Abstract
BACKGROUND:
Antenatal fetoscopic endoluminal tracheal occlusion (FETO) has been introduced as an effective intervention to improve the outcome of severe congenital diaphragmatic hernia (CDH).
OBJECTIVE:
We report our early experience with FETO.
DESIGN:
A retrospective chart review of case series.
SETTING:
Tertiary health care center.
PATIENTS AND METHODS:
18–45 years old, with single fetuses diagnosed with left severe CDH (lung-head ratio <1 measured between 27–29 weeks of gestational age (GA) and liver up or observed/expected lung-to-head ratio <25%, normal echocardiogram and karyotype were included. FETO was performed between 28–30 weeks of gestation and removed after 4–6 weeks or at birth during an ex utero intrapartum treatment (EXIT) procedure.
MAIN OUTCOME MEASURES:
FETO represents a viable option for severe type of CDH fetuses with reasonable outcomes. FETO performance in low volume centers may be feasible with reasonable outcomes. Good outcome of postnatal care with no potential antenatal complications may affect FETO adoption in some societies.
SAMPLE SIZE:
5
RESULTS:
14 pregnant women were referred for assessment and only 7 met the inclusion criteria. Two were excluded initially (late referral and spouse refusal) and a 3rd excluded later due to failure of FETO due to faulty balloons. The median age of the mothers was 28 years and the gestational age was 29 weeks. Median observed/expected lung-to-head ratio was 23%. Among patients who had successful FETO, one had the balloon removed fetoscopically 4 weeks after insertion and one was removed 8 weeks after insertion during an elective EXIT procedure and both have survived. The other two had premature labor after 1 and 5 weeks after FETO and balloon removed during an emergency EXIT procedures, and both died within 24 hours of birth.
CONCLUSION:
FETO represents a viable option for severe type of CDH fetuses with reasonable outcome. FETO performance in a low volume centers may be feasible with reasonable outcomes. Good outcome of postnatal care with no potential antenatal complications may affect FETO adoption in some societies.
LIMITATIONS:
Retrospective nature of the study may imply inaccuracy, but we believe data from electronic medical records is highly accurate.
INTRODUCTION
Congenital diaphragmatic hernia (CDH) affects 1 in every 2500–5000 newborns1 and carries high mortality.2 Several postnatal options and lately antenatal interventions were developed that lead to improvement in survival by 15–20% in the high risk group.3–5 However, despite improvement of neonatal intensive care and ventillatory strategies, a subset of CDH continued to have dismal outcomes.2 Fetoscopic endoluminal tracheal occlusion (FETO) has been introduced as an antenatal intervention that could impact the pathogenesis of the disease and improve outcome in the very severe CDH group. FETO was shown to be feasible and effective in many observational studies6,7 and two recent randomized clinical trial (The TOTAL trial (https://www.totaltrial.eu/) has shown its effectiveness.8 We report our early experience at with FETO.
PATIENTS AND METHODS
Pregnant women, 18–45 years old, with single fetuses diagnosed with left severe CDH (lung-head ratio <1 measured between 27–29 weeks of GA and liver up or observed/expected lung-to-head ratio <25%, normal echocardiogram and karyotype with no major fetal anomalies were included. FETO was performed between 28–30 weeks of gestation and removed after 4–6 weeks if possible or at birth with an ex utero intrapartum treatment (EXIT) procedure. Data were collected prospectively including demographic, clinical, and outcome data between January 2018 and June 2022. Descriptive data were generated for patients who underwent FETO (5 patients).
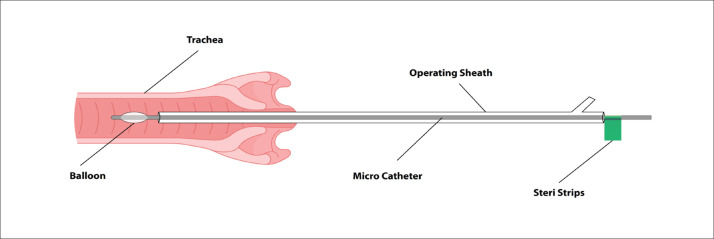
The procedure was performed using an operating fetoscopy sheath (11630 KF, STORZ) introduced via an 11 FR introducer sheath (brite tip SHEATH, Cordis) inserted using Seldinger technique under US guidance. A miniature straight forward telescope 0° (11630 AA, SRORZ) was utilized and the trachea was entered via the fetal oral cavity. Once at the trachea, the sheath was stabilized manually and the fetoscope was removed. The flow-dependent microcatheter with progressive suppleness for detachable balloons (BALTACCI-BDPE100, BALT Delivery Microcatheter) was utilized and prepared pre-procedural and the balloon (GOLDBAL2, BALT) was mounted on its tip after testing its size and competency and a mark (steristrip) was placed on the microcatheter to ensure the exit of the balloon from the sheath and its inflation safely (Figure 1). It is inserted at the main working channel and once inserted up to the mark, the balloon is inflated with 0.7 mL of distilled water under US guidance. When the balloon is inflated, we detach the delivery microcatheter from the balloon and reinsert the fetoscope to check balloon position in the trachea and its fitness. Postoperatively, 100 mg indomethacin rectal suppository is given followed by another dose in 12h interval.
Figure 1.
Fetoscopy with sheath/balloon in fetal trachea.
RESULTS
Fourteen pregnant women were referred for assessment at King Faisal Specialist Hospital and Research Centre-Riyadh and only 7 met the inclusion criteria. Two were excluded initially (late referral and spouse refusal). Five patients underwent FETO, 4 were successful and one failed (Table 1). FETO in the latter patient failed due to faulty balloons despite repeated successful intrauterine intubation of the trachea. The median duration of FETO was 23 minutes. There was no complications of the procedure. The median age of the mothers was 28 years and the gestational age was 29 weeks. TThe lung-to-head ratio was 0.8 (0.7–0.9). The median observed/expected lung-to-head ratio was 23% (22%-24%) and all had liver up in the chest. One balloon was removed antenatally in one patient 4 weeks after FETO fetoscopically, and the baby was born at 38 weeks of gestation by elective C-section. The second was removed 8 weeks after FETO during EXIT after failing to remove it fetoscopically 6 weeks after FETO due to the size and position of the fetus. Both were repaired postanatally (age 2 and 3 days respectively) using composite patch (MarlexTM, CR Bard, Murray Hill, NJ). Both survived and were discharged at 1 month and 2 weeks old respectively. The other two had premature labor after 1 and 5 weeks after FETO and the balloon was removed during emergency EXIT procedures, and both died within 24 hours of birth due to respiratory failure secondary to prematurity and pulmonary hypertension.
Table 1.
Characteristics of patients who had fetoscopic endoluminal tracheal occlusion and their outcomes.
| Serial Number | Mother's age (years) and parity | LHR:a OE LHR (%) | GAb at balloon insertion (weeks) | GA and mode of balloon removal (weeks)/GA at birth (weeks) | Outcome/Gender (Male=M, Female=F) |
|---|---|---|---|---|---|
| 1 | 37 (G9P5+3) | 0.8:23 | 27+5 | 32 Fetoscopic/38 elective C-section | Alive and well (5 y/F) |
| 2 | 27 (G4P1+2) | 0.7:22 | 29+5 | 30+4 (premature labor)/Emergency EXIT) | Death at 19h of age/M |
| 3 | 31 (G3P2) | 0.9:22 | 29+6 | 34 (premature labor)/Emergency EXIT) | Death at 1h of age/F |
| 4 | 28 (G2P1) | 0.8:24 | 28+4 | 36/36 (elective EXIT) | Alive and well (3 years/M) |
| 5 | 27 (G1P1) | 0.8:23 | 29+2 | NA (insertion failed)/39 SVD | Death at 36h of age/M |
Lung/head ratio
Gestational age, OE LHR: Observed/expected lung-to-head ratio (%); EXIT: Ex Utero Intrapartum Treatment procedure.
The other three patients (including the failed FETO) were followed up. One was born at 40 weeks of GA but the baby died 24 hours after birth. The second was born on the 39th weeks of GA and had CDH repair 2nd day of life with good outcome. The last one, who had a failed FETO, was born with spontaneous vaginal delivery at 39th week of gestation but died at 36 hours after birth with severe pulmonary hypertension.
DISCUSSION
CDH survival has improved over the years with many postnatal interventions starting from conventional ventilation, high frequency jet ventilation, surfactant treatment, permissive hypercapnia, pulmonary hypertension treatment with nitric oxide and other medications, and inotropes. Despite this improvement, survival was poor.2 Extracorporeal membrane oxygenation (ECMO) was introduced (1977) as a management option for severe types of CDH. Although ECMO initially was met with high enthusiasm and high expectations, variability of its inclusion criteria among centers, its complications, and comparable survival rates to conventional management have limited its use in many centers.9,10 Surgical repair, although considered an emergency earlier, it is now done after stabilization of the patient. The later approach acknowledges that surgical repair impose some stress on CDH patients, hence stabilization preoperatively ensures some reserve postoperatively for these sick patients.
Early accurate antenatal diagnosis, as early as 14 weeks of gestation, and poor outcomes in a certain group of CDH patients managed postnatally, have led to consideration of antenatal intervention.2,11–13 Earlier, surgical repair was contemplated antenatally with some enthusiasm,14 but later was shown to have high mortality rate15 in high-risk groups and considered aggressive given the dismal results and the negative impact of open fetal surgery on the mothers' future pregnancies. Based on the fact that congenital high airway obstruction syndrome has resulted in hyperplastic lungs at birth, which was shown experimentally,16–18 tracheal occlusion was entertained as a therapeutic option for CDH. Tracheal occlusion is believed to work by increasing airway pressure, hence promoting proliferation of alveoli which increases its airspace and possibly accelerates the maturation of the pulmonary vasculature.19 Moreover, it was also noticed that intrauterine reversal of tracheal occlusion (plug-unplug sequence) have improved lung maturation by enhancing type II pneumocytes regeneration.20 Few methods of intrauterine tracheal occlusion were developed and attempted. Early options including external tracheal clips and intraluminal foam were technically feasible but conveyed a high risk on mothers, induced premature labor, required an EXIT, and had modest outcomes.21–23 Subsequently, FETO was developed taking into account the plug-unplug sequence where few occlusive options that can be reversed were developed.24,25 Currently, the most popular devise used in FETO is the detachable balloon.26–28 There are three randomized clinical trials, to our knowledge, in the English literature8,29,30 that have attempted to address the benefits of FETO over standard postnatal care (Table 2). The first one29 had been terminated early due to an unexpected high survival rate in postnatal care group; a rate that was significantly higher than the one reported from the same center earlier.2 This may be explained by the trial effect. The said trial also have included less severe CDH patients and intervention was performed with a combination of laparotomy and fetoscopy and utilized a two occlusion methods (clips and balloon). The second trial30 found a significant difference between the two arms in favor of the FETO group. This may reflect suboptimal neonatal care. Moreover, there were some concerns related to its methods since it included right-sided CDH (usually has poor outcome compared to the left) and there was no correction of severity assessment to GA.31 The latest8 was multicenter (The TOTAL trials), and recruited more patients. Nonetheless, the recruitment took a long time (9 years) and 72 (43%) eligible candidates declined to participate (28 terminated pregnancy, 25 had FETO outside the trial, 17 had expectant management during pregnancy, and 1 case was lost follow up). The latter may be explained by the fact that FETO was already established in clinical practice by the time the trial was conducted. FETO has shown survival benefits for severe types of CDH in clinical trials when compared to standard postnatal care.8,30
Table 2.
Randomized clinical trials assessing benefits of FETO.
| Author (publication year) | Trial duration (years) | No. of patients received treatment (FETO: postnatal care) | Occlusion (mode: device) | Primary outcome | Survival % (FETO: Postnatal care) |
|---|---|---|---|---|---|
| Harrison MR, et al (2003)29,a | 2 | 11:13 | Laparotomy/fetoscopy: tracheal clips (2), balloon (9) | Survival at 90 days | 73:77 |
| Ruano R, et al (2012)30 | 2 | 19:19 | Fetoscopy:balloon | Survival at 6 months | 52:5.3 |
| Deprest J, et al (2021)8 | 9 | 40:40 | Fetoscopy:balloon | Survival at discharge | 40:15 |
Trial randomized patients with lung/head ratio >1
Applicability of FETO at centers with low number of cases is challenging. The latter could be due to low incidence, lack of advanced antenatal care (lower detection rate), and societal acceptance of fetal interventions. At our center, we had 14 pregnant women referred with antenatally detected CDH over 4.5 years period; of those, only 7 (1.5 cases/year) of them were deemed eligible for FETO (severe CDH). We predict the annual number of CDH in Saudi Arabia to be around 64 cases, based on the disease incidence and local number of deliveries.32 Accordingly, five cases will be annually eligible for FETO due to their severe CDH (based on the reported 8% among all CDH patients in the recent trial).8 This means that we only have seen around 20% of FETO-eligible CDH cases in the country. We believe that nationalization of the fetal program with centralization of its services may help identifying these severe CDH cases early and referring them for FETO at appropriate time if applicable. Our experience has shown that FETO can be performed safely and effectively in a specialized tertiary center; however, the low volume of such complex high-risk procedures should discourage the adoption of FETO by other multiple regional referral centers. When we compared our limited experience with the intervention arm of the last trial,8 the results were comparable (Table 3) acknowledging the difference in methodology between the datasets. On the other hand, advancement of postnatal/neonatal care over the last few decades has significantly improved the survival of severe CDH cases which could limit the adoption of CDH to very few severe cases. Consequently, 72 FETO-eligible subjects have declined to participate during the latest randomized clinical trial.8 Among them, 17 (24%) had expectant postnatal care with 31% survival. This survival rate is higher than the one reported among the randomized patients who received expectant postnatal care (15%). In our small series, three were managed with expectant postnatal care and one survived (33%). On the other hand, some authors have suggested that FETO may be justified in centers with suboptimal neonatal care since it improves fetal lungs antenatally, hence improves survival.33
Table 3.
Comparison between our FETO patients and FETO group of latest trial.
| Current series (n=5) | Treatment arm (TOTAL trial), (n=40) | |
|---|---|---|
| Gestationl age at balloon insertion (weeks) | 29 (27–29) | 28.6 (28–29) |
| Mothers age at FETO (median, years) | 28 | 32 |
| Survival to discharge | 40% | 40% |
| Liver herniation (%) | 100% | 90% |
| Observed/expected lung-to-head ratio % | 23 | 21 |
| Duration of occlusion (days) | 30 | 34 |
Gestational age is median (IQR).
In conclusion, our data have shown that FETO represents a viable option for severe type of CDH fetuses with reasonable outcome. FETO performance in a low case-load centers can be feasible and safe with comparable outcomes. Good outcome of postnatal care with no potential antenatal complications coupled with some societal factors may affect FETO adoption in some populations.
Funding Statement
None.
CONFLICT OF INTEREST:
None.
REFERENCES
- 1.Langham MRJ, Kays DW, Ledbetter DJ, Frentzen B, Sanford LL, Richards DS. Congenital diaphragmatic hernia. Epidemiology and outcome. Clin Perinatol 1996; 23:671–688 [PubMed] [Google Scholar]
- 2.Adzick NS, Harrison MR, Glick PL, Nakayama DK, Manning FA, deLorimier AA. Diaphragmatic hernia in the fetus: prenatal diagnosis and outcome in 94 cases. Journal of pediatric surgery. 1985. Aug 1;20(4):357–61. [DOI] [PubMed] [Google Scholar]
- 3.Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. Journal of pediatric surgery. 2002. Mar 1;37(3):357–66. [DOI] [PubMed] [Google Scholar]
- 4.Heiss KU, Manning PE, Oldham KT, Coran AG, Polley TZ Jr, Wesley JR, et al. Reversal of mortality for congenital diaphragmatic hernia with ECMO. Annals of surgery. 1989. Feb;209(2):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawyer SF, Falterman KW, Goldsmith JP, Arensman RM. Improving survival in the treatment of congenital diaphragmatic hernia. Ann Thoracic Surg 1986; 41:75–78 [DOI] [PubMed] [Google Scholar]
- 6.Harrison MR, Mychaliska GB, Albanese CT, Jennings RW, Farrell JA, Hawgood S, et al. Correction of congenital diaphragmatic hernia in utero IX: fetuses with poor prognosis (liver herniation and low lungto-head ratio) can be saved by fetoscopic temporary tracheal occlusion. J Pediatr Surg 1998;33:1017–23. [DOI] [PubMed] [Google Scholar]
- 7.Wada S, Ozawa K, Sugibayashi R, Suyama F, Amari S, Ito Y, et al. Feasibility and outcome of fetoscopic endoluminal tracheal occlusion for severe congenital diaphragmatic hernia: A Japanese Experience. J Obstet Gynaecol Res 2020; 46:2598–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deprest JA, Nicolaides KH, Bianchi A, Gratacos E, Ryan G, Persico N, et al. Randomized Trial of Fetal Surgery for Severe Left Diaphragmatic Hernia. N Engl J Med 2021; 385:107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azarow K, Messineo A, Pearl R, Filler R, Barker G, Bohn D. Congenital diaphragmatic hernia- a tale of two cities: the Toronto experience. J Pedaitr Surg 1997; 32:395–400 [DOI] [PubMed] [Google Scholar]
- 10.AlShanafey S, Giacomantonio M, Henteleff H. Congenital diaphragmatic hernia: experience without extracorporeal membrane oxygenation. Pediatr Surg Int 2002; 18:28–31 [DOI] [PubMed] [Google Scholar]
- 11.Adzick NS, Vacanti JP, Lillehel CW, O'Rourke PP, Crone RK, Wilson JM. Fetal diaphragmatic hernia: ultrasound diagnosis and clinical outcome in 38 cases. J Pediatr Surg 1989; 24:654–7 [DOI] [PubMed] [Google Scholar]
- 12.Burgos CM, Frenckner B, Luco M, Harting MT, Lally PA, Lally KP, et al. Prenatally versus postnatally diagnosed congenital diaphragmatic hernia–Side, stage, and outcome. Journal of pediatric surgery. 2019. Apr 1;54(4):651–5. [DOI] [PubMed] [Google Scholar]
- 13.Gallot D, Boda C, Ughetto S, Perthus I, Robert-Gnansia E, Francannet C, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol 2007; 29:276–83 [DOI] [PubMed] [Google Scholar]
- 14.Harrison MR, Adzick NS, Longaker MT, Goldberg JD, Rosen MA, Filly RA, et al. Successful repair in utero of a fetal diaphragmatic hernia after removal of herniated viscera from the left thorax. N Engl J Med 1990; 322:1582–4 [DOI] [PubMed] [Google Scholar]
- 15.Harrison MR, Langer JC, Adzick NS, Golbus MS, Filly RA, Anderson RL, et al. Correction of congenital diaphragmatic hernia in utero, V. Initial clinical experience. J Pediatr Surg 1990;25:47–55 [DOI] [PubMed] [Google Scholar]
- 16.Carmel JA, Friedman F, Adams FH. Fetal tracheal ligation and lung development. Am J Dis Child 1965; 109:452–6 [DOI] [PubMed] [Google Scholar]
- 17.Beierle EA, Langham MR, Jr, Cassin S. In utero lung growth of fetal sheep with diaphragmatic hernia and tracheal stenosis. J Pediatr Surg 1996; 31:141–6 [DOI] [PubMed] [Google Scholar]
- 18.Skarsgard ED, Meuli M, VanderWall KJ, Bealer JF, Adzick NS, Harrison MR. Fetal endoscopic tracheal occlusion (‘Fetendo-PLUG) for congenital diaphragmatic hernia. J Pediatr Surg 1996; 31:1335–8 [DOI] [PubMed] [Google Scholar]
- 19.Khan PA, Cloutier M, Piedboeuf B. Tracheal occlusion: a review of obstructing fetal lung to make them grow and mature. Am J Med Genet C Semin Med Genet 2007;145C(2):125–38 [DOI] [PubMed] [Google Scholar]
- 20.Flageole H, Evard VA, Piedboeuf B, Laberge JM, Lerut TE, Deprest JA. The plug unplug sequence: an important step to achieve type II pneumocyte maturation in the fetal lamb model. J Pediatr Surg 1998;33:299–303 [DOI] [PubMed] [Google Scholar]
- 21.Harrison MR, Adzick NS, Flake AW, VanderWall KJ, Bealer JF, Howell LJ, et al. Correction of congenital diaphragmatic hernia in utero VIII: Response of the hypoplastic lung to tracheal occlusion. J Pediatr Surg 1996; 31: 1339–48 [DOI] [PubMed] [Google Scholar]
- 22.Hirose S, Harrison MR. The ex utero intrapartum treatment (EXIT) procedure. Semin Neonatol 2003; 8:207–14 [DOI] [PubMed] [Google Scholar]
- 23.Flake AW, Crombleholme TM, Johnson MP, Howell LJ, Adzick NS. Treatment of severe congenital diaphragmatic hernia by fetal tracheal occlusion: clinical experience with fifteen cases. Am J ObstetGynecol 2000; 183:1059–66 [DOI] [PubMed] [Google Scholar]
- 24.Bealer JF, Skarsgard ED, Hedrick MH, Meuli M, VanderWall KJ, Flake AW, et al. The ‘PLUG’ odyssey: adventures in experimental fetal tracheal occlusion. J Pediatr Surg 1995; 30:361–4 [DOI] [PubMed] [Google Scholar]
- 25.Luks FI, Gilchrist BF, Jackson BT, Piasecki GJ. Endoscopic tracheal obstruction with an expanding device in a fetal lamb model: preliminary considerations. Fetal Diagn Ther 1996; 11:67–71 [DOI] [PubMed] [Google Scholar]
- 26.Harrison MR, Albanese CT, Hawgood SB, Farmer DL, Farrell JA, Sandberg PL, et al. Fetoscopic temporary tracheal occlusion by means of detachable balloon for congenital diaphragmatic hernia. Am J Obstet Gynecol 2001; 185:730–3 [DOI] [PubMed] [Google Scholar]
- 27.Quintero R, Bornick PW, Allen MH, Johnson PK. Minimally invasive intraluminal tracheal occlusion in a human fetus with left sided congenital diaphragmatic hernia at 27 weeks via direct fetal laryngoscopy. Prenatal and Neonatal Medicine 2000; 5:134–40 [Google Scholar]
- 28.Deprest J, Gratacos E, Nicolaides KH. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol 2004; 24:121–6 [DOI] [PubMed] [Google Scholar]
- 29.Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, et al. A randomized trail of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med 2003; 349:1916–24 [DOI] [PubMed] [Google Scholar]
- 30.Ruano R, Yoshisaki CT, da Silva MM, Ceccon ME, Grasi MS, Tannuri U, et al. a randomized controlled trial of fetal endoscopic tracheal occlusion versus postnatal management of severe isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2012;39:20–7 [DOI] [PubMed] [Google Scholar]
- 31.Jani JC, Nicolaides KH. Fetal surgery for severe congenital diaphragmatic hernia?. Ultrasound Obstet Gynecol 2012; 39:7–9 [DOI] [PubMed] [Google Scholar]
- 32.Saudi census 2022. General Authority for Statistics, Kingdom of Saudi Arabia. https://www.stats.gov.sa [Google Scholar]
- 33.Cruz-Martinez R, Martinez-Rodriguez M, Gamez-Varela A, Nieto-Castro B, Luna-García J, Juárez-Martínez I, et al. Survival outcome in severe left-sided congenital diaphragmatic hernia with and without fetal endoscopic tracheal occlusion in a country with suboptimal neonatal management. Ultrasound Obstet Gynecol 2020; 56:516–21. [DOI] [PubMed] [Google Scholar]