Abstract
Objectives:
Laparoscopic myomectomy (LM) is a procedure with a high rate of postoperative adhesions, which can lead to complications such as bowel obstruction and subsequent surgical problems, making anti-adhesion measures important. Various anti-adhesion materials are already on the market and have shown efficacy, but dextrin hydrogel spray (AdSpray™), an anti-adhesion material launched in 2017, has not yet been reported in the field of gynecology, although there are papers showing its usefulness in the surgical field such as repeat hepatectomy and ileostomy closure. Thereby, we investigated the postoperative status of AdSpray™ in LM.
Materials and Methods:
We report 24 cases in which AdSpray™ was used at Teine Keijinkai Hospital from 2018 to 2021 for LM, followed by cesarean section (CS) from 2019 to 2022. Adverse effects related to AdSpray™ and the presence of uterine adhesions in CS were examined.
Results:
Adhesions were observed in 4 (16.7%) cases, none of which resulted in significant adverse effects of AdSpray™.
Conclusion:
AdSpray™ was effective in preventing adhesion and may be an option as an anti-adhesion material in LM.
Keywords: Adhesion, AdSpray™, cesarean section, dextrin hydrogel spray, laparoscopic myomectomy
INTRODUCTION
Laparoscopic myomectomy (LM) is a function-preserving procedure, so it is not uncommon to perform a subsequent cesarean section (CS) or myomectomy, etc., In post-LM surgery, adhesions of the intestinal tract or bladder to the uterus can cause complications. In addition, adhesions can cause bowel obstruction and infertility, so the occurrence of adhesions must be minimized. Therefore, the use of anti-adhesive materials in LM is important.
Adhesion barriers have already been used in film or sheet form and have received some positive feedback, but there are few reports on the effectiveness of gel type, AdSpray™ (Terumo Corporation, Tokyo, Japan), which was launched in 2017, in preventing adhesions.
AdSpray™ is a site-specific sprayable adhesion barrier gel based on a dextrin polymer. It is composed of N-hydroxysuccinimide-modified carboxymethyl dextrin polymer with trehalose (alpha-linked disaccharide), and the second part of this is a standard alkaline sodium hydrogen carbonate/sodium carbonate buffer agent. When sprayed together, the dextrin polymers link to form a hydrogel barrier within 10 s, a hydrogel predominantly of dextrin polymers and microbubbles consisting of 60%–95% water with solids. These microbubbles within the gel provide an opaqueness that allows easier visualization of the gel placement, thickness, and coverage.[1] The nozzle is designed to facilitate spraying in laparoscopic surgery: the nozzle can be inserted through a 5-mm trocar, allowing spraying from any trocar, and its tip can be curved intraperitoneally to target most sites. This formulation may offer advantages compared to some other available products because it can be uniformly applied to targets with three-dimensional field such as the pelvis and allowing precise control over the amount of barrier agents applied. AdSpray™, as well as INTERCEED® and Seprafilm®, has been also retrospectively reported to be correlated with a lower adhesion severity scores around the liver.[2]
No publications on the incidence of adhesions after AdSpray™ use in gynecologic surgery have yet been reported as of November 2023, as far as we could find in MEDLINE and Google Scholar. Therefore, we report a study of the occurrence of adhesions in patients who underwent LM using AdSpray™ and were subsequently observed for the presence of adhesions during CS.
MATERIALS AND METHODS
The study was conducted in accordance with the Declaration of Helsinki and was approved by Teine Keijinkai Hospital Ethics Committee with (approval number 2-019016-00; approval date: 08/21/2019). Written consent to participate in the study was obtained in all cases. We investigated the presence of adhesions in 24 patients who used AdSpray™ as an adhesion barrier at the time of LM from 2018 to 2021 and subsequently underwent CS from 2019 to 2022 at Teine Keijinkai Hospital. The results of LM were examined about the operation time, amount of hemorrhage, total weight and number of enucleated fibroids, location of fibroids, and duration from LM to CS. Adhesions in CS were defined as present if the surgical record of the CS showed adhesions around the uterus, and were evaluated by Zuhlke’s adhesion classification system [Table 1].[3] Adverse effects which were likely to derive from AdSpray™, such as allergic reaction, infection, and the occurrence of ileus after LM, were examined from clinical records.
Table 1.
Zuhlke’s adhesion classification system
| Grade | Status of Adhesions |
|---|---|
| 0 | No adhesions |
| 1 | Filmy adhesions and easily separated by blunt dissection |
| 2 | Blunt dissection is possible but sharp dissection is necessary, beginning of vascularization |
| 3 | Lysis is possible by only sharp dissection, clear vascularization |
| 4 | Lysis is possible by only sharp dissection, organs are strongly attached |
LM was performed by insufflation in all cases, the myometrium was incised with an ultrasonic incision device, the myometrium was sutured with multifilament or barb thread, and AdSpray™ was applied to the targeted area by mixing two liquids set in a special nozzle [Figure 1]. The pressure of the spray is set at 0.1 MPa, and the gas is evacuated through another hole in the nozzle so that insufflation pressure is not increased and venous thrombosis is prevented.
Figure 1.
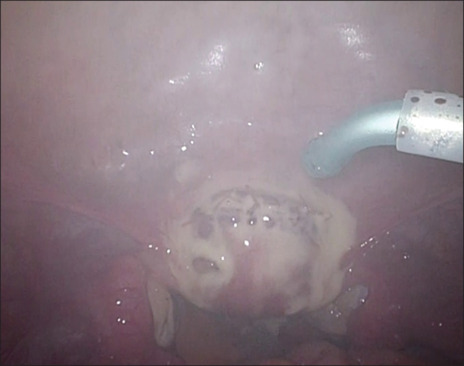
Image of applying AdSpray™ with curved nozzle
RESULTS
The backgrounds of patients and the results of LM are noted in Table 2. The median of duration from LM to CS was 16 months. The locations of fibroids in LM were both sides of the anterior and posterior walls of the uterus in 66.7% of 24 cases. All cases of LM were performed by six gynecological endoscopy technology-accredited doctors.
Table 2.
Backgrounds
| Median (range) | |
|---|---|
| LM | |
| Age | 37.5 (28–45) |
| BMI | 22.2 (17.5–31.4) |
| Operation time (min) | 109 (62–179) |
| Amount of hemorrhage (g) | 20 (10–700) |
| Weight of enucleated fibroids (g) | 58 (0.2–468) |
| Number of enucleated fibroids | 7 (1–19) |
| Location of fibroids | |
| A | 6 cases |
| P | 2 cases |
| A and P | 16 cases |
| Age at CS | 39 (30–46) |
| Duration from LM to CS (months) | 16 (10–55) |
LM: Laparoscopic myomectomy, BMI: Body mass index, CS: Cesarean section, A: Anterior wall of uterus, P: Posterior wall of uterus
Adhesions were observed in 4 (16.7%) cases, and no adverse effects derived from AdSpray™ were seen [Table 3].
Table 3.
Incidence of adhesion and adverse effect
| Number of cases | |
|---|---|
| n | 24 |
| Number of cases of adhesion (%) | 4 (16.7) |
| Adverse effect | 0 |
The intra-abdominal findings of the four cases with adhesions are shown in Table 4. The grade of Zuhlke’s adhesion classification was 1–3, and there were no severe adhesions. In case 2 and 4, adhesions were present on posterior uterine wall and absent on anterior one, which did not interfere with CS, therefore adhesion stripping was not necessary. In case 1, membranous adhesions were manually removed. In case 3, adhesions between uterus and mesentery were removed electrocautery. In the other one case, membranous adhesions in both the anterior and posterior walls of the uterus were manually removed.
Table 4.
Cases of adhesion
| Case | Age at LM | Duration from LM to CS (months) | Zuhlke’s adhesion grade | Status of adhesion | Influence on CS |
|---|---|---|---|---|---|
| 1 | 39 | 12 | 1 | Membranous adhesions were noted between the anterior uterine wall and the ventral peritoneum, and between the posterior uterine wall and the rectum | Adhesions were removed manually |
| 2 | 37 | 10 | 3 | Adhesions were noted between the uterine posterior wall and the omentum | Adhesion stripping was unnecessary |
| 3 | 32 | 55 | 3 | Adhesions were noted between the posterior uterus and the mesentery | Adhesions were removed with electrocautery |
| 4 | 31 | 23 | 2 | Adhesions were noted between the left side of the posterior uterine wall and the intestinal fatty tissue | Adhesion stripping was unnecessary |
LM: Laparoscopic myomectomy, CS: Cesarean section
DISCUSSION
Uterine fibroids can cause symptoms such as excessive menstruation and abdominal mass sensation, as well as infertility and complications during pregnancy. However, since pregnancy after LM can lead to uterine rupture, CS is often performed before the onset of labor, and adhesions during LM can lead to complications such as ileus, as well as risk of bladder and bowel injury during subsequent CS. Considering that the recurrence rate is more than 60% after LM[4] and that complications are more common in revision surgery, it is important to perform adhesion prophylaxis at the time of LM to prevent subsequent problems.
Physical barriers are commonly used to prevent peritoneal adhesion, and several products are commercially available serving as adhesion barriers. Typically, these barriers are classified into three types based on their physical properties: solid films or membranes, solutions, and hydrogels. In Japan, solid films and membranes represented by hyaluronate carboxymethylcellulose (Seprafilm®) and oxidized regenerated cellulose (INTERCEED®) have been utilized for decades. With the increasing adoption of laparoscopic surgery, INTERCEED® and a new hydrogel barrier called AdSpray™ are routinely applied. Table 5 provides a summarized comparison of each type of adhesion barriers.[5,6,7]
Table 5.
A summary overview comparing each type of adhesion barriers (5–7)
| Adhesion barrier types | Materials | Clinical evidence | Usability |
|---|---|---|---|
| Solid membranes | Oxidized regenerated cellulose membrane | Shown to be more effective than Seprafilm® in some pelvic operations when completely hemostatic Reported to have no significant anti-adhesion effect in certain animal models Significantly reduced the overall incidence of adhesions |
Applied more easily than Seprafilm because of its flexibility and good adhesiveness on wounds |
| Solution (not available in Japan) | Icodextrin solution | Reduced adhesion in several procedures Adverse effects were reported such as extravasation, transient labial edema, incidence of small-bowel obstruction |
Solution can diffuse throughout the peritoneal cavity |
| Hydrogel | Gel-based dextrin polymer | Reduced incidence and severity of peritoneal adhesion in laparotomy | Easily applied during laparoscopy using a dedicated sprayer |
AdSpray™ is a new anti-adhesion material with a special nozzle designed for easy and safe use. It has been shown to be effective in preventing uterine adhesions in porcine ileostomies.[8] In humans, an randomized control trial investigated the occurrence of adhesions in patients who underwent a temporary ileostomy with open rectal resection and subsequent ileostomy, in which the incidence of adhesions was significantly lower in the AdSpray™ group (53%) than in the control group (91%).[9] As to the safety of AdSpray™, adverse effects related to device have not been reported. All events were estimated as not related or unlikely to be related.[10] In cases of this study, adverse effects were not shown.
Regarding post-LM adhesions, INTERCEED® has been shown to be effective, for example, the adhesion rate between the INTERCEED® treated group and the control group was 40% versus 88%[11] and 15.9% versus 22.6%[12] in the other report. As to the use of Seprafilm® during myomectomy, it was reported to decrease both the incidence and degree of adhesions compared to the untreated group.[6] Although not directly comparable, the 16.7% adhesion rate with the use of AdSpray™ seems comparable to the 15.9% adhesion rate with INTERCEED®.[12]
The limitation of this study was that confounding factors were not analyzed enough. Possible confounding factors regarding the occurrence of adhesions during LM include the site and length of the uterine incision, suture method, and thread type.[13] However, It is difficult to classify those factors clearly. Cases in this study included many multiple fibroids, so it was difficult to categorize the location and the length of incision, and suturing methods and threads were various in one operation. In this study, confounding factors could not be mentioned because it was not possible to align types of cases.
Although this study does not contain so many cases, it has significance that this report is the first study of post-LM adhesions using AdSpray™, observed in the following CS as a second-look operation.
CONCLUSION
Since there are few reports on dextrin hydrogel spray in gynecologic surgery, we investigated the adhesion status at the time of CS in cases of pregnancy after LM. In contrast to the reportedly high incidence of adhesions after LM without adhesion barriers, the incidence in cases using AdSpray™ was 16.7%. Compared to previous literature using INTERCEED® in LM, the effectiveness of AdSpray™ is not so different.
Although we cannot assert the effectiveness of AdSpray™ because it is not a case–control study, we believe that the incidence is sufficiently low.
Author contributions
All authors contributed to the data generation at the time of surgery, and Wada planned and wrote the manuscript. All authors have read and agreed to the final version of the manuscript.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We greatly thank Ms. Kawabata and Ms. Kameya for constructing the database of operations.
REFERENCES
- 1.Cezar C, Korell M, Tchartchian G, Ziegler N, Senshu K, Herrmann A, et al. How to avoid risks for patients in minimal-access trials: Avoiding complications in clinical first-in-human studies by example of the ADBEE study. Best Pract Res Clin Obstet Gynaecol. 2016;35:84–96. doi: 10.1016/j.bpobgyn.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Okubo S, Shindoh J, Kobayashi Y, Matsumura M, Hashimoto M. Adhesions as a risk factor for postoperative morbidity in patients undergoing repeat hepatectomy and the potential efficacy of adhesion barriers. J Hepatobiliary Pancreat Sci. 2022;29:618–28. doi: 10.1002/jhbp.1047. [DOI] [PubMed] [Google Scholar]
- 3.Zühlke HV, Lorenz EM, Straub EM, Savvas V. Pathophysiology and classification of adhesions. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990:1009–16. [PubMed] [Google Scholar]
- 4.Kotani Y, Tobiume T, Fujishima R, Shigeta M, Takaya H, Nakai H, et al. Recurrence of uterine myoma after myomectomy: Open myomectomy versus laparoscopic myomectomy. J Obstet Gynaecol Res. 2018;44:298–302. doi: 10.1111/jog.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang J, Xiang Z, Bernards MT, Chen S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 2020;116:84–104. doi: 10.1016/j.actbio.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad G, Kim K, Thompson M, Agarwal P, O’Flynn H, Hindocha A, et al. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2020;3:CD000475. doi: 10.1002/14651858.CD000475.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ten Broek RP, Stommel MW, Strik C, van Laarhoven CJ, Keus F, van Goor H. Benefits and harms of adhesion barriers for abdominal surgery: A systematic review and meta-analysis. Lancet. 2014;383:48–59. doi: 10.1016/S0140-6736(13)61687-6. [DOI] [PubMed] [Google Scholar]
- 8.Kai M, Maeda K, Tasaki M, Kira S, Nakamura S, Chino N, et al. Evaluation of a spray-type, novel dextrin hydrogel adhesion barrier under laparoscopic conditions in a porcine uterine horn adhesion model. J Minim Invasive Gynecol. 2018;25:447–54. doi: 10.1016/j.jmig.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Suto T, Watanabe M, Endo T, Komori K, Ohue M, Kanemitsu Y, et al. The Primary result of prospective randomized multicenter trial of new spray-type bio-absorbable adhesion barrier system (TCD-11091) against postoperative adhesion formation. J Gastrointest Surg. 2017;21:1683–91. doi: 10.1007/s11605-017-3503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cezar C, Tchartchian G, Korell M, Ziegler N, Senshu K, De Wilde MS, et al. Long term follow-up concerning safety and efficacy of novel adhesion prophylactic agent for laparoscopic myomectomy in the prospective randomized ADBEE study. Best Pract Res Clin Obstet Gynaecol. 2016;35:97–112. doi: 10.1016/j.bpobgyn.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de-novo adhesion formation after laparoscopic myomectomy: A randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Hum Reprod. 1995;10:3133–5. doi: 10.1093/oxfordjournals.humrep.a135873. [DOI] [PubMed] [Google Scholar]
- 12.Tinelli A, Malvasi A, Guido M, Tsin DA, Hudelist G, Hurst B, et al. Adhesion formation after intracapsular myomectomy with or without adhesion barrier. Fertil Steril. 2011;95:1780–5. doi: 10.1016/j.fertnstert.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann A, Torres-de la Roche LA, Krentel H, Cezar C, de Wilde MS, Devassy R, et al. Adhesions after laparoscopic myomectomy: Incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190–7. doi: 10.4103/GMIT.GMIT_87_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


