficit. Possible biochemical mechanisms underlying such a deficit are emerging with evidence from studies on the impact of type 2 inflammation on olfaction using mouse models, including a direct effect of IL-4 on olfactory sensory neurons and IL-13-induced loss of neurons from the olfactory epithelium.30,31
Dupilumab improved symptoms and objective CRSwNP outcomes in the SINUS trials, 18 but the effect of baseline LoS severity on dupilumab treatment effect was unknown. We found that dupilumab significantly improved all analyzed CRSwNP outcomes and HRQoL versus placebo in patients with moderate LoS and in patients with severe LoS at baseline. For the patient-reported endpoints, NC, RS-VAS, and SNOT-22, the magnitude of improvements with dupilumab at Week 24 were greater in the severe baseline LoS subgroup than the moderate baseline LoS subgroup. This may reflect the greater baseline severity, and therefore greater scope for improvement, observed in these measures in the severe baseline LoS subgroup compared with the moderate baseline LoS subgroup. For the objective endpoints, NPS and LMK-CT, baseline differences between LoS subgroups were proportionately smaller and no difference was observed between LoS subgroups in the magnitude of dupilumab treatment effect at Week 24. Looking at Week 52 (SINUS-52 study only), a similar pattern of results was seen except that the difference in magnitude of improvement in RS-VAS with dupilumab between LoS subgroups did not reach statistical significance. The similarity of magnitude of improvement in NPS and LMK-CT with dupilumab regardless of subjective degree of smell loss at baseline further supports a pathophysiology of smell loss that can be reversed independently of reductions in polyp size and sinus opacity. 29
Limitations of this analysis include that it was post hoc and that the LoS score is not validated. In addition, the SINUS studies involved patients with severe CRSwNP uncontrolled by standard of care, which limits the generalizability of our findings to patients with milder disease. As patients had to have an NPS score of ≥5 for inclusion in the SINUS studies, the associations we found may not apply in patients with NPS ≤4. Moreover, there was little variation in LoS scores (83% severe, 15% moderate), with a smaller subset of patients with mild smell loss, which limited the variability available for association analyses.
In conclusion, this analysis shows that increased severity of smell loss is associated with greater severity of NC, RS-VAS, LMK-CT, greater impact on HRQoL (SNOT-22), and greater prevalence of respiratory comorbidities in patients with severe CRSwNP. Dupilumab significantly improved NPS, NC, LMK-CT, RS-VAS, and SNOT-22 in subgroups with moderate LoS and severe LoS at baseline.
Supplemental Material
Supplemental material, sj-docx-1-ajr-10.1177_19458924241274501 for Association Between Smell Loss, Disease Burden, and Dupilumab Efficacy in Chronic Rhinosinusitis with Nasal Polyps by Zachary M. Soler, Zara M. Patel, Joaquim Mullol, Jose Mattos, Scott Nash, Changming Xia, Zhixiao Wang, Kinga Borsos, Mark Corbett, Juby A. Jacob-Nara, Harry Sacks, Paul Rowe, Yamo Deniz and Andrew P. Lane in American Journal of Rhinology & Allergy
Acknowledgments
The authors thank Asif Khan (Sanofi) for insights and guidance. Medical writing/editorial assistance provided by Neil Anderson, PhD, of Adelphi Group, Macclesfield, UK, funded by Sanofi and Regeneron Pharmaceuticals, Inc. according to the Good Publication Practice guideline.
Footnotes
Author Contributions: All authors contributed to the concept and design of this study and to the data analysis and interpretation. All authors contributed to drafting or revising the manuscript, agreed on the journal to which the article would be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Zachary M. Soler has been a consultant or advisory board member for Lyra, Novartis, Olympus, and Optinose; he has received speaker's fees from GlaxoSmithKline and Regeneron Pharmaceuticals Inc.; and held the post of medical director at Healthy Humming. Zara M. Patel has been a consultant or advisory board member for Dianosic, InfiniteMD, Mediflix, Medtronic, and Optinose and the Chief Medical Officer of Olfera Therapeutics. Joaquim Mullol has participated in a speakers’ bureau or advisory board or received a research grant from AstraZeneca, Genentech, Inc., GlaxoSmithKline, Glenmark, Menarini, Mitsubishi-Tanabe, Merck Sharp & Dohme, Viatris (Mylan-MEDA), Novartis, Procter & Gamble, Regeneron Pharmaceuticals Inc., Sanofi, and the NOUCOR/Uriach Group. Jose Mattos has no financial disclosures or conflicts of interest. Scott Nash, Changming Xia, Zhixiao Wang, Harry Sacks, and Yamo Deniz are employees of Regeneron Pharmaceuticals and may hold stock and/or stock options in the company. Kinga Borsos is a former employee of Sanofi and may hold stock and/or stock options in the company. Mark Corbett, Juby A. Jacob-Nara, and Paul Rowe are employees of Sanofi and may hold stock and/or stock options in the company. Andrew P. Lane is a member of the advisory boards of Sanofi and Regeneron Pharmaceuticals Inc.
Ethical Approval: The SINUS-24 and SINUS-52 study protocols were approved by Copernicus Group IRB (protocol EFC14146, tracking no. SAN4-16-406; protocol EFC14280, tracking no. SAN4-16-417).
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This research was sponsored by Sanofi (Bridgewater, NJ, USA) and Regeneron Pharmaceuticals, Inc. (Tarrytown, NY, USA).
ORCID iDs: Zachary M. Soler https://orcid.org/0000-0002-4304-595X
Zara M. Patel https://orcid.org/0000-0003-2072-982X
Jose Mattos https://orcid.org/0000-0001-8766-1626
Andrew P. Lane https://orcid.org/0000-0001-6369-5469
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Bachert C, Marple B, Schlosser RJ, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020;6(1):86. [DOI] [PubMed] [Google Scholar]
- 2.Mullol J, Azar A, Buchheit KM, et al. Chronic rhinosinusitis with nasal polyps: Quality of life in the biologics era. J Allergy Clin Immunol Pract. 2022;10(6):1434–1453.e1439. [DOI] [PubMed] [Google Scholar]
- 3.Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. [DOI] [PubMed] [Google Scholar]
- 4.Mullol J, Bachert C, Amin N, et al. Olfactory outcomes with dupilumab in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2022;10(4):1086–1095.e1085. [DOI] [PubMed] [Google Scholar]
- 5.Laidlaw TM, Mullol J, Woessner KM, et al. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9(3):1133–1141. [DOI] [PubMed] [Google Scholar]
- 6.Schlosser RJ, Dubno JR, Eckert MA, et al. Unsupervised clustering of olfactory phenotypes. Am J Rhinol Allergy. 2022;36(6):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochet M, El-Hage W, Richa S, et al. Depression, olfaction, and quality of life: A mutual relationship. Brain Sci. 2018;8(5):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Head K, Chong LY, Hopkins C, et al. Short-course oral steroids alone for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4(4):Cd011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Corso E, Pipolo C, Cantone E, et al. Survey on use of local and systemic corticosteroids in the management of chronic rhinosinusitis with nasal polyps: Identification of unmet clinical needs. J Pers Med. 2022;12(6):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen DT, Bonfort G, Arous F, et al. Evaluation of residual symptoms: A method to assess surgical outcomes for nasal polyposis. Am J Rhinol Allergy. 2016;30(2):e36–e41. [DOI] [PubMed] [Google Scholar]
- 11.DeConde AS, Mace JC, Levy JM, et al. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riva G, Tavassoli M, Cravero E, et al. Long-term evaluation of nasal polyposis recurrence: A focus on multiple relapses and nasal cytology. Am J Otolaryngol. 2022;43(2):103325. [DOI] [PubMed] [Google Scholar]
- 13.Wu AW, Ting JY, Platt MP, et al. Factors affecting time to revision sinus surgery for nasal polyps: A 25-year experience. Laryngoscope. 2014;124(1):29–33. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111(14):5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111(14):5153–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. [DOI] [PubMed] [Google Scholar]
- 17.Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. [DOI] [PubMed] [Google Scholar]
- 19.Alt JA, Mace JC, Buniel MC, et al. Predictors of olfactory dysfunction in rhinosinusitis using the brief smell identification test. Laryngoscope. 2014;124(7):E259–E266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(2):139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito T, Tsuzuki K, Yukitatsu Y, et al. Correlation between olfactory acuity and sinonasal radiological findings in adult patients with chronic rhinosinusitis. Auris Nasus Larynx. 2016;43(4):422–428. [DOI] [PubMed] [Google Scholar]
- 22.Lee SE, Amin N, Mannent LP, et al. The relationship of sinus opacification, olfaction and dupilumab efficacy in patients with CRSwNP. Rhinology. 2023;61(6):531–540. [DOI] [PubMed] [Google Scholar]
- 23.Han JK, Bachert C, Lee SE, et al. Estimating clinically meaningful change of efficacy outcomes in inadequately controlled chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2022;132(2):265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. 2009;34(5):447–454. [DOI] [PubMed] [Google Scholar]
- 25.Claeys N, Teeling MT, Legrand P, et al. Patients unmet needs in chronic rhinosinusitis with nasal polyps care: A patient advisory board statement of EUFOREA. Front Allergy. 2021;2:761388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soler ZM, Smith TL, Alt JA, et al. Olfactory-specific quality of life outcomes after endoscopic sinus surgery. Int Forum Allergy Rhinol. 2016;6(4):407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi HA, Lane AP. Olfaction now and in the future in CRSwNP. Am J Rhinol Allergy. 2023;37(2):168–174. [DOI] [PubMed] [Google Scholar]
- 28.Gelardi M, Piccininni K, Quaranta N, et al. Olfactory dysfunction in patients with chronic rhinosinusitis with nasal polyps is associated with clinical-cytological grading severity. Acta Otorhinolaryngol Ital. 2019;39(5):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantone E, De Corso E, Ricciardiello F, et al. Olfaction recovery following dupilumab is independent of nasal polyp reduction in CRSwNP. J Pers Med. 2022;12(8):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara Y, Jha MK, Mattoo H, et al. Interleukin 4 directly activates olfactory neurons and induces loss of smell in mice. J Allergy Clin Immunol. 2023;151(2, Supplement):AB128. [Google Scholar]
- 31.Saraswathula A, Liu MM, Kulaga H, et al. Chronic interleukin-13 expression in mouse olfactory mucosa results in regional aneuronal epithelium. Int Forum Allergy Rhinol. 2023;13(3):230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ajr-10.1177_19458924241274501 for Association Between Smell Loss, Disease Burden, and Dupilumab Efficacy in Chronic Rhinosinusitis with Nasal Polyps by Zachary M. Soler, Zara M. Patel, Joaquim Mullol, Jose Mattos, Scott Nash, Changming Xia, Zhixiao Wang, Kinga Borsos, Mark Corbett, Juby A. Jacob-Nara, Harry Sacks, Paul Rowe, Yamo Deniz and Andrew P. Lane in American Journal of Rhinology & Allergy