Abstract
Objective
Recently, we revealed that triggering receptor expressed on myeloid cells-like 2 (TREML2) modulated inflammation by regulating microglial polarization and NLRP3 inflammasome activation. However, the role of TREML2 in Alzheimer’s disease (AD) pathogenesis remains poorly understood. In this study, we tried to observe the impact of TREML2 on neuropathological hallmarks (including amyloid-β (Aβ) pathology, hyperphosphorylated tau and neuroinflammation) and cognitive deficiency in senescence-accelerated mouse prone substrain 8 (SAMP8) mice, an animal model of sporadic AD.
Methods
A lentiviral-based strategy was employed to manipulate TREML2 levels in the brain of SAMP8 mice. Enzyme-linked immunosorbent assay was used to detect the protein levels of inflammatory cytokines, Aβ42 and hyperphosphorylated tau. The mRNA levels of microglial polarization markers were assessed by qRT-PCR. Morris water maze test was performed to evaluate the spatial cognitive functions.
Results
TREML2 overexpression elevated inflammatory cytokines levels, induced microglial M1-type polarization, and exacerbated Aβ and tau pathology in SAMP8 mice. Contrastingly, knocking down TREML2 mitigated inflammatory cytokines release, promoted microglial M2-type polarization, ameliorated Aβ and tau pathology, and rescued cognitive deficiency in SAMP8 mice.
Conclusion
This study offers the first in vivo evidence that TREML2 contributes to the pathogenesis of AD. Furthermore, this study also proves that inhibition of TREML2 signaling may represent a potential treatment strategy for this disease.
Keywords: TREML2, neuroinflammation, Alzheimer’s disease, microglia, cognitive deficiency
Introduction
Alzheimer’s disease (AD), the most common type of neurodegenerative diseases, is a growing global health concern.1 It is characterized by amyloid-β (Aβ) deposition, tau hyperphosphorylation, neuroinflammation, and progressive decline of cognitive function.1 Notably, late-onset AD accounts for over 95% of all AD cases.2 Genetic factors play a pivotal role in the etiology of late-onset AD,3 and numerous susceptibility genes have been identified through genome-wide association studies and high-throughput sequencing research in recent years.4–7
Triggering receptor expressed on myeloid cells-like 2 (TREML2) is a newly confirmed susceptibility gene for late-onset AD.3,8 It encodes an inflammation-associated transmembrane receptor.8 Unlike its family member TREM2, TREML2 does not require adapter protein DAP12 for signaling.9 Within the brain, TREML2 is expressed by microglia, and its levels gradually increase during normal aging and the progression of AD.10 Our recent study has demonstrated that TREML2 activated microglial NLRP3 inflammasome, modulated the expression of microglial polarization markers such as iNOS, ARG1 and CD206, and facilitated microglia-mediated inflammatory cytokine release.10 Despite these findings, the precise role of TREML2 in the pathogenesis of AD remains enigmatic.
Aging is the most significant known risk factor for late-onset AD.11 Senescence-accelerated mouse prone substrain 8 (SAMP8) is a naturally occurring mouse line possessing accelerated aging characteristics.12 Compared to its normal aging control mouse line senescence-accelerated mouse resistant substrain 1 (SAMR1), SAMP8 mouse begin to exhibit hallmarks of AD including elevated Aβ levels, tau hyperphosphorylation, neuroinflammation and cognitive deficits at 4–5 months of age, and was utilized as an animal model for late-onset AD.13 Therefore, in this study, we manipulated TREML2 levels in the brain of SAMP8 mice via a lentivirus-based strategy, and tried to observe its impact on neuropathological hallmarks and cognitive deficiency.
Materials and Methods
Animals
Five-month-old SAMP8 mice and their age-matched SAMR1 controls were purchased from Beijing Zhishan Institute of Health Medicine.12 The mice were maintained in standard conditions, including a 12-h bright/dark cycle, a temperature of 23 ± 2°C, and a humidity of 60 ± 5%. The mice were provided ad libitum access to food and water. The animal experiments were conducted in accordance with ARRIVE guidelines,14 and all procedure was reviewed and approved by the Ethics Committee of Nanjing First Hospital (approval No. DWSY-24051507). In addition, the welfare of the laboratory animals was reviewed and approved by Nanjing First Hospital according to the Guidelines for the ethical review of laboratory animal welfare People’s Republic of China (National Standard GB/T 35892–2018).15
Intracerebral Lentiviral Particles Injection
Mouse TREML2 lentiviral activation particles (Santa Cruz Biotechnology, Cat# sc-435918-LAC), mouse TREML2 shRNA lentiviral particles (Santa Cruz Biotechnology, Cat# sc-154630-V), and their control lentiviral particles (control lentiviral activation particles: Santa Cruz Biotechnology, Cat# sc-437282; control shRNA lentiviral particles: Santa Cruz, Biotechnology, Cat# sc-108080) were obtained from Santa Cruz Biotechnology Company. SAMP8 mice were randomly assigned to 5 groups: SAMP8 group, SAMP8+Control overexpression (OE) group, SAMP8+TREML2 OE group, SAMP8 + Control knockdown (KD) group, and SAMP8 + TREML2 KD group. Injection of lentiviral particles was performed in the cerebral cortex (two deposits) and hippocampus (one deposit) of each hemisphere with 2 μL lentiviral particles using a micropipette attached to a 10 μL syringe as described.16,17 Stereotactic coordinates of injection sites from bregma were (1) anteroposterior: −0.3, mediolateral: 2, dorsoventral: −1.5 mm and anteroposterior: −2, mediolateral: 1.2, dorsoventral: −1.2 mm for the cerebral cortex; and (2) anteroposterior: −2; mediolateral: 1.2; dorsoventral: −2 mm for the hippocampus as described.16 As reported by Dodart et al, neuronal losses were observed in the brain over a period of three months following lentiviral-mediated gene delivery, potentially due to the long-term neurotoxicity of the lentivirus.18 Considering these factors, ‘3 months’ was chosen as the time point for investigation.
Morris Water Maze Test
Three months after lentiviral particles injection, Morris water maze (MWM) test was employed to evaluate the spatial cognitive functions of SAMP8 mice as described previously.19 Mice were given four training trials per day for five consecutive days. The swimming speed and the path lengths to the submerged platform were recorded using a video tracking system (Jiangsu SANS Biotechnology Company).
Western Blot
Western blot was conducted as described previously.20 After the MWM test, mice were sacrificed. The whole brain was excised, homogenized, and the total protein was extracted. Equal amounts of protein from different samples were separated using a sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk for 2 hours at room temperature, and were incubated with anti-TREML2 (1:500, Santa Cruz Biotechnology, Cat# sc-390343) or anti-β-Actin (1:1000, Cell Signaling Technology, Cat# 4970) primary antibodies overnight at 4°C. Next, the membranes were washed three times with Tris-buffered saline with 0.1% Tween® 20 Detergent, followed by incubation with anti-mouse or anti-rabbit secondary antibodies for 2 hours. After another three-time washings, the membranes were exposed to the chemiluminescent kit (Tanon, Cat# 180–5001) for protein band detection. The optical density of the protein bands was measured using Quantity One software (Bio-Rad Laboratories, USA).
Real-Time PCR
Real-time PCR (RT-PCR) was carried out as previously described.21 The total RNA was extracted from brain tissue using TRIzolTM reagents (Thermo Fisher Scientific, Cat# 15596018). Subsequently, reverse transcription of total RNA was conducted using PrimeScript TM RT kit (Takara Bio, Cat# RR047A). The PCR reactions were carried out as previously described.21 The specific PCR primers used listed in Supplementary Table S1.
Immunofluorescence Staining for Aβ
See Methods for immunofluorescence staining section of Supplementary Information file for details.
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) was conducted as previously described.22 The Interleukin (IL)-1β (Abcam, Cat# ab197742), IL-6 (Abcam, Cat# ab100713), and tumor necrosis factor-α (TNF-α, Abcam, Cat# ab208348) ELISA kits were procured from Abcam. The Aβ42 ELISA kit (Thermo Fisher Scientific, Cat# KMB3441) and hyperphosphorylated tau at Ser199 site ELISA kit (Thermo Fisher Scientific, Cat# KMB7041) was obtained from Thermo Fisher Scientific. The hyperphosphorylated tau at Thr205 site ELISA kit (Cell Signaling Technology, Cat#51580) was provided by Cell Signaling Technology. All experimental procedures were meticulously performed in accordance with the manufacturer’s instructions.
Statistics
Data were analyzed using the GraphPad Prism software (version 8.3.0; GraphPad Software; San Diego, CA, USA), and were expressed as mean ± standard deviation (SD). The path length data obtained from the MWM test were analyzed using two-way repeated measures analysis of variance followed by Bonferroni’s multiple comparisons. Other data were analyzed using one-way analysis of variance followed by Tukey’s post hoc test. P<0.05 was considered statistically significant.
Results
TREML2 Protein Levels in the Brains of SAMP8 Mice Following Lentivirus-Based TREML2 Manipulation
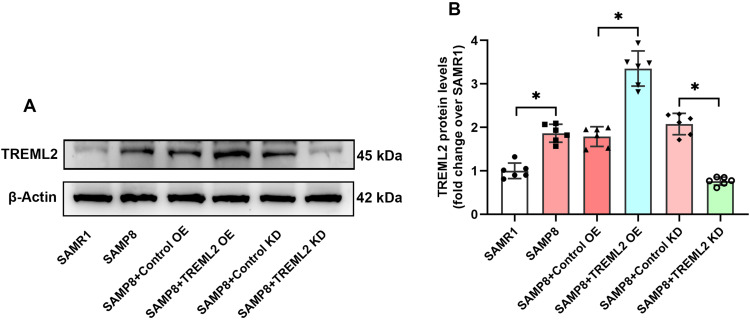
Western blots analysis showed that the TREML2 protein levels in the brains of SAMP8 mice were obviously higher than those of SAMR1 controls (P<0.05, Figure 1A and B). Lentiviral-mediated TREML2 overexpression further elevated the levels of TREML2 in the brains of SAMP8 mice by 87.6% (P<0.05, Figure 1A and B). Contrastingly, lentiviral-mediated TREML2 knockdown significantly reduced TREML2 protein levels by 63.3% in the brains of SAMP 8 mice (P<0.05, Figure 1A and B).
Figure 1.
TREML2 protein levels in the brains of SAMP8 mice following TREML2 manipulation. (A) The levels of TREML2 in the brains of mice were determined using Western blot. (B) Quantitative analysis of TREML2 levels. Data were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Columns represent mean ± SD. N = 6 per group. *P<0.05.
The Effects of TREML2 on Neuroinflammation in SAMP8 Mice
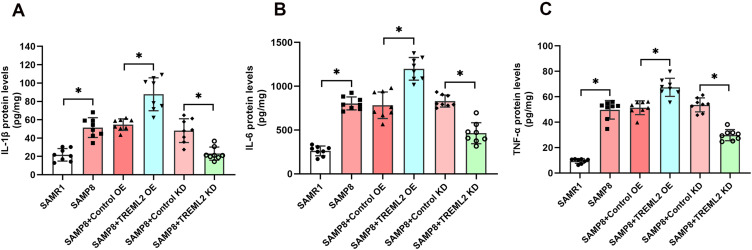
As shown in Figure 2A–C, the levels of IL-1β, IL-6, and TNF-α were substantially increased in the brains of SAMP8 mice when compared with those of SAMR1 controls (P<0.05, Figure 2A–C). TREML2 overexpression further increased the levels of IL-1β (by 60.5%, P<0.05), IL-6 (by 53.0%, P<0.05), and TNF-α (by 31.3%, P<0.05) in the brains of SAMP8 mice, whereas the increments of these inflammatory cytokines were significantly mitigated by TREML2 knockdown (Figure 2A–C, for IL-1β: reduced by 52.2%, P<0.05; for IL-6: reduced by 55.9%, P<0.05; for TNF-α: reduced by 44.3%, P<0.05).
Figure 2.
The effects of TREML2 on neuroinflammation in SAMP8 mice. The levels of IL-1β (A), IL-6 (B), and TNF-α (C) in the brains of mice were detected using ELISA. Data were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Columns represent mean ± SD. N = 8 per group. *P<0.05.
The Effects of TREML2 on Microglial Polarization in the Brains of SAMP8 Mice
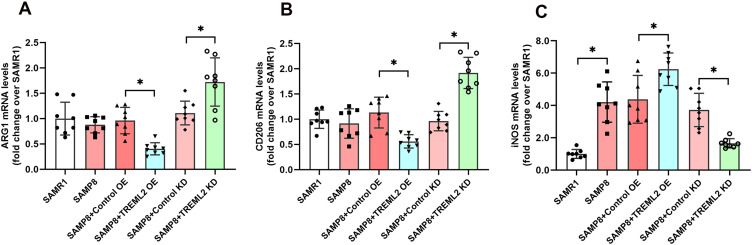
In the brain, activated microglia exhibit either M1-type polarization or M2-type polarization.23 As shown in Figure 3A–C, the expression of iNOS, a M1-type polarization marker, was observably increased in the brains of SAMP8 mice when compared with those of SAMR1 controls (P<0.05). TREML2 overexpression further elevated the expression of iNOS by 42.6% (P<0.05), while this increment was suppressed by TREML2 knockdown by 55.5% (P<0.05, Figure 3C). The expressions of ARG1 and CD206, two M2-type polarization markers, were slightly decreased in the brains of SAMP8 mice when compared to SAMR1 mice. However, these decrements did not achieve statistical significance. Overexpression of TREML2 further reduced the expressions of ARG1 (by 58.0%, P<0.05) and CD206 (by 50.5%, P<0.05) in the brains of SAMP8 mice, whereas TREML2 knockdown reversed these decrements (Figure 3A and B, for ARG1: increased by 55.2%, P<0.05; for CD206: increased by 99.2%, P<0.05).
Figure 3.
The effects of TREML2 on microglial polarization in the brains of SAMP8 mice. The mRNA expression of M2-type microglial polarization markers ARG1 (A) and CD206 (B) as well as M1-type microglial polarization marker iNOS (C) in the brains of mice were measured using RT-PCR. Data were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Columns represent mean ± SD. N = 8 per group. *P<0.05.
The Effects of TREML2 on Aβ and Tau Pathologies in the Brains of SAMP8 Mice
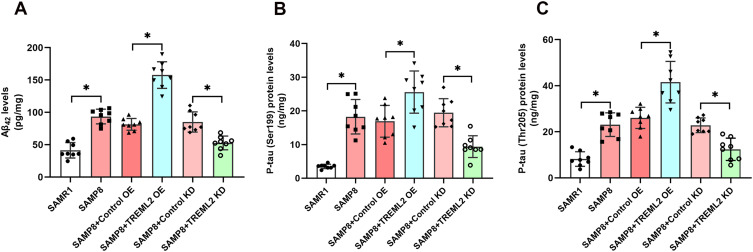
As shown in Figure 4A–C, the levels of Aβ42 and hyperphosphorylated tau (Ser199 and Thr205 sites) were significantly elevated in the brains of SAMP8 mice when compared with those of SAMR1 controls (P<0.05). TREML2 overexpression further increased the levels of Aβ42 in the brains of SAMP8 mice by 93.5% (P<0.05), whereas TREML2 knockdown markedly ameliorated this increment by 38.1% (P<0.05, Figure 4A). The impacts of TREML2 on Aβ42 levels in the brains of SAMP8 mice were confirmed by immunofluorescence staining (Supplementary Figure S1). Similarly, overexpression of TREML2 further facilitated tau hyperphosphorylation at Ser199 (increase by 51.3%, P<0.05) and Thr205 (increase by by 59.5%, P<0.05) sites, while TREML2 knockdown markedly attenuated tau hyperphosphorylation at Ser199 (reduced by 51.8%, P<0.05) and Thr205 (reduced by 45.8%, P<0.05) sites in the brains of SAMP8 mice (Figure 4B and C).
Figure 4.
The effects of TREML2 on Aβ and tau pathologies in the brains of SAMP8 mice. (A) The levels of Aβ42 in the brains of mice were tested by ELISA. The levels of hyperphosphorylated tau at Ser199 (B) and Thr205 (C) sites were assessed using ELISA. Data were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Columns represent mean ± SD. N = 8 per group. *P<0.05.
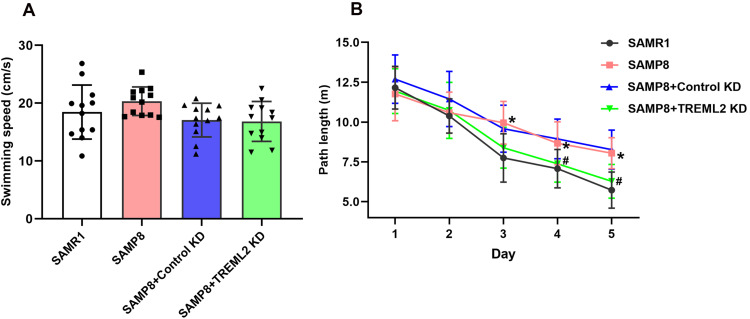
TREML2 Knockdown Rescues Cognitive Deficits in SAMP8 Mice
In the MWM test, no significant difference was observed in the swimming speed between SAMP8 mice and SAMR1 controls. In addition, the swimming speed of SAMP8 mice was not significantly affected by TREML2 knockdown (Figure 5A). Overall, SAMP8 mice performed worse than SAMR1 mice in the MWM test (P<0.05). As shown in Figure 5B, SAMP8 mice exhibited longer path lengths on days 3, 4, and 5 than SAMR1 controls in the MWM test (P<0.05). These findings indicated that SAMP8 mice manifested obvious spatial cognitive impairments at 8 months of age. TREML2 knockdown mitigated cognitive deficits in SAMP8 mice, since SAMP8 +TREML2 KD group showed a distinct reduction in path lengths on days 4 and 5 when compared with those of SAMP8 + Control KD in the MWM test (P<0.05, Figure 5B).
Figure 5.
TREML2 knockdown rescues cognitive deficits in SAMP8 mice. (A) The swimming speeds of mice before the MWM test. (B) The path lengths of mice over 5 consecutive days during the MWM test. Data for panel A were analyzed by one-way analysis of variance followed by Tukey’s post hoc test. Data for panel B were analyzed by two-way repeated measures analysis of variance followed by Bonferroni’s multiple comparisons. Columns represent mean ± SD. N = 12 per group. *P<0.05, vs SAMR1 controls; #P<0.05, vs SAMP8+Control KD group.
Discussion
Firstly, our study demonstrated elevated TREML2 levels in the brains of SAMP8 mice, a sporadic AD animal model, compared to their age-matched control mice. This finding is further supported by Sierksma et al, who reported similar TREML2 elevations in the brains of APP/PS1 mice, a transgenic AD model, compared to their age-matched wild-type controls.24 Additionally, our previous study revealed a gradual increase in brain TREML2 levels during AD progression in APP/PS1 mice.10 Collectively, these observations suggested a potential role for TREML2 in the pathogenesis of AD.
To gain deeper insights into the involvement of TREML2 in the pathogenesis of AD, we employed a lentiviral-based approach to manipulate TREML2 levels in the brains of SAMP8 mice. Our findings revealed that TREML2 overexpression led to elevated levels of inflammatory cytokines in the brains of these mice, while TREML2 knockdown effectively mitigated these increases. These observations align with our previous in vitro study, where we demonstrated that TREML2 overexpression potentiated the LPS-induced release of inflammatory cytokines by microglia, while TREML2 knockdown attenuated this effect.10 Based on these findings, we speculated that TREML2 contributes to neuroinflammation in AD.
In the brain, activated microglia exhibit either M1-type polarization or M2-type polarization.25 M1-type polarization is characterized by pro-inflammatory status, while M2-type polarization is characterized by enhanced anti-inflammatory activity.25 Given that TREML2 contributed to neuroinflammation in AD, we investigated whether the effect of TREML2 on neuroinflammation was attributed to the alterations in microglia polarization. Our findings demonstrated that overexpression of TREML2 in the brains of SAMP8 mice enhanced the expression of the M1-type polarization marker iNOS while suppressing the levels of two M2-type polarization markers, ARG1 and CD206. Conversely, knocking down TREML2 expression led to increased expression of ARG1 and CD206 and reduced iNOS levels. These observations were consistent with our previous findings in LPS-induced primary microglia.10 Based on these evidences, it appeared that TREML2 contributed to neuroinflammation by promoting M1-type microglial polarization.
Aβ is the most characteristic pathological feature of AD.26 In our study, we observed that Aβ levels increased following TREML2 overexpression, while TREML2 knockdown resulted in a significant reduction of brain Aβ levels. These findings suggested that TREML2 aggravated brain Aβ pathology in the context of AD. Multiple lines of evidence suggested that microglia play a role in brain Aβ clearance, and M1-type microglial polarization is associated with reduced Aβ phagocytosis.27 Therefore, the exacerbation of brain Aβ pathology here might be attributed to TREML2-induced M1-type microglial polarization. Tau hyperphosphorylation represents another pathological hallmark of AD.28 In this study, we demonstrated that tau hyperphosphorylation was enhanced by TREML2 overexpression and attenuated by TREML2 knockdown, indicating that TREML2 promoted tau pathology. Since tau hyperphosphorylation is a downstream event triggered by Aβ,29 the worsening of tau pathology might be attributed to TREML2-induced exacerbation of brain Aβ pathology. Additionally, neuroinflammation has been reported to induce tau hyperphosphorylation.30 Therefore, it is plausible that the increased neuroinflammation caused by TREML2 might also contribute to the deterioration of tau pathology.
AD-related pathologies including neuroinflammation, Aβ deposition and tau hyperphosphorylation lead to the dysfunction of neurons and synapses, ultimately resulting in cognitive impairments.31 In this study, we revealed that knockdown of TREML2 rescued cognitive impairments in SAMP8 mice. To our knowledge, this is the first study reporting a beneficial effect of TREML2 knockdown on cognitive impairments in a mouse model of AD. This beneficial outcome might be attributed to the mitigation of AD-related pathologies resulting from TREML2 knockdown.
This study also has some limitations. Specifically, SAMP8 mice serve as an animal model for aging rather than being specific to AD. Although Aβ levels gradually increase in their brains, they do not develop amyloid plaques. Consequently, our findings may not accurately reflect the role of TREML2 during AD progression and should be further validated in animal models with pure human Aβ pathology, such as APP/PS1 transgenic mice.
Conclusion
Summarily, in the current study, we reveal that TREML2 overexpression elevates neuroinflammation, induces microglial M1-type polarization, and exacerbates Aβ and tau pathology in SAMP8 mice. Conversely, knocking down TREML2 mitigates neuroinflammation, induces microglial M2-type polarization, ameliorates Aβ and tau pathology, and rescues cognitive deficiency in SAMP8 mice. This study offers the first in vivo evidence that TREML2 contributes to the pathogenesis of AD. Furthermore, this study also proves that inhibition of TREML2 signaling may represent a potential treatment strategy for this disease.
Acknowledgments
The authors have no acknowledgments to report.
Funding Statement
This study was supported by the grants from the National Science and Technology Innovation 2030-Major program of “Brain Science and Brain Inspired Intelligence Research” (2021ZD0201807) (to YDZ), National Natural Science Foundation of China (81974156) (to TJ), Natural Science Foundation of Jiangsu Province (BK20221175) (to TJ), and Nanjing Medical Science and Technology Development Foundation (YKK23111) (to TJ).
Data Sharing Statement
The data used to support the findings of this study are available on request from the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi: 10.1016/S0140-6736(20)32205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers. 2015;1(1):1–18. doi: 10.1038/nrdp.2015.56 [DOI] [PubMed] [Google Scholar]
- 3.Jiang T, Wan Y, Zhou J-S, et al. A missense variant in TREML2 reduces risk of Alzheimer’s disease in a Han Chinese population. Mol Neurobiol. 2017;54(2):977–982. doi: 10.1007/s12035-016-9706-8 [DOI] [PubMed] [Google Scholar]
- 4.Neumann H, Daly MJ. Variant TREM2 as risk factor for Alzheimer’s disease. New Engl J Med. 2013;368(2):182–184. doi: 10.1056/NEJMe1213157 [DOI] [PubMed] [Google Scholar]
- 5.Tan MS, Yu JT, Jiang T, Zhu XC, Guan HS, Tan L. Genetic variation in BIN1 gene and Alzheimer’s disease risk in Han Chinese individuals. Neurobiol Aging. 2014;35(7):e1781–1788. doi: 10.1016/j.neurobiolaging.2014.01.151 [DOI] [PubMed] [Google Scholar]
- 6.Jiang T, Yu JT, Wang YL, et al. The genetic variation of ARRB2 is associated with late-onset Alzheimer’s disease in Han Chinese. Curr Alzheimer Res. 2014;11:408–412. doi: 10.2174/1567205011666140317095014 [DOI] [PubMed] [Google Scholar]
- 7.Tan L, Yu JT, Zhang W, et al. Association of GWAS-linked loci with late-onset Alzheimer’s disease in a northern Han Chinese population. Alzheimer’s Dementia. 2012;9:546–553. [DOI] [PubMed] [Google Scholar]
- 8.Wang S-Y, Gong P-Y, Zhang Y-D, Jiang T. The role of TREML2 in Alzheimer’s disease. J Alzheimers Dis. 2020;76(3):799–806. doi: 10.3233/JAD-200406 [DOI] [PubMed] [Google Scholar]
- 9.Colonna M. The biology of TREM receptors. Nat Rev Immunol. 2023;23:1–15. doi: 10.1038/s41577-022-00806-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang SY, Fu XX, Duan R, et al. The Alzheimer’s disease-associated gene TREML2 modulates inflammation by regulating microglia polarization and NLRP3 inflammasome activation. Neural Regen Res. 2023;18(2):434–438. doi: 10.4103/1673-5374.346468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang T, Yu JT, Tian Y, Tan L. Epidemiology and etiology of Alzheimer’s disease: from genetic to non- genetic factors. Curr Alzheimer Res. 2013;10(8):852–867. doi: 10.2174/15672050113109990155 [DOI] [PubMed] [Google Scholar]
- 12.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp Gerontology. 1997;32(1–2):105–109. doi: 10.1016/S0531-5565(96)00036-8 [DOI] [PubMed] [Google Scholar]
- 13.Morley JE, Armbrecht HJ, Farr SA, Kumar VB. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer’s disease. BBA. 2012;1822(5):650–656. doi: 10.1016/j.bbadis.2011.11.015 [DOI] [PubMed] [Google Scholar]
- 14.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 2020;40(9):1769–1777. doi: 10.1177/0271678X20943823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacArthur Clark JA, Sun D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018 [Issued 6 February 2018 Effective from 1 September 2018]. Anim Models Exp Med. 2020;3(1):103–113. doi: 10.1002/ame2.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang T, Tan L, Zhu XC, et al. Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39(13):2949–2962. doi: 10.1038/npp.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang T, Zhang YD, Gao Q, et al. TREM1 facilitates microglial phagocytosis of amyloid beta. Acta Neuropathol. 2016;132(5):667–683. doi: 10.1007/s00401-016-1622-5 [DOI] [PubMed] [Google Scholar]
- 18.Dodart JC, Marr RA, Koistinaho M, et al. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2005;102(4):1211–1216. doi: 10.1073/pnas.0409072102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan R, Xue X, Zhang -Q-Q, et al. ACE2 activator diminazene aceturate ameliorates Alzheimer’s disease-like neuropathology and rescues cognitive impairment in SAMP8 mice. Aging. 2020;12(14):14819. doi: 10.18632/aging.103544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu -X-X, Duan R, Wang S-Y, et al. Lamotrigine protects against cognitive deficits, synapse and nerve cell damage, and hallmark neuropathologies in a mouse model of Alzheimer’s disease. Neural Regen Res. 2023;18(1):189–193. doi: 10.4103/1673-5374.343888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang T, Wan Y, Zhang YD, et al. TREM2 overexpression has no improvement on neuropathology and cognitive impairment in aging APPswe/PS1dE9 mice. Mol Neurobiol. 2017;54(2):855–865. doi: 10.1007/s12035-016-9704-x [DOI] [PubMed] [Google Scholar]
- 22.Duan R, Wang SY, Wei B, et al. Angiotensin-(1-7) Analogue AVE0991 modulates astrocyte-mediated neuroinflammation via lncRNA SNHG14/miR-223-3p/NLRP3 pathway and offers neuroprotection in a transgenic mouse model of Alzheimer’s disease. J Inflamm Res. 2021;14:7007–7019. doi: 10.2147/JIR.S343575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Yao H, Liu W, et al. Microglia polarization in Alzheimer’s disease: mechanisms and a potential therapeutic target. Front Aging Neurosci. 2021;13:772717. doi: 10.3389/fnagi.2021.772717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sierksma A, Lu A, Mancuso R, et al. Novel Alzheimer risk genes determine the microglia response to amyloid‐β but not to TAU pathology. EMBO Mol Med. 2020;12(3):e10606. doi: 10.15252/emmm.201910606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo S, Wang H, Yin Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci. 2022;14:815347. doi: 10.3389/fnagi.2022.815347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korczyn AD. The amyloid cascade hypothesis. Alzheimer’s Dementia. 2008;4(3):176–178. doi: 10.1016/j.jalz.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5 [DOI] [PubMed] [Google Scholar]
- 28.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847 [DOI] [PubMed] [Google Scholar]
- 29.Frisoni GB, Altomare D, Thal DR, et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci. 2022;23(1):53–66. doi: 10.1038/s41583-021-00533-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Yu Y. Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation. J Neuroinflammation. 2023;20(1):165. doi: 10.1186/s12974-023-02853-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int j Nanomed. 2019:5541–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available on request from the corresponding author.