Abstract
Background
Anecdotal reports of tumour regression with histamine type 2 receptor antagonists (H2RAs) have lead to a series of trials with this class of drug as adjuvant therapy to try and improve outcomes in patients with resected colorectal cancers. There was a plausible scientific rationale suggesting merit in this strategy. This included improved immune surveillance (by way of increasing tumour infiltrating lymphocytes), inhibiting the direct proliferative effect of histamine as a growth factor for colorectal cancer and, in the case of cimetidine, inhibiting endothelial expression of E‐selectin (a cell adhesion molecule thought to be critical for metastatic spread).
Objectives
To determine if H2RAs improve overall survival when used as pre‐ and/or postoperative therapy in colorectal cancer patients who have had surgical resection with curative intent. We also stratified the results to see if there was an improvement in overall survival in terms of the specific H2RA used.
Search methods
Randomised controlled trials were identified using a sensitive search strategy in the following databases: MEDLINE (1964 to present), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2009), EMBASE (1980 to present) and Cancerlit (1983 to present).
Selection criteria
Criteria for study selection included: patients with colorectal cancer surgically resected with curative intent; H2RAs used i) at any dose, ii) for any length of time, iii) with any other treatment modality and iv) in the pre‐, peri‐ or post‐operative period. The results were stratified for the H2RA used.
Data collection and analysis
The literature search retrieved 142 articles. There were six studies included in the final analysis, published from 1995 to 2007, including a total of 1229 patients. All patients were analysed by intention to treat according to their initial allocation. Log hazard ratios and standard errors of treatment effects (on overall survival) were calculated using the Cochrane statistical package RevMan Version 5. Hazard ratios and standard errors were recorded from trial publications or, if not provided, were estimated from published actuarial survival curves using a spreadsheet designed for this purpose (http://www.biomedcentral.com/content/supplementary/1745‐6215‐8‐16‐S1.xls).
Main results
Of the six identified trials, five used cimetidine as the experimental H2RA, whereas one used ranitidine. There was a trend towards improved survival when H2RAs were utilised as adjuvant therapy in patients having curative‐intent surgery for colorectal cancer (HR 0.70; 95% CI 0.48‐1.03, P = 0.07). Analysis of the five cimetidine trials (n = 421) revealed a statistically significant improvement in overall survival (HR 0.53; 95% CI 0.32 to 0.87).
Authors' conclusions
Of the H2RAs evaluated cimetidine appears to confer a survival benefit when given as an adjunct to curative surgical resection of colorectal cancers. The trial designs were heterogeneous and adjuvant therapy has evolved since these trials were performed. Further prospective randomised studies are warranted.
Keywords: Humans; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/methods; Cimetidine; Cimetidine/therapeutic use; Colorectal Neoplasms; Colorectal Neoplasms/drug therapy; Colorectal Neoplasms/mortality; Colorectal Neoplasms/surgery; Histamine H2 Antagonists; Histamine H2 Antagonists/therapeutic use; Randomized Controlled Trials as Topic; Ranitidine; Ranitidine/therapeutic use
Plain language summary
Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer
Colorectal cancer (bowel cancer) is the third most commonly diagnosed cancer in the world. Surgery is the primary curative treatment for those with early stage disease. However, a number of patients relapse after primary surgery, presumably due to cancer cells that have spread undetected to other parts of the body. In general, once colorectal cancer has spread it is no longer curable. Hence, adjuvant treatments are given around the time of surgery to eliminate any remnant cells to improve a patient's chance of cure.
Histamine type 2 receptor antagonist drugs (H2RAs) were originally developed as a treatment for peptic ulcers. However, anecdotal reports surfaced of tumour shrinkage with the use of these drugs. This launched a number of trials to see if these medications could be used to improve a patient's chance of cure following surgery for colorectal cancer.
This Cochrane review found six studies that adopted this strategy. There was wide variability amongst the trials in respect to a) the dose used, b) the timing in relation to surgery and c) for how long the H2RA drug was used for. When the results of the trial were analysed together it appeared that there was no survival benefit with the use of these medications. When the studies using cimetidine (a particular H2RA which has a theoretical additional mechanism of action in preventing tumour spread) were analysed, there appeared to be a survival benefit for patients receiving cimetidine.
Given the variability amongst the trials the results can only be considered as speculative, as opposed to strong evidence for this approach. Furthermore, these trials were conducted in a time where the approach to staging and treatment would be considered sub optimal by today's standards. Hence, further trials in the future are warranted.
Summary of findings
Summary of findings for the main comparison. Effect of H2RAs on survival in patients with early stage colorectal cancer.
| Trial | Number of patients | Hazard ratio | 95% Confidence interval |
| Svendsen 1995 | 148 | 0.79 | 0.33‐1.90 |
| Adams 1997 | 34 | 0.44 | 0.05‐3.77 |
| Finlay 1999 | 115 | 0.42 | 0.13‐1.40 |
| Matsmoto 2002 | 64 | 0.30 | 0.11‐0.82 |
| Nielsen 2002 | 560 | 0.93 | 0.80‐1.09 |
| Huang 2007 | 60 | 0.68 | 0.25‐1.86 |
Background
Histamine type 2 receptor antagonists (H2RAs) were originally developed as a treatment for peptic ulcers. By inhibiting the action of histamine on the gastric parietal cell, acid production was reduced. The USA FDA approved the use of cimetidine in 1979 for the treatment of peptic ulcers and subsequently three further H2RAs have been marketed: ranitidine, famotidine and nizatidine.
Following increasing use of this class of drug, anecdotal reports surfaced demonstrating that H2RAs could heal malignant stomach ulcers and induce regression in a number of other malignancies (Taylor 1988; Harland 1989; Armitage 1979). This spurred a series of scientific studies and clinical trials to determine the nature and utility of the effect of H2RAs in cancer.
At least three mechanisms mediating these effects have been demonstrated. The first is enhancement of immune function. Histamine enhances the activity of T‐suppressor lymphocytes through their H2 receptor, thereby suppressing immune function (Melmon 1972; Nanda 1985; Kumar 1990). Blockade of the H2 receptors inhibits this effect and results in non‐specific immune enhancement. Cancer patients commonly have impaired immune function associated with increased activity of T‐suppressor cells and H2RAs are able to restore certain immune parameters including cell‐mediated immunity, antigen‐presenting ability of dendritic cells and anti‐tumour activity of natural killer cells (Flodgren 1985; Katoh 1996; Kubota 2002). Moreover, the use of histamine is markedly increased as part of the post‐surgical inflammatory milieu, and clinical trials have demonstrated that H2RAs can prevent or minimise the post‐operative immunosuppression that commonly occurs following major surgery (Adams 1994a; Hansbrough 1986). Another immune‐mediated mechanism of H2RAs relates to the observation that epithelial tumours commonly secrete high levels of histamine into the local tumour microenvironment, which prevents tumour infiltrating lymphocytes (TILs) from invading such cancers and recognising tumour cell antigens (Lin 2004; Adams 1997a). This has been shown to confer a worse prognosis in bowel cancers (Adams 1997a). Inhibition of H2 receptors by H2RAs protects TILs from this effect of histamine, resulting in an increase in TILs in some clinical trials, which is thought likely to improve immune surveillance (Adams 1997a; Lin 2004).
A second mechanism by which H2RAs may have an anti‐cancer effect is through inhibiting the direct proliferative effect of histamine as a growth factor for colorectal cancers (Adams 1994b). In vitro data has shown that histamine increases the growth of some human tumour cell lines, an effect inhibited by H2RAs (Adams 1994b).
A third anticancer mechanism that appears to be unique to cimetidine, of all the H2RAs, is its ability to inhibit endothelial cell expression of E‐selectin (Kobayashi 2000). The ligands for this cell adhesion molecule are commonly expressed on tumour cell plasma membranes as well as by neutrophils and other blood cells, and are sialylated forms of the Lewis blood group antigens: sLea (also known as CA19‐9) and sLex. This binding of tumour cells to endothelial cells appear to be a critical mechanism for metastatic spread via vascular pathways (Kannagi 2004; Tozeren 1995). In vitro work has shown that cimetidine inhibits binding of human colorectal cancer cells to activated human umbilical vein endothelial cells in a dose‐dependent fashion (Kobayashi 2000). Consistent with this, cimetidine showed dose‐dependent inhibition of blood‐borne liver metastases in an in vivo xenograft model of human colorectal cancer (Kobayashi 2000).
These mechanisms suggest that the use of H2RAs in the peri‐operative and post‐operative setting might usefully modulate the immune system in a cancer patient's favour, and that cimetidine in particular could potentially inhibit metastases by preventing circulating tumour cells from adhering to vascular endothelium.
However, the hypergastrinaemia that results from H2RA administration has raised concern that these medications may promote tumour growth. Gastrin has a trophic effect on the cells of the intestine (Koh 1999) and a proportion of colorectal tumours have gastrin receptors (Imdahl 1995). Despite these observations the link between gastrin and colorectal cancer remains controversial. Observational studies in patients with Zollinger‐Ellison syndrome (Orbuch 1996) and chronic proton pump inhibitor use (Robertson 2007) have shown no increased incidence of colorectal cancer. The levels of gastrin in these two cohorts exceed the levels seen with H2RAs (Trudeau 1970), with cimetidine having the smallest elevation in gastrin levels (Ohsawa 2002).
We are therefore conducting this review to determine the clinical effects of the use of H2RAs in the peri‐operative or post‐operative settings in those patients who have had surgical resection of a colorectal cancer with curative intent. By pooling the data of these trials we hope to determine whether H2RAs, and cimetidine in particular, confer survival benefits in this setting.
Objectives
To determine if H2RAs improve overall survival when used as peri‐ and/or postoperative therapy in colorectal cancer patients who have had surgical resection with curative intent. In conjunction with this, we will stratify the results to see if there is an improvement in overall survival in terms of the specific H2RA used.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials (RCTs) were considered for inclusion. Duplicate publications of the same data were excluded.
Types of participants
Participants could be of any age or sex, and must have had colorectal cancers that were surgically resected with curative intent.
Types of interventions
H2RAs could have been used at any time in the perioperative and/or postoperative periods.
H2RAs could be used for any length of time.
H2RAs could be used at any dose or by any route of administration.
H2RAs could be used with any other non‐surgical treatment modality (if any) as long as it was the use of the H2RAs as the point of difference between the treatment and control arm.
Types of outcome measures
Primary outcomes
The primary outcome measure was overall survival.
Secondary outcomes
Nil.
Search methods for identification of studies
Electronic searches
We identified randomised controlled trials using the following databases: MEDLINE (1964 to present), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library (2009 to present), EMBASE (1980 to present) and Cancerlit (1983 to present). The search will be based on the Cochrane Highly Sensitive Search Strategy for identifying Randomised Controlled Trials as published in the Cochrane handbook (Lefebvre 2008). Search strategy last performed on 16th December 2011.
MEDLINE (Ovid)
1. exp Colorectal Neoplasms/
2. ((colorect* or colon* or rect* or anus) adj3 (cancer or neoplasm* or carcinom* or tumour* or tumour* or polyp* or adenom*)).mp.
3. (sigmoid neoplasm* or adenomatous polyp* or hereditary nonpolyp*).mp.
4. 1 or 2 or 3
5. exp Histamine H2 Antagonists/
6. (H2RA or H2RAs or (histamin* adj3 antagonist*)).mp.
7. (ranitidin* or cimetidin* nizatidin* or famotidin*).mp. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier]
8. 5 or 6 or 7
9. 4 and 8
10. randomised controlled trial.pt.
11. controlled clinical trial.pt.
12. randomized.ab.
13. placebo.ab.
14. clinical trial.sh.
15. randomly.ab.
16. trial.ti.
17. 10 or 11 or 12 or 13 or 14 or 15 or 16
18. humans.sh.
19. 17 and 18
20. 9 and 19
EMBASE (Ovid)
1. exp colon tumour/
2. exp rectum tumour/
3. ((colorect* or colon* or rect* or anus) adj3 (cancer or neoplasm* or carcinom* or tumour* or tumour* or polyp* or adenom*)).mp.
4. (sigmoid neoplasm* or adenomatous polyp* or hereditary nonpolyp*).mp.
5. 1 or 2 or 3 or 4
6. exp histamine H2 receptor agonist/
7. (H2RA or H2RAs or (histamin* adj3 antagonist*)).mp.
8. (ranitidin* or cimetidin* nizatidin* or famotidin*).mp.
9. 6 or 7 or 8
10. 5 and 9
11. randomised controlled trial/
12. randomizations/
13. controlled study/
14. multicenter study/
15. phase 3 clinical trial/
16. phase 4 clinical trial/
17. double blind procedure/
18. single blind procedure/
19. ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).ti,ab.
20. (random* or cross* over* or factorial* or placebo* or volunteer*).ti,ab.
21. 16 or 13 or 17 or 19 or 12 or 18 or 14 or 11 or 20 or 15
22. "human*".ti,ab.
23. (animal* or nonhuman*).ti,ab.
24. 23 and 22
25. 23 not 24
26. 21 not 25
27. 10 and 26
Cochrane Library
#1 MeSH descriptor Colorectal Neoplasms explode all trees
#2 ((colorect* or colon* or rect* or anus) near3 (cancer or neoplasm* or carcinom* or tumour* or tumour* or polyp* or adenom*)):ti,ab,kw
#3 (sigmoid neoplasm* or adenomatous polyp* or hereditary nonpolyp*):ti,ab,kw
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Histamine H2 Antagonists explode all trees
#6 (H2RA or H2RAs or (histamin* adj3 antagonist*)):ti,ab,kw
#7 (ranitidin* or cimetidin* nizatidin* or famotidin*):ti,ab,kw
#8 (#5 OR #6 OR #7)
#9 (#4 AND #8)
MEDLINE search (Appropriate changes will be made for the specific databases):
1. randomized.ab.
2. clinical trials.tw.
3. placebo.ab.
4. Clinical Trial/
5. randomly.ab.
6. trial.ti.
7. #1 or #2 or #3 or #4 or #5 or #6
8. exp rectum tumour/ Why not colorectal cancer?
9. ((colorectal or colonic or colon or rectal or anus) adj3 (cancer or neoplasm* or carcinom* or tumour* or tumour* or polyp* or adenom*)).tw.
10. (sigmoid neoplasm* or adenomatous polyp* or hereditary nonpolyp*).tw.
11. #8 or #9 or #10
12. exp histamine H2 receptor antagonist/
13. (H2RA or H2RAs or (histamin* adj3 antagonist*)).tw.
14. #13 or #12
15. SURGERY/
16. Cancer Surgery/
17. (surgical or surgery or surgeries or resection? or operat* or preoperat* or perioperat* or postoperat*).tw.
18. #17 or #16 or #15
19. (ranitidin* or cimetidin* nizatidin* or famotidin*).tw.
20. exp Rectum Tumor/su [Surgery] Why not colorectal cancer?
21. #7 and #14 and #20
22. #11 and #18 and #7 and #19 and #14
23. #22 or #21
Searching other resources
The reference list of the relevant studies were searched for further studies. Additional databases relating to ongoing studies were also searched (ClinicalTrials.gov, Current Controlled Trials, International Clinical Trials Registry, Australian New Zealand Clinical Trials Registry).
Data collection and analysis
Selection of studies
Two reviewers (SD and MJ) independently selected the studies to be included in the review. Any differences were resolved by consensus after critical reading of the whole article.
Data extraction and management
The extraction and registration of data from each study was undertaken independently by each author using a form specifically designed for this review. The data extracted included details of the methodology used, the characteristics of the study participants, the type of interventions undertaken, the comparison groups and the results obtained (including the follow‐up period).
Assessment of risk of bias in included studies
The authors independently assessed the methods section of the RCTs, considering the randomisation process, the presence or not of adequate allocation concealment, the blinding of the care provider, patients and outcome assessors, the description or not of follow up and the description of losses of included patients. Each study was classified into categories A, B or C following the criteria set out in the Cochrane Collaboration Handbook in order to estimate the selection bias, performance bias, attrition bias and detection bias.
Measures of treatment effect
All patients were analysed by intention to treat, according to their initial allocation. Log hazard ratios and standard errors of treatment effects (on overall survival) were calculated using the Cochrane statistical package RevMan Version 5. Hazard ratios and standard errors were recorded from trial publications or, if not provided, will be estimated from published actuarial survival curves using a spreadsheet designed for this purpose (http://www.biomedcentral.com/content/supplementary/1745‐6215‐8‐16‐S1.xls).
Dealing with missing data
Authors of included studies were contacted in order to obtain any missing data.
Assessment of heterogeneity
Statistical heterogeneity between studies was tested using a chi‐squared test, with cut‐off point of P < 0.1. I2 was used to estimate the total variation across studies that is due to heterogeneity: <25% is considered as low level heterogeneity, 25% to 50% as moderate level, and higher than 50% as high level (Higgins 2002).
Assessment of reporting biases
A funnel plot was produced to investigate possible publication bias.
Data synthesis
Meta‐analysis of log hazard ratios for overall survival and their respective standard errors were processed by RevMan 5. The study results were combined and analysed using the generic inverse‐variance method.
Subgroup analysis and investigation of heterogeneity
The pooled analysis of the trials was undertaken using the random‐effects model. If significant heterogeneity is evident, causes for this will be sought.
A potential source of heterogeneity is the specific type of H2RA used, hence a sensitivity analysis was performed to examine the impact of this variable.
Sensitivity analysis
Sensitivity analysis was performed to explore whether heterogeneity resulted from low quality trials or not.
Results
Description of studies
Results of the search
The literature search retrieved 142 articles. A screening of abstracts to determine if the publications were randomised controlled trials, using an H2RA for early stage colorectal cancer narrowed the search to 18 articles. Of the 18 articles obtained, a number of the publications were duplications of the same trials.
Included studies
There were 6 studies included in the analysis, published from 1995 to 2007, including a total of 1229 patients. The trials varied in terms of inclusion/exclusion criteria, course of treatment and extent of follow‐up.
The treatment duration ranged from 5 days to 5 years. The timing of the use of H2RAs in relation to surgery also varied: pre‐, intra‐ and post‐operative administration of H2RAs were used to varying extents in the different trials (see table below). One trial (Nielsen 2002) used ranitidine, while the other five used cimetidine. Apart from Nielsen 2002 (Scotland and Denmark), the remaining trials were single country trials. The stage of patients was not uniform amongst the trials, including patients with disease stages I through IV. In the trials that included stage IV disease (Adams 1994c; Finlay 1999; Matsumoto 2002; Nielsen 2002) data was presented in a format that could exclude patients that had metastatic disease at baseline. After excluding ineligible patients there were 981 patients included in this meta‐analysis.
In addition to the H2RAs, two trials used other concurrent adjuvant treatments: patients in the Matsumoto 2002 trial received IV mitomycin C 24 hours post‐operatively then oral 5‐fluorouracil for 12 months, and those in the Huang 2007 trial received 6 months of adjuvant IV 5‐fluorouracil and folinic acid. One trial (Finlay 1999) investigated the effect of different doses of cimetidine.
The authors were all contacted to provide individual patient data. However, this was unable to be retrieved for any of the listed trials. As a result hazard ratio estimations using the published Kaplan‐Meier curves were calculated. Only two trials (Nielsen 2002; Finlay 1999) produced numbers at risk at different time points during follow up.
In this non‐blinded study, 50 patients who were electively scheduled for resection of their colon or rectal cancer were randomised to standard care or to have oral cimetidine 400mg BD for 5 to10 days prior to surgery in a single Australian hospital then IV cimetidine 200mg Q6H for 3 days peri‐operatively. Eight patients were excluded from the survival analysis: seven in the treatment group (three withdrew consent, one was referred for radiotherapy thus delaying surgery, one underwent emergency surgery after three doses of cimetidine because of tumour perforation and two had non‐malignant histology) and one in the control group (non‐malignant histology). No additional adjuvant treatments were given. An interim analysis published in 1994 (Adams 1994c) included 34 patients with median follow up of 30 months (four patients in each arm were excluded due to metastatic disease at baseline); a Kaplan‐Meier survival curve was provided. There were no significant differences in patient characteristics between the two treatment groups. A subsequent publication (Adams 1997a) that evaluated the effects of cimetidine on TILs in this patient cohort did not update the overall survival data.
192 patients who underwent resection of a colon (n = 123) or rectal cancer (n = 69) in one of three Danish hospitals were randomised within three weeks of surgery to two years of oral cimetidine 400mg BD or placebo. No additional adjuvant treatments were given. 148 of these patients had surgery with curative intent (Dukes A ‐ 28 patients, Dukes B ‐ 72, Dukes C ‐ 47); three patients were excluded ‐ who had lymphoma (n = 1), carcinoid (n = 1) or took the medication for less than two weeks (n = 1). There were no significant differences in baseline characteristics of the two treatment arms following block randomisation. With a median observation time of 40 months in surviving patients, Kaplan‐Meier curves for cancer‐specific survival were published for patients who had undergone non‐curative resection (n = 41), curative resection (n = 148) and Dukes C patients (n = 47). The curative resection group have been included in this analysis.
115 patients who were undergoing curative resection for their colon or rectal cancer were randomised to low dose (400mg BD) oral cimetidine, high dose (800mg BD) cimetidine or placebo for 5 to 10 days pre‐operatively. It was a double‐blind, placebo‐controlled trial. Despite metastatic disease being an exclusion criterion there were approximately five patients with (estimated from stage distribution graph) "Dukes D" cancer. These patients were distributed in all three treatment arms and were unlikely to have had an impact on the outcome. Subjects were followed for a median of 472 days at the time of publication. A Kaplan‐Meier curve for overall survival was produced, grouping both low‐ and high‐dose cimetidine versus placebo. There was a duplicate publication of the trial (Kelly 1999) which recruited an additional 10 patients over an extra month and had longer follow up (mean of 972 days). The Finlay 1999 paper was used instead of the Kelly 1999 paper in this meta‐analysis as it provided a) the numbers of patients at risk of events on the Kaplan‐Meier curve, allowing for greater precision for HR estimation, and b) it displayed the data with one comparison (cimetidine vs. placebo). The Kelly 1999 paper displayed three individual arms on the Kaplan‐Meier curve (low dose, high dose, placebo). Calculating separate HRs for each dose level would distort (double count) the control arm numbers for this meta‐analysis.
72 patients with primary colorectal (CRC) and tumour stage of T2 or T3 (excluding those with prior chemotherapy, radiotherapy or immunotherapy, multiple cancers or severe operative complications) were recruited into this unblinded study in which all patients received adjuvant chemotherapy (IV mitomycin C 8mg/m2 within 24 hours of surgery then, starting 2 weeks later, oral 5‐fluorouracil 200mg daily for 1 year). Patients were randomised to no additional therapy or to take oral cimetidine 800mg daily for 1 year. The primary aim of the trial was to determine whether cimetidine would reduce appetite loss and reflux oesophagitis in those undergoing adjuvant oral chemotherapy. Eight patients were excluded: those who did not undergo curative resection (n = 2), did not receive adequate drug administration (n = 3) and whose tumour stage was considered inappropriate (n = 3); there were equally distributed across the two study arms. In 64 included patients (46 colon cancer and 18 rectal cancer) improved 39‐month survival was reported at a mean follow up of 31 months (Matsumoto 1995) but the published survival curve only included colon cancer patients. The subsequent report (Matsumoto 2002) included all patients in the survival analyses with a mean follow up of 10.7 years, and has been incorporated into this meta‐analysis.
823 patients were involved in this randomised, double‐blind, placebo‐controlled trial, the only one that used ranitidine (100mg IV BD from immediately prior to skin incision then 150mg po BD for 5 years). No adjuvant chemotherapy or radiotherapy was given. 83 patients were recruited in Scotland and after an interim analysis showing no survival benefit (at a median observation period of 40 months), these patients were lost to follow up. The remaining 740 patients recruited in Denmark (419 with colon cancer, 321 with rectal cancer) had been observed for a median period of 6.8 years at the time of the final, planned analysis (Nielsen 2002). Of these, 560 underwent curative resection, including 10 of 190 patients with metastatic disease, and are included in this meta‐analysis. While Kaplan‐Meier survival curves were published including numbers at risk at different time points, Hazard Ratios (HRs) and log rank analyses, the HR for survival in curative resection patients was not reported and had to be estimated from the published curves.
60 patients who underwent resection of rectal cancer were randomised to cimetidine (200mg QID orally for 1 week preoperatively then 400mg BD IV for 7 days from surgery, then 200mg QID orally for 2 years) or control. There were no significant differences in clinical or tumour characteristics between the two groups (n = 30 in each). All patients received adjuvant chemotherapy with 6 cycles of 5‐fluorouracil and folinic acid. Median follow up was 54 months. The HR was estimated from the published Kaplan‐Meier survival curves.
Note. The trial was translated from its publication language (Mandarin)
| Trial | Number | Treatment | Additional treatment | Median/Mean follow up |
| Adams 1994c | 14 | Cimetidine 400mg po bd for 5 days pre‐operatively. Followed by 200mg iv q6hrly for 3 days post‐operatively. | Nil | 30 months |
| 20 | No placebo | |||
| Svendsen 1995 | 78 | Cimetidine 400mg po bd for 2 years post‐operatively. | Nil | 40 months |
| 70 | Placebo | |||
| Finlay 1999 | 34 | Cimetidine 400mg po bd for 5 days pre‐operatively. | Nil | 14 months |
| 37 | Cimetidine 800mg po bd for 5 days pre‐operatively. | |||
| 34 | Placebo | |||
| Matsumoto 2002 | 34 | Cimetidine 800mg po daily for 1 year post‐operatively. | 8mg/m2 mitomycin C peri‐operatively, plus 200mg/m2 of oral 5‐FU daily for 1 year. | 10.7 years |
| 30 | No placebo | |||
| Nielsen 2002 | 282 | Ranitidine 100mg IV intra‐operatively, followed by 150mg po bd for 5 years | Nil | 6.8 years |
| 278 | Placebo | |||
| Huang 2007 | 30 | Cimetidine 200mg po qid for one week pre‐op, followed by 400mg IV bd for one week post‐op, then to continue 200mg po qid for 2 years. | 6 cycles of 5‐Fluorouracil | 54 months |
| 30 | No placebo |
Excluded studies
A number of studies were excluded as they were duplications of one of the six included studies. The excluded studies in these cases either had shorter follow up and/or were often looking at different outcome parameters (as opposed to overall survival).
Two excluded trials of interest were Kapoor 2005 and Lin 2004. Kapoor 2005 had published a paper looking at the efficacy of famotidine in augmenting TILs in patients with early stage CRC. In this small study 23 patients were randomised to receive either famotidine 40mg orally for 1 week pre‐operatively (n = 11) or placebo (n = 12). They concluded that famotidine significantly increased the rate of tumour lymphocytic infiltration and, follow up of 10 months, recurrent disease was seen in five patients in the placebo arm and in only one patient in the treatment arm. Mean overall survival was 21 months for the treatment group versus 14.9 months for the placebo group (P = 0.83) but no survival curve was published. Lin 2004 looked at the effect of peri‐operative cimetidine on immune modulating cells (peripheral blood lymphocytes, natural killer cells and tumour infiltrating lymphocytes) in patients with gastro‐intestinal malignancies. This small study (n = 49) included patients with both stomach and bowel cancer and also at patients with all stages of disease. There was no survival outcome data published. For these two publications attempts to contact the authors to provide additional information for further analysis have proven to be unsuccessful.
Tavani 1998 was a large (n = 955) case‐control study to determine the effect of cimetidine on the incidence (not outcome) of colorectal cancer.
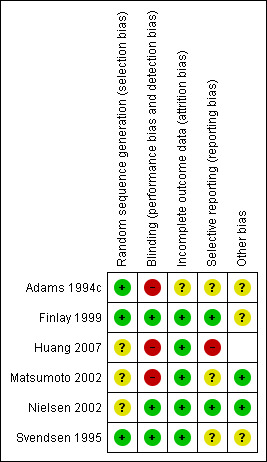
Risk of bias in included studies
The six trials were well conducted with multiple routine measures/procedures in place to reduce bias. The only areas that were identified as having a high risk of bias were: three trials were not placebo‐controlled (Adams 1994c; Huang 2007 and Matsumoto 2002) and one trial (Huang 2007) utilised a different statistical method to analyse the outcome measure from what was specified in the protocol.
Allocation
All studies had a randomisation process, however the method of randomisation was not explicit in three trials (Huang 2007; Matsumoto 2002; Nielsen 2002). Numbered envelopes were used by Adams 1994c and Finlay 1999. Block randomisation was used in Svendsen 1995 trial.
See Figure 1.
1.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Three trials were placebo‐controlled (Svendsen 1995; Finlay 1999 and Nielsen 2002). Given the relatively low toxicity of H2RAs it is likely that both the patient and doctor would have remained blinded to the treatment received. The remaining three trials were unblinded and there was concern of performance bias were in the two trials (Adams 1994c; Huang 2007) that delivered H2RAs pre‐operatively. This could have potentially had an impact on the surgery that was undertaken as it was likely known to both surgeon and patient which arm of the trial the patient was on. There was also a high risk of performance bias in the other non‐placebo controlled trial (Matsumoto 2002). Although the trial medication was only taken post‐operatively (based on eligible histology), given that the patients in both arms of the trial had additional adjuvant chemotherapy (mitomycin C and 5‐fluorouracil), the knowledge of which arm of the trial the patient was on could have theoretically had a differential impact on the delivery of the standard adjuvant therapy.
An additional concern for the all the trials, given the high incidence of H2RA use in the community, was the risk of 'cross over' to the H2RA arm in the follow‐up period.
Detection bias was not a concern in this trial as overall survival was the endpoint. One trial (Svendsen 1995) reported cancer‐specific survival only but, given the low toxicity profile of H2RAs (Richter 1989) and the lack of significant impact of perioperative ranitidine on surgical complications and mortality in a study in 905 patients having surgery for CRC (Nielsen 1999), the authors believe that the data produced would have produced a similar HR to that expected for overall survival data and have thus incorporated this study into the meta‐analysis.
See Figure 1.
Incomplete outcome data
The reasons for exclusions of patients from the primary analysis in the six trials were on the whole justified (mainly due to incorrect histological diagnosis or ineligible AJCC cancer stage). Two trials excluded patients when they had not received adequate drug delivery which could have been a potential source of bias. Although the outcome data was not a strict intention‐to‐treat analysis, this only totaled n = 4 patients for the whole meta‐analysis and is unlikely to have had a significant impact on the analysis.
There were no major concerns in regards to loss of patients in follow up. Four trials (Finlay 1999; Svendsen 1995; Huang 2007; Matsumoto 2002) reported no loss of patients during the follow up period. Nielsen 2002 was a trial conducted in two countries (Denmark and Scotland) and when a 40 month interim analysis indicated futility of ranitidine all the Scottish patients (n = 83) were lost to follow up. However, all the Danish patients (n = 740) were followed up. It was unclear if there was a significant attrition in Adams 1994c. It must be noted that the length of follow up differed widely amongst the six trials. The mean/median length of follow up were 2.5 years, 1.2 years, 4.5 years, 10.7 years, 6.8 years and 3.3 years for Adams 1994c; Finlay 1999; Huang 2007; Matsumoto 2002; Nielsen 2002 and Svendsen 1995 respectively.
See Figure 1.
Selective reporting
Trial protocols were not available for any of the six trials so it was generally unclear if there were potential concerns of reporting bias. A possible source of reporting bias is the lack of updated survival data for trials with short follow up (especially Adams 1994c and Svendsen 1995). There was a duplicate publication of the Adams 1994c trial three years later (Adams 1997a) reporting on TILs but no updated survival curves were published.
In the Huang 2007 publication they state that the will use log‐rank to compare the survival rates in the methodology section. In the results section they used chi‐squared methodology to compare survival rates at different points. It is unclear what the E (expected) survival value that was used at different time points (or how they derived them). It is also unclear if there was a chi squared correction performed (as there was only one degree of freedom (i.e. two categories: cimetidine and control)).
See Figure 1.
Other potential sources of bias
Although difficult to make strong conclusions when only six trials were included in this review, the forest plot (Figure 2) comparing the effect of outcome versus the standard error of the included trials suggests a possible publication bias with the trials skewed towards the left (towards a positive treatment effect for H2RAs).
2.
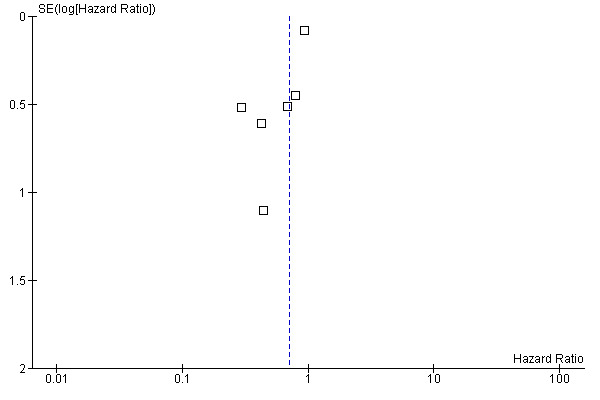
Funnel plot of comparison: 1 Effect of H2RAs on survival in patients with colorectal cancer, outcome: 1.1 Overall Survival.
Effects of interventions
See: Table 1
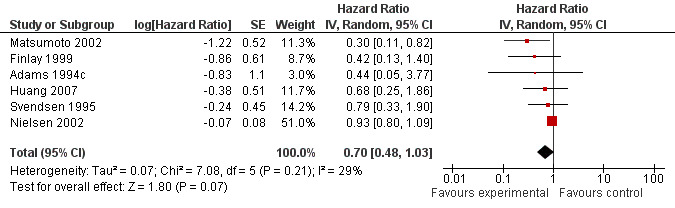
In all the included trials the effect of the intervention was displayed by way of a Kaplan‐Meier survival curve. By using a validated statistical spreadsheet (http://www.biomedcentral.com/content/supplementary/1745‐6215‐8‐16‐S1.xls) we were able to calculate the HR for the addition of an H2RA as adjuvant treatment for early stage CRC. Although patient numbers in most of the trials was small, all showed a trend towards improved survival with the addition of ranitidine or cimetidine.
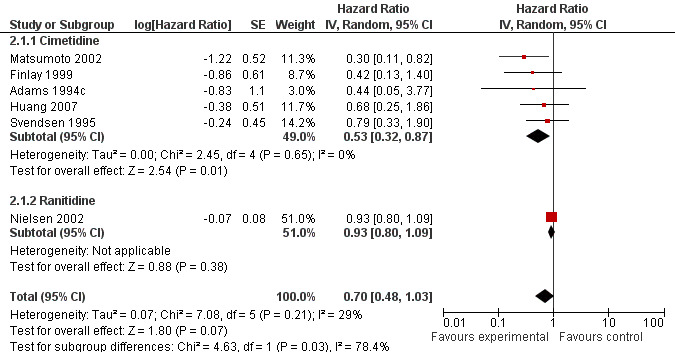
There was an overall non‐significant trend towards an improvement in survival when the results of all the trials are combined (HR 0.70; 95% CI 0.48 to 1.03, Figure 3). The effect appears more pronounced when the effect of cimetidine is looked at in isolation (n = 421) with a resulting significant improvement in survival (HR 0.53; 95% CI 0.32 to 0.87, Figure 4). In one cimetidine trial (Matsumoto 2002) its effect on survival was evaluated retrospectively in different groups characterised immunohistochemically by tumour expression of the sialyl Lewis antigen ligands for E‐selectin. The hazard ratios estimated for patients whose tumours were designated as positive for sLea and sLex were HR 0.14 (95% CI 0.04 to 0.58) and HR 0.18 (95% CI 0.02 to 1.59) respectively.
3.
Forest plot of comparison: 1 Effect of H2RAs on survival in patients with early stage colorectal cancer, outcome: 1.1 Overall Survival.
4.
Forest plot of comparison: 2 Overall Survival, outcome: 2.1 Cimetidine versus Ranitidine.
Only one included trial (Nielsen 2002) examined the effect of ranitidine on overall survival (HR 0.93; 95% CI 0.80‐1.09). Protocol‐specified subgroup analyses revealed improved survival from the addition of ranitidine in the groups who did not receive blood transfusions (HR 0.6; 95% CI 0.4 to 0.9) or those who had neither blood transfusions nor post‐operative infections (HR 0.6; 95% CI 0.4 to 0.9).
| Trial | Number of patients | Hazard ratio | 95% Confidence interval |
| Svendsen 1995 | 148 | 0.79 | 0.33‐1.90 |
| Adams 1994c | 34 | 0.44 | 0.05‐3.78 |
| Finlay 1999 | 115 | 0.42 | 0.13‐1.40 |
| Matsumoto 2002 | 64 | 0.30 | 0.11‐0.82 |
| Nielsen 2002 | 560 | 0.93 | 0.80‐1.09 |
| Huang 2007 | 60 | 0.68 | 0.25‐1.86 |
Discussion
Summary of main results
There was a trend towards improved survival when H2RAs were utilised as adjuvant therapy in patients having curative‐intent surgery for CRC (HR 0.70; 95% CI 0.48‐1.03, P = 0.07). Of the six included trials, one used ranitidine (Nielsen 2002) and the other five (Adams 1994c; Finlay 1999; Huang 2007; Matsumoto 2002; Svendsen 1995) used cimetidine as the H2RA. The Nielsen 2002 trial was the largest (n = 560) and demonstrated the least effect from the addition of the H2RA (HR 0.93; 95% CI 0.80‐1.09), whereas the five cimetidine trials together (n =421) revealed a statistically significant improvement in survival (HR 0.53; 95% CI 0.32 to 0.87). Heterogeneity among all 6 trials was moderate (P = 0.21, I2 = 29%; Higgins 2002) but was low among the cimetidine trials (P = 0.65, I2 = 0%) and the test for subgroup differences according to H2RA type used was significant (P = 0.03, I2 = 78.4%), indicating a significant difference in the effect of ranitidine compared to cimetidine.
Overall completeness and applicability of evidence
While these six trials were relatively small (with a total of 981 patients being included in this meta‐analysis), most had adequate data without significant loss of patients during the observation period, which ranged from 1.3 to 10.7 years. There was significant diversity in the timing, dose and duration of H2RA employed, ranging from as little as five days pre‐operatively (Finlay 1999) to five years post‐operatively (Nielsen 2002). This variability draws into question the significance of the differing trial designs which, despite showing similar trends towards improved survival, do not clearly indicate when an H2RA may be optimally used.
Further concerns about the applicability of the evidence lies in the fact that, apart from Huang 2007, the trials were conducted approximately twenty years ago. Since this time there have been significant improvements in the care and survival of patients diagnosed with CRC. CT‐based staging is commonplace prior to elective surgery for CRC, ensuring that patients are treated appropriately for disease stage. It is unclear from the included trial publications what staging measures were undertaken to exclude patients with metastatic disease. Indeed several trials included significant numbers with metastases at diagnosis, and these numbers may have been higher if they were staged in the current era, and this group could have been appropriately excluded. Furthermore all six trials included patients staged as Dukes A, B and C. Certainly most of the patients with Dukes A CRC and a significant number of Dukes B patients would have had excellent outcomes from surgery alone and it would have required much larger trials to show any incremental gains in survival from H2RAs.
Surgical techniques (e.g. laparoscopic resection, improved nodal harvests, total mesorectal excision) and post‐operative care, including the use of leukocyte‐depleted blood transfusions (Jensen 1996) and other measures to enhance post‐operative recovery and reduce complications, have also resulted in improved outcomes. Moreover patients in four of the trials did not receive any adjuvant chemotherapy or radiotherapy (for rectal cancer patients). Only Huang 2007 and Matsumoto 2002 utilised additional adjuvant chemotherapy, though the oral 5‐fluorouracil chemotherapy employed in the latter trial has not been proven effective. The current standard of care for Dukes C (and high‐risk Dukes B) colon cancer includes adjuvant 5‐fluorouracil‐based chemotherapy (often with oxaliplatin), with improved survival of 10‐15% at 5 years expected compared to patients treated with surgery alone (Hsiao 2011; Abrams 2011). For rectal cancer patients with radiologically‐staged T3 or T4 tumours or positive regional nodes, preoperative radiation with or without concurrent and/or adjuvant chemotherapy would be given in many parts of the world, again with significantly improved survival and reduced local recurrence (Gerard 2006).
It is possible that the outcomes with cimetidine may have been enhanced by a pharmacokinetic interaction with the 5‐fluorouracil‐based chemotherapy in two trials where it was given concurrently (Matsumoto 2002 and Huang 2007) as cimetidine has been shown to reduce the clearance of 5‐fluorouracil by 28% (Harvey 1984). However the HRs seen with these two trials differ greatly (0.30 and 0.69 respectively) while other trials where no adjuvant chemotherapy was given show intermediate results.
Even if the apparent survival benefit seen with cimetidine in particular is substantiated in subsequent large trials with a similar HR, the absolute magnitude of the benefit is likely to be considerably smaller than that seen in this collection of trials (about 30% greater survival at 3 years), due to the improved outcomes in the control arms that may be seen with modern management. Furthermore the contribution made by H2RAs through reducing post‐operative immunosuppression could also be diminished because modern care minimises the immunological insult of surgery. Another effect of these changes in care could be a reduction in the magnitude and duration of inflammatory cytokine production, which might reduce the likelihood of E‐selectin expression and thus tumour cell metastasis. This could diminish the impact of the cimetidine‐mediated E‐selectin inhibition and blockade of tumour cell adhesion to endothelium.
Quality of the evidence
Individual patient data was unable to be obtained for the six trials, nor HR and 95% confidence intervals for the main comparisons. Thus a less precise estimate of the effectiveness of the intervention was derived by evaluating the published survival curves in a spreadsheet designed for this purpose (http://www.biomedcentral.com/content/supplementary/1745‐6215‐8‐16‐S1.xls). The precision of the estimate would be greater in the two trials (Nielsen 2002; Finlay 1999) that reported number of patients at risk at regular time points on the Kaplan‐Meier curve.
There was little data on compliance (and toxicity) of the intervention, however H2RAs are generally well tolerated and good compliance would be expected. There could have been concern in regard to possible contamination by H2RAs in the control arm, as these drugs were frequently used to treat the common ailment of gastro‐oesophageal reflux. However this would have, if anything, reduced the impact of the intervention when compared to the control arm.
A funnel plot of the included trials appeared asymmetric which raises the possibility of reporting bias, with only the positive results being published but a thorough search of the literature, trials registries, conference proceedings and the published trials manuscripts in this setting revealed no other trials evaluating the impact of H2RAs on survival in CRC. Some of the trials that reported data early in the observation of patients have not published updated reports of survival with longer follow up.
Potential biases in the review process
Nil.
Agreements and disagreements with other studies or reviews
No other reviews published at present.
Authors' conclusions
Implications for practice.
The results of this meta‐analysis indicate a trend towards improved survival outcomes when H2RAs are used as peri‐operative and/or adjuvant treatment for early stage CRC. In particular the survival benefit seen in trials using cimetidine, as opposed to a trial using ranitidine, reached statistical significance in the meta‐analysis, which is consistent with its differing mechanisms of action to those of other H2RAs in this setting. However given the variability in the trial design, particularly in terms of the timing and duration of H2RA administration, these results should be judged as hypothesis‐generating rather than as demonstrating an effective treatment option. In addition the benefits suggested by this meta‐analysis come from trials where patient management at that time would be considered sub optimal today, and better outcomes are expected now. Thus it is unlikely that the size of the apparent benefits from H2RA administration in CRC patients around the time of surgery could be replicated currently.
Implications for research.
The result of this meta‐analysis supports the call for large‐scale trials with cimetidine in this setting (Eaton 2002). CRC is one of the most common cancers worldwide and there remains a significant clinical need for further treatment improvements in this disease. There is a significant minority of patients who, in spite of having early stage disease, relapse following optimal surgery, chemotherapy and/or radiation. Further trials utilising H2RAs should be considered as these treatments are inexpensive, well‐tolerated, have a plausible biological rationale and can be administered alongside standard adjuvant chemotherapy. In addition the quantum of benefit likely to be derived from their use could exceed that of oxaliplatin at considerably less toxicity, cost and inconvenience. Given the results of this meta‐analysis and the spectrum of biological actions of cimetidine compared to other H2RAs the authors believe that future research should primarily utilize cimetidine.
The results also lend support to the biological and clinical significance of the ability of cimetidine to block E‐selectin expression in addition to the mechanisms common to all H2RAs, as evidenced by the greater impact on survival than ranitidine, and the similar benefits seen with very short perioperative administration in the trials by (Adams 1994c) and (Finlay 1999) compared to the others that used it for 1‐2 years post‐operatively. The results from the ranitidine trial (Nielsen 2002) provide indirect supportive evidence for the significance of E‐selectin blockade, in that ranitidine was effective (HR 0.6; 95% CI 0.4 to 0.9) only in those patient groups where inflammatory cytokine production from postoperative infections and blood transfusions was avoided. It is interesting to see from this trial the substantial clinical benefit from ranitidine that is mediated by histamine antagonism alone. To this cimetidine adds the benefits of E‐selectin inhibition, so it is likely that cimetidine may confer significantly greater benefits than another agent that blocks E‐selectin‐mediated cell adhesion alone. The importance of E‐selectin blockade in the survival benefit conferred by cimetidine has significance for the timing and duration of dosing. Ideally cimetidine administration should commence prior to surgery and continue for as long as the inflammatory cytokines remain elevated (which has yet to be clarified, especially with open versus laparoscopic surgery). Most patients are swallowing water within a day of surgery for CRC so oral administration is likely to be adequate for most patients.
Other factors to consider in cimetidine dosing is the potential for interaction with chemotherapy and radiation. Apart from the pharmacokinetic interaction with 5‐fluorouracil already noted (Harvey 1984) cimetidine may also inhibit uptake of oxaliplatin into CRC cells, though at concentrations about 100‐fold higher than those achieved with oral administration in the usual dose range (Zhang 2006). Thus cimetidine has the potential to increase toxicity of chemotherapy as well as potentially enhance or compromise its efficacy. It would be prudent, as well as informative, in any future trials utilizing cimetidine concurrently with chemotherapy, to carefully evaluate its impact on toxicity as well as relapse and survival. With regard to radiation, all the H2RAs have been shown to have radioprotective properties, possibly through anti‐oxidant properties (Kojima 2002; Mozdarani 2003), and would be best avoided concurrently with radiation.
Further trials aimed at detecting disease‐free or overall survival improvements should be designed with appropriate statistical power to detect a clinically‐meaningful improvement. In addition, given the results from the (Matsumoto 2002) trial where the benefits of cimetidine appeared to be confined to the patients whose tumours expressed sLea (CA19‐9) or sLex, their expression and interaction with the impact of cimetidine on relapse and survival would greatly inform the identification of patients who are most likely to benefit from the use of this medication (as it could be determined on preoperative diagnostic biopsies). The expression of these tumour antigens in different ethnic groups is also poorly studied and should be evaluated prospectively.
Finally, future trials could evaluate whether H2RAs can still usefully impact on post‐operative immunocompetence, perhaps most easily by assessing cell‐mediated immunity with skin‐prick testing pre‐ and post‐operatively. Whether cimetidine administration in the peri‐ or post‐operative period may still confer a substantial survival benefit remains to be proven in large, well‐designed prospective trials.
History
Protocol first published: Issue 2, 2009 Review first published: Issue 8, 2012
| Date | Event | Description |
|---|---|---|
| 16 December 2011 | Amended | Review completed |
Acknowledgements
Dr Jayne Tierney. Statistician.
Data and analyses
Comparison 1. Effect of H2RAs on survival in patients with colorectal cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival | 6 | Hazard Ratio (Random, 95% CI) | 0.70 [0.48, 1.03] |
1.1. Analysis.
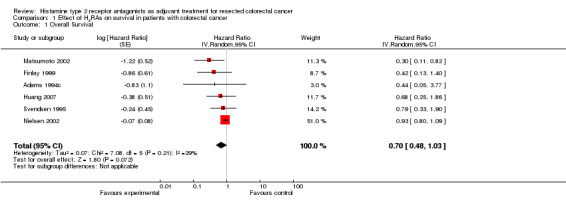
Comparison 1 Effect of H2RAs on survival in patients with colorectal cancer, Outcome 1 Overall Survival.
Comparison 2. Overall Survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cimetidine versus Ranitidine | 6 | Hazard Ratio (Random, 95% CI) | 0.70 [0.48, 1.03] | |
| 1.1 Cimetidine | 5 | Hazard Ratio (Random, 95% CI) | 0.53 [0.32, 0.87] | |
| 1.2 Ranitidine | 1 | Hazard Ratio (Random, 95% CI) | 0.93 [0.80, 1.09] |
2.1. Analysis.
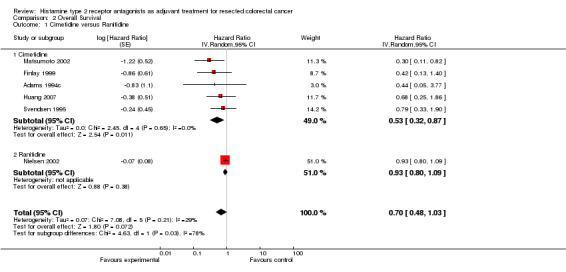
Comparison 2 Overall Survival, Outcome 1 Cimetidine versus Ranitidine.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adams 1994c.
| Methods | Randomised controlled trial | |
| Participants | 50 consecutive patients undergoing elective colorectal resection gave consent. | |
| Interventions | Cimetidine 400mg po bd for 5 days pre‐operatively. Followed by 200mg iv q6hrly for 3 days post‐operatively. No placebo in control arm. | |
| Outcomes | Primary endpoint: tumour lymphocyte concentration Secondary endpoint: overall survival |
|
| Notes | Five patients were withdrawn from the treatment arm (three withdrew consent, one required radiotherapy, one required emergency surgery). Eight patients discovered to have metastatic cancer and were excluded from the analysis (four in treatment arm, four in control arm). Therefore treatment effect assessed on 34 patients ‐ n = 14 on treatment arm (Dukes A ‐ 5 patients, Dukes B ‐ 4 patients, Dukes C ‐ 5 patients) versus n = 20 in the control arm (Dukes A ‐ 5 patients, Dukes B ‐11 patients, Dukes C ‐ 4 patients). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbered envelope |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo and hence unblinded. As treatments were given pre‐ and post‐operatively, this knowledge could have had an impact on the surgery that had taken place. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The exclusions of the patients (from 50 consented patients to the 34 used in this analysis) were justifiable. Unclear if there is any significant missing data. Median follow‐up 30 months. |
| Selective reporting (reporting bias) | Unclear risk | Unclear what all the primary/secondary outcomes were in the trial. |
| Other bias | Unclear risk | Further paper published in 1997 looking at tumour infiltrating lymphocytes. No updated survival data included. |
Finlay 1999.
| Methods | Randomised controlled trial | |
| Participants | Patients with presumed localised colorectal cancer undergoing elective surgery. N = 115. | |
| Interventions | Patients randomised to : ‐ Low dose (400mg bd) cimetidine, ‐ High dose (800mg bd) cimetidine, or ‐ Placebo for 5 to 10 days pre‐operatively. |
|
| Outcomes | Primary endpoint: tumour lymphocyte concentration Secondary endpoint: overall survival |
|
| Notes | Duplicate paper published in the same year (Kelly 1999). Had ten additional patients (n = 125) in their analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbered envelope. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Treatment group details were withheld from all subject and investigators until after completion of all subjects' treatment and analysis of results." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat efficacy analysis with 104 of 115 randomised patients. Reasons for exclusion of eleven patients stated (one unresectable tumour, nine benign adenomas, one intestinal lymphoma), which were evenly spread across the three treatment arms. Median follow up 427 days. No patients lost in follow up. |
| Selective reporting (reporting bias) | Low risk | The published reports include all the pre‐specified outcomes. |
| Other bias | Unclear risk | Unclear where the 10 extra patients in the Kelly 1999 publication came from (patients recruited from 1995 to 1997). |
Huang 2007.
| Methods | Randomised controlled trial. | |
| Participants | 60 patients who underwent surgical removal of rectal cancer. | |
| Interventions | Patients randomised to: ‐ Control: 6 cycles of post‐operative 5‐fluorouracil. ‐ Intervention: peri‐operative cimetidine (200mg qid orally for one week pre‐operatively, 400mg qid intravenously for one week post‐operatively followed by 200mg qid orally from day 8 post‐operatively till 2 years) plus 6 cycles of post‐operative 5‐fluorouracil. |
|
| Outcomes | 5 year overall survival | |
| Notes | Paper translated from Mandarin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo and hence unblinded. As treatments were given pre‐ and post‐operatively, this knowledge could have had an impact on the surgery that had taken place. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | States that all patients were followed‐up. Median follow‐up was 54 months. |
| Selective reporting (reporting bias) | High risk | In the methodology they state that the will use log‐rank to compare the survival rates. In the results they used chi‐squared methodology to compare survival rates at different points. It is unclear what the E (expected) survival value that was used at different time points (or how they derived them). It is also unclear if there was a chi squared correction performed (as there was only one degree of freedom (i.e.. two categories: cimetidine and control)). |
Matsumoto 2002.
| Methods | Randomised controlled trial. | |
| Participants | 72 patients with T2/T3 primary colon or rectal tumours undergoing curative resection. 8 were excluded which were "equally distributed between the treatment groups" (three did not undergo curative resection, three did not have adequate drug delivery, three patients had inappropriate stage). Hence, N=64. | |
| Interventions | Patient randomised to : ‐ Control: 8mg/m2 of mitomycin with 24 hours following the operation + 200mg of daily oral 5‐fluorouracil from two weeks till 1 year. ‐ Intervention: 800mg of daily oral cimetidine from two weeks post‐operatively to 1 year + 8mg/m2 of mitomycin with 24 hours following the operation + 200mg of daily oral 5‐fluorouracil from two weeks till 1 year. |
|
| Outcomes | Overall survival | |
| Notes | In an interim report the aim of the study was stated as to assess if cimetidine could "reduce appetite loss and reflux oesophagitis in colorectal cancer patients". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients 'randomly assorted into two groups'. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No placebo. Although eligibility and enrolment into the study was done on a post‐operative histological specimen (T2/T3), given that patients also received adjuvant (post‐operative) chemotherapy in this trial there was a potential risk of performance bias in the administration of adjuvant chemotherapy which could have had an impact on overall survival. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for patient exclusions given (Nb. Three patients with inadequate drug delivery were removed from the analysis, and was not an intention to treat sample. However, given the small numbers it is unlikely this had any effect on the outcome). No patients lost to follow up. Mean follow up of 10.7 years. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Unclear what the primary outcome of the trial was (overall survival vs. rates of reflux oesophagitis/appetite loss). |
| Other bias | Low risk | No other concerns identified. |
Nielsen 2002.
| Methods | Randomised controlled trial | |
| Participants | Patients scheduled for elective resection of primary tumours were included. N = 740. Of this 560 were undergoing curative resection. | |
| Interventions | Before skin incision ranitidine 100 mg or placebo was given intravenously twice daily followed by oral ranitidine 150 mg or placebo twice daily for five years. | |
| Outcomes | Overall survival. | |
| Notes | The original study compromised of two sites (Denamrk and Scotland). An observer‐blinded analysis performed after 40 months showed that there was no effect of the ranitidine and the study was discontinued in accordance of the protocol. After discontinuation all the Scottish (n = 83) were lost to follow‐up. All the Danish patients (n = 740) were followed until death. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were 'double blind randomised'. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, placebo controlled trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Despite losing all the Scottish data (n = 83) the reviewers believe that this was unlikely to have an effect on the primary outcome. None of the 740 Danish patients were lost to follow up. Median observation period of 6.8 years. |
| Selective reporting (reporting bias) | Low risk | The published reports contained all the pre‐specified outcomes. |
| Other bias | Low risk | No other concerns identified. |
Svendsen 1995.
| Methods | Randomised controlled trial. | |
| Participants | Patients who had undergone resection or an exploratory operation for adenocarcinoma of the colon and rectum were included. N=192. Based on the presence of residual tumour tissue patients were stratified into those who were: a) curatively operated on (n=148), or b) non‐curatively operated on (n=41). |
|
| Interventions | Cimetidine 400mg twice daily starting within three weeks of the operation till two years post‐operatively. | |
| Outcomes | Cancer‐specific mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised into groups of ten. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo controlled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients excluded after primary inclusion due to carcinoid tumour (n = 1), lymphoma (n = 1) and a violation of study protocol ‐ less than two weeks of protocol medication (n = 1). Median follow‐up of 40 months. No patients lost during follow up. |
| Selective reporting (reporting bias) | Unclear risk | Used cancer‐specific mortality (as opposed to overall survival). |
| Other bias | Unclear risk | Used 90% confidence intervals. Stated that was done to diminish the chance of a false negative conclusion. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 1994a | Duplication (Adams 1994c) |
| Adams 1994b | Duplication (Adams 1994c) |
| Adams 1997b | Duplication (Adams 1994c) |
| Kapoor 2005 | Unable to extract enough data from publication. No reply from authors. |
| Kelly 1999 | Duplication (Finlay 1999) |
| Li 1999 | No survival data (Duplication with Lin 2004). |
| Lin 2004 | No survival data. |
| Matsumoto 1995 | Duplication (Matsumoto 2002) |
| Moesgaard 1998 | No survival data. (Duplication Nielsen 2002) |
| Nielsen 1998 | Duplication (Nielsen 2002) |
| Tavani 1998 | Retrospective case control study on the effect of cimetidine on the incidence (not outcome) of colorectal cancer. |
| Umemoto 1996 | Duplication (Matsumoto 2002) |
Differences between protocol and review
No PFS/toxicity data.
Contributions of authors
Dr Sanjeev Deva: Protocol development. Data collection and data management. Analysis and interpretation of data. Writing the review.
Dr Michael Jameson: Conceiving and designing the review. Data collection. Analysis and interpretation of data. Writing and providing advice on the review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
No declarations of interest.
New
References
References to studies included in this review
Adams 1994c {published data only}
- Adams WJ, Morris DL. Short‐course cimetidine and survival with colorectal cancer. Lancet 1994;344:1768. [PubMed] [Google Scholar]
Finlay 1999 {published data only}
- Finlay IL, Dwerryhouse SJ, King J, King DW, Lubowski DZ, Morris DL. The effect of a short course of preoperative cimetidine in the grade of TIL in primary colorectal cancer ‐ a randomised controlled clinical trial.. GI cancer 1999;3(2):121‐7. [Google Scholar]
Huang 2007 {published data only}
- Huang WH, et al. Cimetidine as adjuvant treatment in rectal cancer. Chinese journal of cancer prevention and treatment 2007;14(8):623‐4. [Google Scholar]
Matsumoto 2002 {published data only}
- Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H, Okamoto T. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis‐X and sialyl Lewis‐A epitope expression on tumour cells.. British Journal of Cancer 2002;86(2):161‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2002 {published data only}
- Nielsen HJ, Christensen IJ, Moesgaard F, Kehlet H. Ranitidine as adjuvant treatment in colorectal cancer. British Journal of Surgery 2002;89(11):1416‐22. [DOI] [PubMed] [Google Scholar]
Svendsen 1995 {published data only}
- Svendsen LB, Ross C, Knigge U, Frederiksen HJ, Graversen P, Kjaergård J, et al. Cimetidine as an Adjuvant Treatment in Colorectal Cancer. A double‐blind, randomized pilot study. Diseases of the colon and rectum 1995;38(5):514‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 1994a {published data only}
- Adams WJ, Morris DL, Ross WB, Lubowski DZ, King DW, Peters L. Cimetidine preserves non‐specific immune function after colonic resection for cancer. Aust NZ J Surg 1994;64(12):847‐52. [DOI] [PubMed] [Google Scholar]
Adams 1994b {published data only}
- Adams WJ, Lawson JA, Morris DL. Cimetidine inhibits in vivo growth of human colon cancer and reverses histamine stimulated in vitro and in vivo growth. Gut 1994;35(11):1632‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 1997b {published data only}
- Adams WJ, Morris DL. Pilot study‐‐cimetidine enhances lymphocyte infiltration of human colorectal carcinoma: results of a small randomized control trial. Cancer 1997;80(1):15‐21. [DOI] [PubMed] [Google Scholar]
Kapoor 2005 {published data only}
- Kapoor S, Pal S, Sahni P, Dattagupta S, Kanti Chattopadhyay T. Effect of Pre‐Operative Short Course Famotidine on Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Double Blind, Placebo Controlled, Prospective Randomized Study. Journal of Surgical Research 2005;129(2):172‐5. [DOI] [PubMed] [Google Scholar]
Kelly 1999 {published data only}
- Kelly MD, King J, Cherian M, Dwerryhouse SJ, Finlay IG, Adams WJ, et al. Randomized Trial of Preoperative Cimetidine in Patients with Colorectal Carcinoma with Quantitative Assessment of Tumor‐Associated Lymphocytes. Cancer 1999;85(8):1658‐63. [DOI] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li Y, Bai DJ, Wang K, Yang GL, Yuan HY, Shao H. Effects of perioperative cimetidine administration on natural killer cells in patients with gastrointestinal cancer. Chinese Journal of Cancer Research 1999;11(1):70‐3.. [Google Scholar]
Lin 2004 {published data only}
- Lin CY, Bai DJ, Yuan HY, Wang K, Yang GL, Hu MB, et al. Perioperative cimetidine administration promotes peripheral blood lymphocytes and tumor infiltrating lymphocytes in patients with gastrointestinal cancer: Results of a randomized controlled clinical trial.. World Journal of Gastroenterology 2004;10(1):136‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsumoto 1995 {published data only}
- Matsumoto S. Cimetidine and survival with colorectal cancer. Lancet 1995;346(8967):115. [DOI] [PubMed] [Google Scholar]
Moesgaard 1998 {published data only}
- Moesgaard F, Jensen LS, Christiansen PM, Thorlacius‐Ussing O, Nielsen KT, Rasmussen NR, et al. The effect of ranitidine on postoperative infectious complications following emergency colorectal surgery: a randomized, placebo‐controlled, double‐blind trial. Inflammation research : Official journal of the European Histamine Research Society 1998;47(1):12‐7. [DOI] [PubMed] [Google Scholar]
Nielsen 1998 {published data only}
- Nielsen HJ, McArdle CS, Moesgaard F, Schulze S, et al. The effect of ranitidine on long‐term survival in primary colorectal cancer. A 40 months interim analysis. GI cancer 1998;2(3):227‐33. [Google Scholar]
Tavani 1998 {published data only}
- Tavani A, Fioretti F, Franceschi S, Vecchia C, Morris D.L, Adams W.J. Pilot study ‐ Cimetidine enhances lymphocyte infiltration of human colorectal carcinoma: Results of a small randomized control trial. Cancer 1998;82(11):2296‐7. [DOI] [PubMed] [Google Scholar]
Umemoto 1996 {published data only}
- Umemoto S. The effect of cimetidine as BRM ‐ Adjuvant immuno‐chemotherapy for colorectal carcinoma. Biotherapy 1996;10(3):511‐5. [Google Scholar]
Additional references
Abrams 2011
- Abrams TA, Brightly R, Mao J, Kirkner G, Meyerhardt JA, Schrag D, et al. Patterns of Adjuvant Chemotherapy Use in a Population‐Based Cohort of Patients With Resected Stage II or III Colon Cancer.. Journal of Clinical Oncology 2011;29(24):3255‐62. [DOI] [PubMed] [Google Scholar]
Adams 1997a
- Adams WJ, Morris DL. Pilot study‐‐cimetidine enhances lymphocyte infiltration of human colorectal carcinoma: results of a small randomized controlled trial. Cancer 1997;80(1):15‐21. [DOI] [PubMed] [Google Scholar]
Armitage 1979
- Armitage JO, Sidner RD. Antitumour effect of cimetidine. Lancet 1979;8121(1):882‐3. [DOI] [PubMed] [Google Scholar]
Eaton 2002
- Eaton D, Hawkins RE. Cimetidine in colorectal cancer ‐‐ are the effects immunological or adhesion‐mediated?. British Journal of Cancer 2002;86(2):159‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flodgren 1985
- Flodgren P, Sjogren HO. Influence in vitro on NK and K cell activities by cimetidine and indomethacin with and without simultaneous exposure to interferon. Cancer Immunol Immunother 1985;19(1):28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerard 2006
- Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon‐Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‐4 rectal cancers: results of FFCD 9203.. Journal of Clinical Oncology 2006;24(28):4620‐5. [DOI] [PubMed] [Google Scholar]
Hansbrough 1986
- Hansbrough JF, Zapata‐Sirvent RL, Bender EM. Prevention of alterations in postoperative lymphocyte subpopulations by cimetidine and ibuprofen. Am J Surg 1986;151(2):249‐55. [DOI] [PubMed] [Google Scholar]
Harland 1989
- Harland CC, Saihan EM. Regression of cutaneous metastatic malignant melanoma with topical diphencyprone and oral cimetidine. Lancet 1989;8660(2):445. [DOI] [PubMed] [Google Scholar]
Harvey 1984
- Harvey VJ, Slevin ML, Dilloway MR, Clark PI, Johnston A, Lant AF. The influence of cimetidine on the pharmacokinetics of 5‐fluorouracil. British Journal of Clinical Pharmacology 1984;18(3):421‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Hsiao 2011
- Hsiao FY, Mullins CD, Onukwugha E, Pandya N, Hanna N. Comparative Effectiveness of Different Chemotherapeutic Regimens on Survival of People Aged 66 and Older with Stage III Colon Cancer: A "Real World" Analysis Using Surveillance, Epidemiology, and End Results‐Medicare Data. Journal of the American Geriatrics Society 2011; (Electronic ahead of publication).;59(9):1717‐23. [DOI] [PubMed] [Google Scholar]
Imdahl 1995
- Imdahl A, Mantamadiotis T, Eggstein S, Farthmann EH, Baldwin GS. Expression of gastrin, gastrin/CCK‐B‐ and gastrin/CCK‐C receptors in human colorectal carcinomas.. Journal Cancer Res Clin Oncol 1995;121(11):661‐6. [DOI] [PubMed] [Google Scholar]
Jensen 1996
- Jensen LS, Kissmeyer‐Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte‐depleted versus buffy‐coat‐poor blood transfusion and complications after colorectal surgery.. Lancet 1996;348(9031):841‐5. [DOI] [PubMed] [Google Scholar]
Kannagi 2004
- Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate‐mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 2004;95(5):377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katoh 1996
- Katoh J, Tsuchiya K, Sato W, Nakajima M, Iida Y. Cimetidine and immunoreactivity. Lancet 1996;9024(348):404‐5. [DOI] [PubMed] [Google Scholar]
Kobayashi 2000
- Kobayashi K, Matsumoto S, Morishima T, Kawabe T, Okamoto T. Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E‐selectin expression. Cancer Research 2000;60(14):3978‐84. [PubMed] [Google Scholar]
Koh 1999
- Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, et al. Overexpression of glycine‐extended gastrin in transgenic mice results in increased colonic proliferation.. Jornal of Clinical Investigations 1999;103:1119‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kojima 2002
- Kojima Y, Kondo T, Zhao QL, Shoji M, Futatsuya R. Protective effects of cimetidine on radiation‐induced micronuclei and apoptosis in human peripheral blood lymphocytes.. Free Radical Research 2002;36(3):255‐63. [DOI] [PubMed] [Google Scholar]
Kubota 2002
- Kubota T, Fujiwara H, Ueda Y, Itoh T, Yamashita T, Yoshimura T, et al. Cimetidine modulates the antigen presenting capacity of dendritic cells from colorectal cancer patients. British Journal of Cancer 2002;86(8):1257‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumar 1990
- Kumar A. Cimetidine: an immunomodulator. DICP; the annals of pharmacotherapy 1990;24(3):289‐295. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies.. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Melmon 1972
- Melmon KL, Bourne HR, Weinstein J, Sela M. Receptors for histamine can be detected on the surface of selected leukocytes. Science 1972;177(50):707‐9. [DOI] [PubMed] [Google Scholar]
Mozdarani 2003
- Mozdarani H. Radioprotective properties of histamine H2 receptor antagonists: present and future prospects.. Journal of Radiation Research (Tokyo) 2003;44(2):145‐9. [DOI] [PubMed] [Google Scholar]
Nanda 1985
- Nanda NK, Nath I. Characteristics of histamine receptors present on suppressor T cells in "healthy individuals". International Journal of Immunopharmacology 1985;7(4):587‐95. [DOI] [PubMed] [Google Scholar]
Nielsen 1999
- Nielsen HJ, McArdle CS, Moesgaard F. The effect of ranitidine on postoperative infectious complications after elective colorectal surgery.. GI Cancer 1999;3(2):87‐95. [Google Scholar]
Ohsawa 2002
- Ohsawa T, Hirata W, Higichi S. Effects of three H2‐receptor antagonists (cimetidine, famotidine, ranitidine) on serum gastrin level. International Journal of Clinical Pharamacological Research 2002;22(2):29‐35. [PubMed] [Google Scholar]
Orbuch 1996
- Orbuch M, Venzon DJ, Lubensky IA, Weber HC, Gibril F, Jensen RT. Prolonged hypergastrinemia does not increase the frequencyof colonic neoplasia in patients with Zollinger‐Ellison syndrome.. Digestive diseases and sciences. 1996;41:604‐13. [DOI] [PubMed] [Google Scholar]
Richter 1989
- Richter JM, Colditz GA, Huse DM, Delea TE, Oster G. Cimetidine and adverse reactions: a meta‐analysis of randomized clinical trials of short‐term therapy.. American Journal of Medicine 1989;87(3):278‐84. [DOI] [PubMed] [Google Scholar]
Robertson 2007
- Robertson DJ, Larsson H, Friis S, Pedersen L, Baron JA, Sorensen HT. Proton Pump Inhibitor Use and Risk of Colorectal Cancer: A Population‐Based, Case–Control Study.. Gastrenterology 2007;133:755‐60. [DOI] [PubMed] [Google Scholar]
Taylor 1988
- Taylor TV, Boom SJ, Blower AL, McMahon RF, Lawler W. Healing of a malignant gastric ulcer with cimetidine. J R Coll Surg Edinb 1988;33(6):339‐340. [PubMed] [Google Scholar]
Tozeren 1995
- Tozeren A, Kleinman HK, Grant DS, Morales D, Mercurio AM, Byers SW. E‐selectin‐mediated dynamic interactions of breast‐ and colon‐cancer cells with endothelial‐cell monolayers. International Journal of Cancer 1995;60(3):426‐31. [DOI] [PubMed] [Google Scholar]
Trudeau 1970
- Trudeau WL, McGuigan JE. Serum gastrin levels in patientswith peptic ulcer disease. Gastroenterology 1970;59:6‐12. [PubMed] [Google Scholar]
Zhang 2006
- Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, et al. Organic cation transporters are determinants of oxaliplatin cytotoxicity.. Cancer Research 2006;66(17):8847‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]


