Abstract
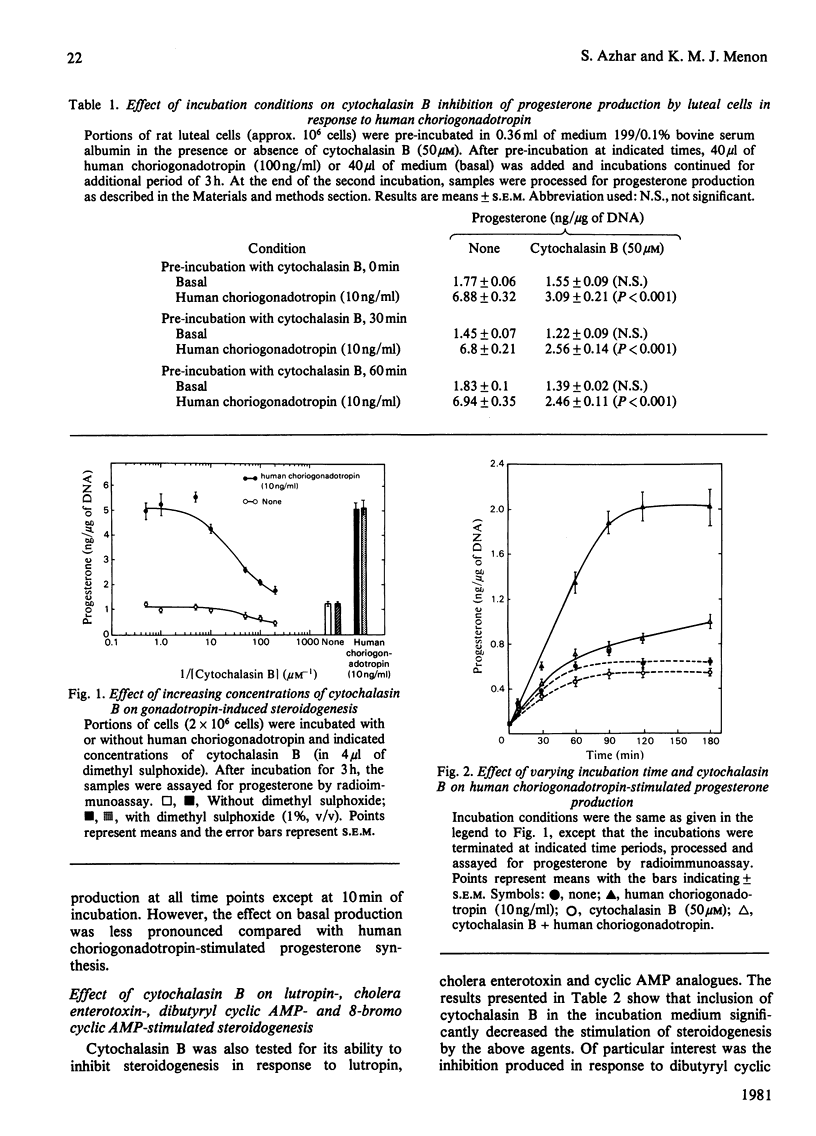
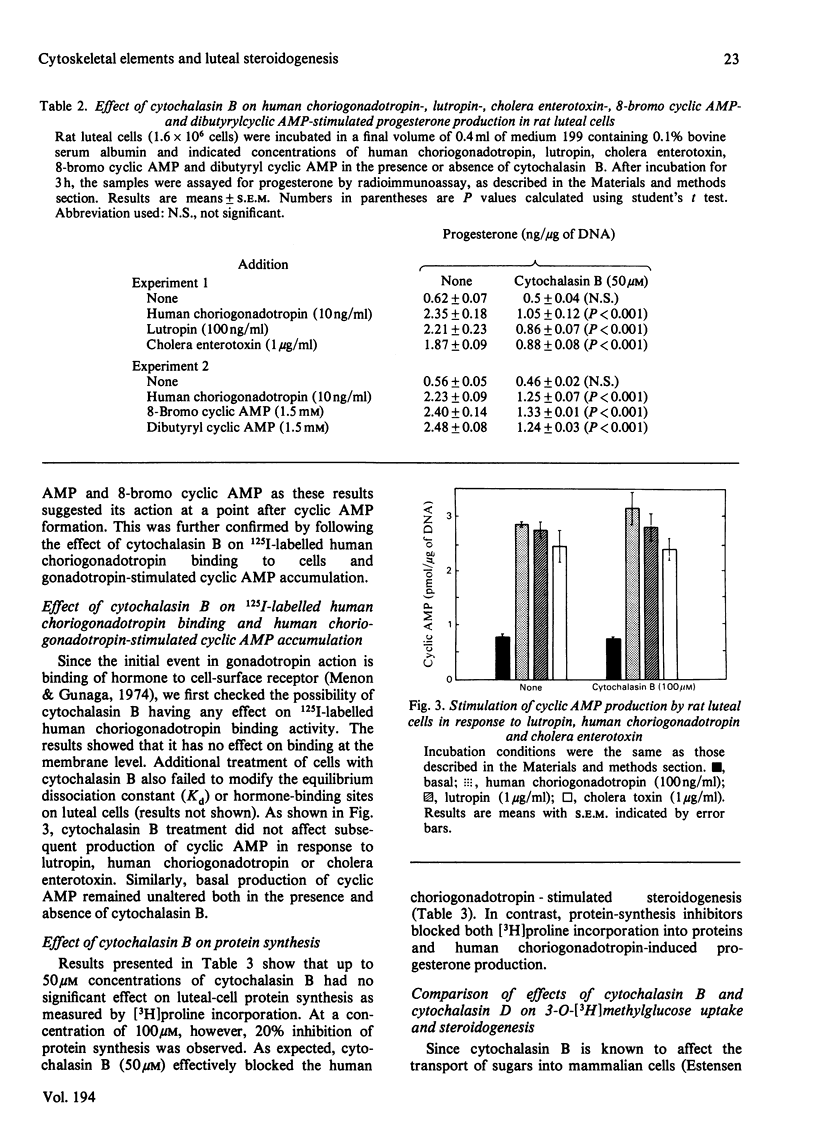
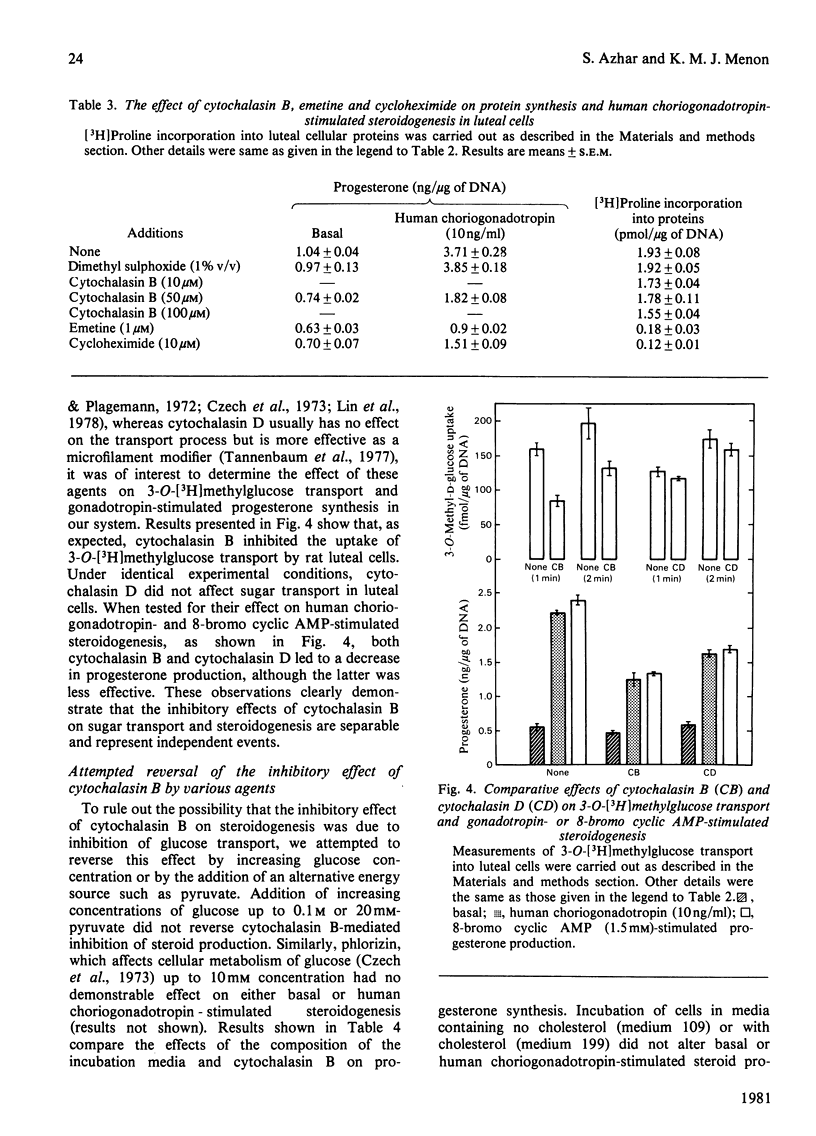
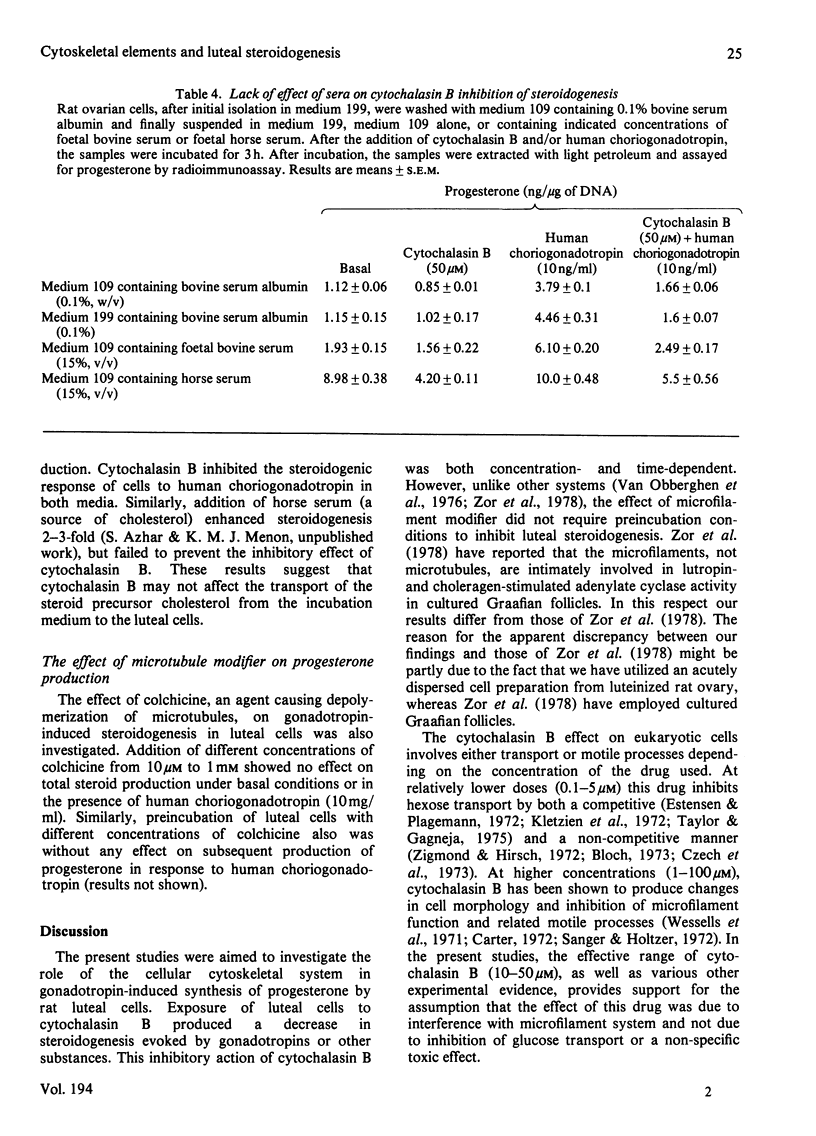
The role of the cellular cytoskeletal system of microtubules and microfilaments on gonadotropin-stimulated progesterone production by isolated rat luteal cells has been investigated. Exposure of luteal cells to human choriogonadotropin resulted in a stimulation of cyclic AMP (4-7-fold) and progesterone (3-4-fold) responses.l Incubation of cells with the microfilament modifier cytochalasin B inhibited the gonadotropin-induced steroidogenesis in a dose- and time-dependent manner. The effect of cytochalasin B on basal production of steroid was less pronounced. Cytochalasin B also inhibited the accumulation of progesterone in response to lutropin, cholera enterotoxin, dibutyryl cyclic AMP and 8-bromo cyclic AMP. The inhibition of steroidogenesis by cytochalasin B was not due to (a) inhibition of 125I-labelled human choriogonadotropin binding to luteal cells, (b) inhibition of gonadotropin-stimulated cyclic AMP formation or (c) a general cytotoxic effect and/or inhibition of protein biosynthesis. Cytochalasin D, like cytochalasin B, inhibited gonadotropin- and 8-bromo cyclic AMP-stimulated steroidogenesis. Although cytochalasin B also blocked the transport of 3-O-methyl-glucose into luteal cells, cytochalasin D was without such an effect. Increasing glucose concentration in the medium, or using pyruvate as an alternative energy source, failed to reverse the inhibitory effect of cytochalasin B. The anti-microtubular agent colchicine failed to modulate synthesis and release of progesterone by luteal cells in response to human choriogonadotropin. These studies suggest that the cellular microfilaments may be involved in the regulation of gonadotropin-induced steroidogenesis. In contrast, microtubules appear to be not directly involved in this process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azhar S., Fitzpatrick P., Menon K. M. Role of gangliosides in gonadotropin and cholera enterotoxin stimulated steroidogenesis in isolated rat ovarian cells. Biochem Biophys Res Commun. 1978 Jul 28;83(2):493–500. doi: 10.1016/0006-291x(78)91017-3. [DOI] [PubMed] [Google Scholar]
- Azhar S., Hajra A. K., Menon K. M. Gonadotropin receptors in plasma membranes of bovine corpus luteum. II. Role of membrane phospholipids. J Biol Chem. 1976 Dec 10;251(23):7405–7412. [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Gonadotropin receptors in plasma membranes of bovine corpus luteum. I. Effect of phospholipases on the binding of 125I-choriogonadotropin by membrane-associated and solubilized receptors. J Biol Chem. 1976 Dec 10;251(23):7398–7404. [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Receptor-mediated gonadotropin action in ovary. Differential effects of various gangliosides and cholera enterotoxin on 125I-choriogonadotropin binding, production of adenosine 3':5'-monophosphate and steroidogenesis in rat ovarian cells. Eur J Biochem. 1979 Feb 15;94(1):77–85. doi: 10.1111/j.1432-1033.1979.tb12873.x. [DOI] [PubMed] [Google Scholar]
- Azhar S., Menon K. M. Receptor-mediated gonadotropin action in the ovary. Regulatory role of cyclic nucleotide phosphodiesterase(s) in intracellular adenosine 3':5'-cyclic monophosphate turnover and gonadotropin-stimulated progesterone production by rat ovarian cells. Biochem J. 1979 Apr 15;180(1):201–211. doi: 10.1042/bj1800201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D., Manning C. P., Redman C. M. The in vivo effect of colchicine on the addition of galactose and sialic acid to rat hepatic serum glycoproteins. J Biol Chem. 1976 Jul 10;251(13):3887–3892. [PubMed] [Google Scholar]
- Berlin R. D. Microtubules and the fluidity of the cell surface. Ann N Y Acad Sci. 1975 Jun 30;253:445–454. doi: 10.1111/j.1749-6632.1975.tb19220.x. [DOI] [PubMed] [Google Scholar]
- Bloch R. Inhibition of glucose transport in the human erythrocyte by cytochalasin B. Biochemistry. 1973 Nov 6;12(23):4799–4801. doi: 10.1021/bi00747a036. [DOI] [PubMed] [Google Scholar]
- Carter S. B. The cytochalasins as research tools in cytology. Endeavour. 1972 May;31(113):77–82. [PubMed] [Google Scholar]
- Chambaut-Guérin A. M., Muller P., Rossignol B. Microtubules and protein secretion in rat lacrimal glands. Relationship between colchicine binding and its inhibitory effect on the intracellular transport of proteins. J Biol Chem. 1978 Jun 10;253(11):3870–3876. [PubMed] [Google Scholar]
- Clark M. R., Menon K. M. Regulation of ovarian steroidogenesis. The disparity between 125I-labelled choriogonadotropin binding cyclic adenosine 3',5'-monophosphate formation and progesterone synthesis in the rat ovary. Biochim Biophys Acta. 1976 Aug 24;444(1):23–32. doi: 10.1016/0304-4165(76)90220-8. [DOI] [PubMed] [Google Scholar]
- Cortese F., Wolf J. Cytochalasin-stimulated steroidogenesis from high density lipoproteins. J Cell Biol. 1978 May;77(2):507–516. doi: 10.1083/jcb.77.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Lynn D. G., Lynn W. S. Cytochalasin B-sensitive 2-deoxy-D-glucose transport in adipose cell ghosts. J Biol Chem. 1973 May 25;248(10):3636–3641. [PubMed] [Google Scholar]
- Dufau M. L., Charreau E. H., Catt K. J. Characteristics of a soluble gonadotropin receptor from the rat testis. J Biol Chem. 1973 Oct 25;248(20):6973–6982. [PubMed] [Google Scholar]
- Ebstensen R. D., Plagemann P. G. Cytochalasin B: inhibition of glucose and glucosamine transport. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1430–1434. doi: 10.1073/pnas.69.6.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Gautvik K. M., Hoyt R. F., Jr, Tashjian A. H., Jr Effects of colchicine and 2-Br-alpha-ergocryptine-methane-sulfonate (CB 154) on the release of prolactin and growth hormone by functional pituitary tumor cells in culture. J Cell Physiol. 1973 Dec;82(3):401–409. doi: 10.1002/jcp.1040820310. [DOI] [PubMed] [Google Scholar]
- Gemmell R. T., Stacy R. D. Effects of colchicine on the ovine corpus luteum: role of microtubules in the secretion of progesterone. J Reprod Fertil. 1977 Jan;49(1):115–117. doi: 10.1530/jrf.0.0490115. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Properties of the luteinizing hormone receptor of isolated bovine corpus luteum plasma membranes. J Biol Chem. 1973 Jul 25;248(14):5042–5049. [PubMed] [Google Scholar]
- Haour F., Saxena B. B. Characterization and solubilization of gonadotropin receptor of bovine corpus luteum. J Biol Chem. 1974 Apr 10;249(7):2195–2205. [PubMed] [Google Scholar]
- Kawano A., Gunaga K. P., Menon K. M. Stimulatory effect of gonadotropins on the synthesis of adenosine 3': 5'-cyclic monophosphate and progesterone by suspensions of rat ovarian interstitial cells. Biochim Biophys Acta. 1975 Mar 14;385(1):88–100. doi: 10.1016/0304-4165(75)90077-x. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F., Springer A. Cytochalasin A and B. Inhibition of sugar uptake in cultured cells. J Biol Chem. 1972 May 10;247(9):2964–2966. [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Interaction of ovarian receptors with human luteinizing hormone and human chorionic gonadotropin. Biochemistry. 1973 Nov 6;12(23):4609–4615. doi: 10.1021/bi00747a011. [DOI] [PubMed] [Google Scholar]
- Lin S., Lin D. C., Flanagan M. D. Specificity of the effects of cytochalasin B on transport and motile processes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):329–333. doi: 10.1073/pnas.75.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K. M., Gunaga K. P. Role of cyclic AMP in reproductive processes. Fertil Steril. 1974 Aug;25(8):732–750. doi: 10.1016/s0015-0282(16)40577-7. [DOI] [PubMed] [Google Scholar]
- Menon K. M., Kiburz J. Isolation of plasma membranes from bovine corpus luteum possessing adenylate cyclase, 125I-hCG binding and Na-K-ATPase activities. Biochem Biophys Res Commun. 1974 Jan 23;56(2):363–371. doi: 10.1016/0006-291x(74)90851-1. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Mousa G. Y., Trevithick J. R. Differentiation of rat lens epithelial cells in tissue culture. II. Effects of cytochalasins B and D on actin organization and differentiation. Dev Biol. 1977 Oct 1;60(1):14–25. doi: 10.1016/0012-1606(77)90107-5. [DOI] [PubMed] [Google Scholar]
- Mrotek J. J., Hall P. F. Response of adrenal tumor cells to adrenocorticotropin: site of inhibition by cytochalasin B. Biochemistry. 1977 Jul 12;16(14):3177–3181. doi: 10.1021/bi00633a021. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Bernstein J. A possible role of microtubules in catecholamine release from the adrenal medulla: effect of colchicine, vinca alkaloids and deuterium oxide. J Pharmacol Exp Ther. 1971 Apr;177(1):102–108. [PubMed] [Google Scholar]
- Rao C. V. Properties of gonadotropin receptors in the cell membranes of bovine corpus luteum. J Biol Chem. 1974 May 10;249(9):2864–2872. [PubMed] [Google Scholar]
- Robinson J., Stevenson P. M., Boyd G. S., Armstrong D. T. Acute in vivo effects on HCG and LH on ovarian mitochondrial cholesterol utilization. Mol Cell Endocrinol. 1975 Mar;2(3):149–155. doi: 10.1016/0303-7207(75)90001-5. [DOI] [PubMed] [Google Scholar]
- Sanger J. W., Holtzer H. Cytochalasin B: effects on cell morphology, cell adhesion, and mucopolysaccharide synthesis (cultured cells-contractile microfilaments-glycoproteins-embryonic cells-sorting-out). Proc Natl Acad Sci U S A. 1972 Jan;69(1):253–257. doi: 10.1073/pnas.69.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer H. R., Abel J. H., Jr, McClellan M. C., Schmitz M., Niswender G. D. Secretory granules and progesterone secretion by ovine corpora lutea in vitro. Endocrinology. 1979 Feb;104(2):476–486. doi: 10.1210/endo-104-2-476. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Schofield J. G., Mira F. Colchicine binding to bovine anterior pituitary slices and inhibition of growth-hormone release. Biochem J. 1975 Jun;148(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum J., Tanenbaum S. W., Godman G. C. The binding sites of cytochalasin D. II. Their relationship to hexose transport and to cytochalasin B. J Cell Physiol. 1977 May;91(2):239–248. doi: 10.1002/jcp.1040910209. [DOI] [PubMed] [Google Scholar]
- Taylor N. F., Gagneja G. L. A model for the mode of action of cytochalasin B inhibition of D-glucose transport in the human erythrocyte. Can J Biochem. 1975 Oct;53(10):1078–1084. doi: 10.1139/o75-148. [DOI] [PubMed] [Google Scholar]
- Thambyrajah V., Azhar S., Menon K. M. Sedimentation behavior of solubilized gonadotropin receptor from plasma membranes of bovine corpus luteum. Biochim Biophys Acta. 1976 Mar 25;428(1):35–44. doi: 10.1016/0304-4165(76)90106-9. [DOI] [PubMed] [Google Scholar]
- Toaff M. E., Strauss J. F., 3rd, Flickinger G. L., Shattil S. J. Relationship of cholesterol supply to luteal mitochondrial steroid synthesis. J Biol Chem. 1979 May 25;254(10):3977–3982. [PubMed] [Google Scholar]
- Trifaró J. M., Collier B., Lastowecka A., Stern D. Inhibition by colchicine and by vinblastine of acetylcholine-induced catecholamine release from the adrenal gland: an anticholinergic action, not an effect upon microtubules. Mol Pharmacol. 1972 Mar;8(2):264–267. [PubMed] [Google Scholar]
- Van Obberghen E., De Meyts P., Roth J. Cell surface receptors for insulin and human growth hormone. Effect of microtubule and microfilament modifiers. J Biol Chem. 1976 Nov 10;251(21):6844–6851. [PubMed] [Google Scholar]
- Vaughan M., Moss J. Mechanism of action of choleragen. J Supramol Struct. 1978;8(4):473–488. doi: 10.1002/jss.400080410. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Wolff J., Williams J. A. The role of microtubles and microfilaments in thyroid secretion. Recent Prog Horm Res. 1973;29:229–285. doi: 10.1016/b978-0-12-571129-6.50010-5. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Modulation of lymphocyte receptor mobility by concanavalin A and colchicine. Ann N Y Acad Sci. 1975 Jun 30;253:455–469. doi: 10.1111/j.1749-6632.1975.tb19221.x. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Cytochalasin B: inhibition of D-2-deoxyglucose transport into leukocytes and fibroblasts. Science. 1972 Jun 30;176(4042):1432–1434. doi: 10.1126/science.176.4042.1432. [DOI] [PubMed] [Google Scholar]
- Zor U., Strulovici B., Lindner H. R. Implication of microtubules and microfilaments in the response of the ovarian adenylate cyclase-cyclic AMP system to gonadotropins and prostaglandin E2. Biochem Biophys Res Commun. 1978 Feb 28;80(4):983–992. doi: 10.1016/0006-291x(78)91342-6. [DOI] [PubMed] [Google Scholar]
- de Petris S. Concanavalin A receptors, immunoglobulins, and theta antigen of the lymphocyte surface. Interactions with concanavalin A and with Cytoplasmic structures. J Cell Biol. 1975 Apr;65(1):123–146. doi: 10.1083/jcb.65.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen S. Cholera toxin. Biol Rev Camb Philos Soc. 1977 Nov;52(4):509–509. doi: 10.1111/j.1469-185x.1977.tb00858.x. [DOI] [PubMed] [Google Scholar]


