ABSTRACT
The performance of many known fluorescent sensors for Ca2+ is pH‐dependent. We set out to address this by preparing a novel calcium sensor based on diacylated 1,10‐diaza‐18‐crown‐6 and containing no basic centres. The sensor is sensitive to sub‐millimolar levels of Ca2+ and partially tolerant of an aqueous environment. However, it suffers from a lack of selectivity over some other divalent cations, and retained pH sensitivity in the acidic range. The relations between these properties and the sensor's structure are discussed.
Abbreviations
- HEPES
(4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid)
- ICT
intramolecular charge transfer
- MeCN
acetonitrile
- MeOH
methanol
- PET
photo‐induced electron transfer
1. Introduction
While calcium indicator Fura‐2 and its intellectual successors remain invaluable tools for intracellular calcium imaging [1, 2] their reliance on ionised carboxylates and basic nitrogens for metal ion coordination renders their use in low pH environments problematic. Crown ethers represent a potential way of avoiding this problem by offering a cation‐binding site which can be polar but uncharged across the pH range. Several N‐aryl aza‐crown‐based sensors have been reported on the principle that Ca2+ complexation by the crown disrupts intramolecular charge transfer (ICT) from the crown nitrogen to a conjugated fluorophore thereby altering fluorescence. It appears, however, that we lack a fundamental understanding of such molecules as there are examples where fluorescence decreases [3, 4] as well as those where it increases, eg [5]. More relevant to this discussion are the repeated reports [6, 7, 8] that the emission from such sensors is affected by H+ rendering them pH‐sensitive (cf [9] where fluorescence from a structurally similar sensor increases below pH 5). Photo‐induced electron transfer (PET) is a complementary phenomenon to ICT as electron transfer occurs through space to quench the excited state of a fluorophore. Multiple examples exist of the exploitation of this process as the basis of sensors for metal ions which feature crown ethers as the metal binding unit (reviewed [10]). Co‐ordination of metal ions by the crown reduces the availability of lone pairs and blocks the PET process resulting in an increase in fluorescence. Outstanding applications of this idea to fluorescent calcium sensors are molecules whose emission increases by over 100x on the addition of Ca2+ [11, 12, 13], though all three sensors feature N‐aryl aza‐crowns so it is likely that response is pH‐dependent (this was shown in one case [12]). Another of these studies [13] found that the addition of even small amounts of water significantly reduced the fluorescent response, a result echoed by studies on a related polyether Ca2+ sensor [14]. More generally the interactions of crown ethers with associated waters form an ongoing area of research [15, 16, 17] into the subtleties of this class of molecules which are still incompletely understood almost 60 years after their discovery.
Multiple studies in the application of crown ethers to calcium‐sensing have demonstrated the coordination of carbonyl oxygen to the metal ion [18, 19, 20, 21, 22, 23, 24] which offers the possibility of designing a metal binding site with 3‐dimensional character (for early studies see [25]). This may explain the effectiveness of the work from Koji Suzuki's lab [26] which reported electrochemical sensors based on diacylated diaza‐crowns, eg 1 (Figure 1), embedded in a polymer membrane (compare [27]). The sensors displayed remarkable sensitivity for Ca2+ (detection of 5 × 10−5 M) and selectivity (up to 105) over related ions. A more recent density functional theory study [28] has supported the idea that such compounds would use both carbonyl oxygens to coordinate the metal ion. We reasoned that a diacylated diaza‐crown would serve as an alternative template for a fluorescent sensor—the ion binding properties would be retained but incorporated fluorophores would give a fluorescent response on calcium binding via blocking PET. The large Stokes’ shift and easy synthetic modification of the naphthalimide moiety have led to its widespread use in fluorescent sensors (reviewed [29]) thus we adopted that as our fluorophore and proposed structure 5 as a fluorescent calcium sensor. We report here our preparation of this molecule and our initial explorations of its properties.
FIGURE 1.
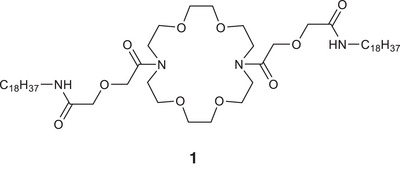
Diacylated crown ether previously used as the basis for an electrochemical calcium sensor.
2. Results and Discussion
Sensor 5 was prepared as shown in Scheme 1. Known [30] anhydride 2 was treated with an excess of methylamine to yield naphthalimide 3. The linker was then installed by using the amino group to perform a ring‐opening reaction on diglycolic anhydride to yield acid 4. Coupling with 1,10‐diaza‐18‐crown‐6 under previously described conditions [26] yielded our desired sensor 5. The photophysical characteristics (λex, λem, ε) of 3 and 4 were broadly in line with previously disclosed analogues [31, 32, 33] but the extinction coefficient of 5 (2300 M−1 cm−1, Figure S1) was rather lower than that of previous compounds [31, 33] and 4. Absorption and emission wavelengths of 5 (λex = 351 nm, λem = 462 nm) were very similar to those previously published for 4‐amidonaphthalimides [31, 32, 33]. We speculate that this drop in ε may be due to a ground‐state interaction between the bis‐amido crown and the naphthalimide.
SCHEME 1.
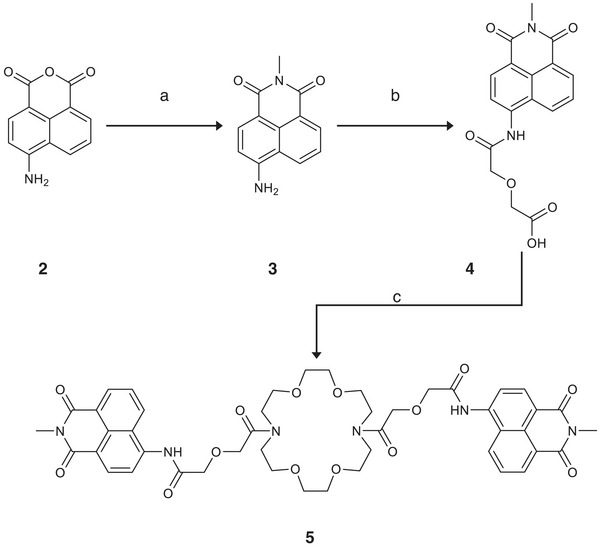
Synthesis of sensor 5 reagents and conditions: (a) MeNH2, EtOH/H2O, reflux, 24 h, quantitative; (b) diglyocolic anhydride, py, reflux, 7 days, 41%; (c) 1,10‐diaza‐18‐crown‐6, N,N‐bis(2‐oxo‐3‐oxazolidinyl)]phosphinic chloride Et3N, DCM, 20%.
While the fluorescence spectra of 5 in acetonitrile (MeCN) and tetrahydrofuran gave single peaks the spectrum in methanol (MeOH) had an additional peak at a longer wavelength (Figure 2A) which we assigned to excimer emission. We initially attributed this to the polarity of the medium [34, 35] but experiments with mixtures of MeCN and (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid) (HEPES) buffer (10 mM, pH 7.4) problematised this. Excimer emission (575 nm) was pronounced with 20% HEPES but monomer emission (462 nm) then increased relative to excimer intensity (Figure 2B) in a linear fashion (Figure S2A) with higher proportions. Absorption was unaffected by variations in the proportion of HEPES (Figures 2B, while excitation spectra for monomer and excimer were almost identical as expected (Figure S2C). Although we do not yet understand why excimer should appear at a low proportion of HEPES and then disappear again at higher proportions it is noteworthy that exactly similar results were obtained when using phosphate‐buffered saline as a buffer in place of HEPES (Figure S2D,E) and that other such sharp discontinuities in fluorescence of naphthalimides in partially aqueous media have been observed previously [32, 36]. Tentatively we attribute the appearance of the excimer peak to an aggregation effect (see [37] for a previous example of this with a naphthalimide) as monomer intensity increases relative to excimer intensity at lower concentrations in both 20% HEPES/MeCN and in MeOH (Figure S2F,G).
FIGURE 2.
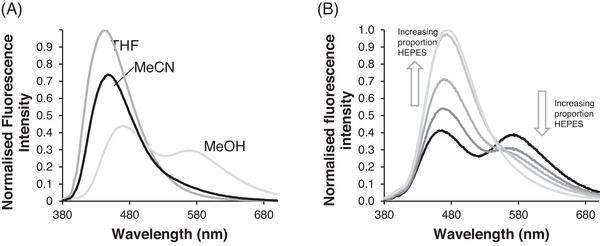
Excimer properties of sensor 5: (A) Emission spectrum in various solvents. (B) Increasing the proportion of (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid) (HEPES) (10 mM, pH 7.4) increases emission from monomer and decreases emission from excimer. For all experiments [5] = 10 µM, λex = 355 nm.
We proceeded to examine the effects of Ca2+ on emission from sensor 5. In 20% HEPES (10 mM, pH 7.4)/MeCN, we were pleased to find that monomer emission increased gradually to a maximum of ca. 2.7‐fold higher than the sensor alone (Figure 3A). We attribute this change to Ca2+ binding inside the crown ether ring blocking PET fluorescence quenching and so giving emission enhancement. This interpretation is supported by the observation of identical fluorescence enhancements with three different calcium salts (Figure S3A) cf [38, 39]. However, we also observed small absorbance changes on adding Ca2+ (Figure S3B,C) which may imply a Ca2+‐induced disruption of the postulated ground‐state interaction between bis‐amido crown ether and napthalimide and may in turn suggest that our sensor does not work by a pure PET mechanism. [40] We also observed a decline in excimer emission (Figure 3A) which followed a biphasic profile (Figure 3C). We hypothesise that this reveals two binding events. The first is the binding of Ca2+ with high affinity inside the crown giving a cationic complex, thus reducing aggregation and thereby excimer emission. The second, low affinity, binding event of Ca2+ is likely to the naphthalimide or the linker. This might also explain the gradual blue shift of the monomer peak (Figure 3A). Consistent with this interpretation we were able to fit the data (Figure 3B) to a 1:2 binding model using BindFit (http://supramolecular.org) [40] and obtained log K 11 = 3.4, log K 12 = 1.5. The equivalent experiment in 40% HEPES (10 mM, pH 7.4) / MeCN gave a smaller fluorescence enhancement (Figure S4A) resulting from less potent binding (log K 11 = 2.8, log K 12 = 1.7; Figure S4B). Further increases in the proportion of aqueous components gave even lower emission enhancements (Figure S4C). A higher proportion of aqueous components renders less favourable the necessary dehydration of both Ca2+ and crown ether before ion binding can occur, which we hypothesise can rationalise these observations. Whilst this performance is short of optimal the ability of our sensor to sense calcium at all in a 40% aqueous system marks it out as significantly more water tolerant than any other fluorescent Ca2+ sensor using unionised groups.
FIGURE 3.
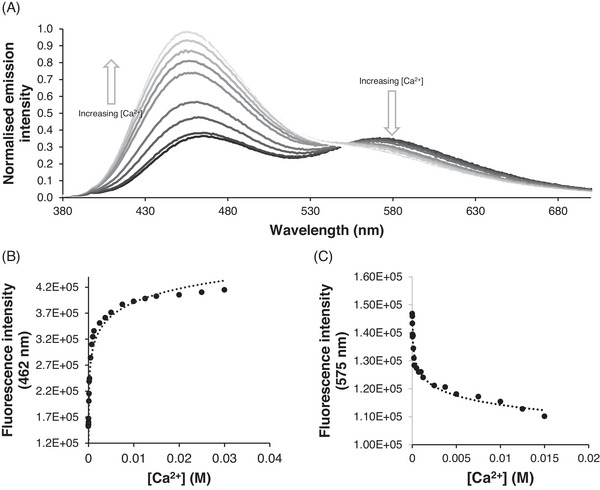
Fluorescence changes on the addition of Ca2+ to sensor 5: (A) Addition of increasing concentrations of Ca2+ leads to an increase in monomer and a decrease of excimer. (B) Titration experiment at monomer wavelength. (C) Titration experiment at excimer wavelength. All experiments were conducted in 20% (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid) (HEPES)/acetonitrile (MeCN), [5] = 10 µM, λex = 355 nm. Curves are simple logarithmic fits.
We next asked if emission from the sensor was pH‐independent, both alone and in the presence of Ca2+. Although fluorescence did not vary with pH in the range of 6.5–9.5 emission from the sensor alone increased significantly at more acidic values and the addition of Ca2+ did not further enhance it (Figure 4A). We postulate that this arises not from protonation of our sensor per se but from its ability to form H‐bonds with H3O+, which would also explain why our sensor shows reduced responses in more aqueous media (Figure S4). To our knowledge H‐bonding of H3O+ has not been systematically studied with aza‐crowns but has been extensively investigated with standard crown ethers [41, 42]. The use of lone pairs on the ring oxygens in this manner reduces the PET quench of naphthalimide emission thereby rendering 5 insensitive to calcium ions. To investigate the selectivity of our sensor we added 1 mM metal perchlorate salts to 10 µM sensor in 20% HEPES/MeCN. We were pleased to discover that our sensor did not respond at all to monovalent cations (Figure 4B and Figure S5A) which we attribute to the preference of relatively hard amide donors for ions with higher charge densities. We also observed virtually no change in emission of 10 µM sensor even with 100 mM Na+ or K+ (Figure S5B). 5 showed only limited selectivity for Ca2+ over other group II ions (Figure 4B and Figure S5C) and over other dications in general (Figure 4B and Figure S5D). In particular, our sensor gave an emission change with Zn2+ comparable to that observed with Ca2+ (Figure 4B and Figures S5D and S6A). This is likely driven by similar binding constants (log K 11 = 4.0, log K 12 = 1.9; Figure S6B), though changes in the form of both the absorbance and emission spectra (Figure S6A,C) suggest that this may derive at least partially from direct interaction between Zn2+ and the napthalimide. The general lack of selectivity towards other divalent cations likely arises from the relative flexibility of our molecule and in particular the ability of the carbonyls to adopt a range of O … M2+ distances. The exceptions to this pattern were Cu2+ and Fe2+ which caused appreciable fluorescence quenching (Figure 4B and Figure S5E) presumably through a paramagnetic mechanism (cf [32]).
FIGURE 4.
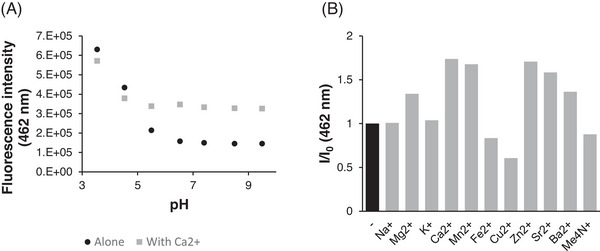
Effect of other species on emission from sensor 5: (A) Emission from 5 (10 µM) is invariant (both alone and in the presence of 5 mM Ca2+) above pH 6.5 but increases at more acidic values. (B) Emission enhancement from 5 in the presence of 1 mM various cations. (Cations were added as perchlorate salts as solutions in acetonitrile (MeCN) with the exception of Me4NClO4 which was added as a solution in dimethyl sulfoxide (DMSO). All experiments were conducted in 20% (4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid) (HEPES) (10 mM, pH 7.4)/MeCN, [5] = 10 µM, λex = 355 nm.
3. Conclusion
In summary, we have presented an example of an alternative template for the design of fluorescent sensors of Ca2+. This compound shows interesting aggregation‐driven behaviour in partially aqueous media, excellent selectivity over monovalent cations and a greater water tolerance than previously disclosed related sensors. Future work will focus on improving selectivity over divalent cations and making a sensor that is functional in aqueous environments for potential biological applications. These investigations are underway in our laboratory and will be reported in due course.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting Information
Acknowledgements
We thank De Montfort University (Frontrunner studentship to O.G.S.), the Royal Society of Chemistry (Undergraduate Research Bursary (U24‐3976923672) to J.C.A.) and Fisher Scientific (New Lab Start‐Up scheme to S.W.) for financial support. We are grateful to Calum Greenhalgh (Loughborough University) for assistance with HRMS. SDG.
Funding: This research was supported by the De Montfort University and Royal Society of Chemistry.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Lock J. T., Parker I., and Smith I. F., “A Comparison of Fluorescent Ca2+ Indicators for Imaging Local Ca2+ Signals in Cultured Cells,” Cell Calcium 58 (2015): 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roopa, Kumar N., Kumar M., and Bhalla V., “Design and Applications of Small Molecular Probes for Calcium Detection,” Chemistry ‐ An Asian Journal 14 (2019): 4493–4505. [DOI] [PubMed] [Google Scholar]
- 3. Bourson J. and Valeur B., “Ion‐Responsive Fluorescent Compounds. 2. Cation‐Steered Intramolecular Charge Transfer in a Crowned Merocyanine,” Journal of Physical Chemistry 93 (1989): 3871–3876. [Google Scholar]
- 4. Kim H. M., Jeong M. Y., Ahn H. C., Jeon S. J., and Cho B. R., “Two‐Photon Sensor for Metal Ions Derived From Azacrown Ether,” Journal of Organic Chemistry 69 (2004): 5749–5751. [DOI] [PubMed] [Google Scholar]
- 5. Fery‐Forgues S., Le Bris M. T., Guetté J. P., and Valeur B., “Ion‐Responsive Fluorescent Compounds. 1. Effect of Cation Binding on Photophysical Properties of a Benzoxazinone Derivative Linked to monoaza‐15‐Crown‐5,” Journal of Physical Chemistry 92 (1988): 6233–6237. [Google Scholar]
- 6. Mateeva N., Enchev V., Antonov L., Deligeorgiev T., and Mitewa M., “Spectroscopic Study of the Complexation of an Aza‐15‐Crown‐5 Containing Chromofluoroionophore With Ba2+ and Ca2+ Cations,” Journal of Inclusion Phenomena and Molecular Recognition in Chemistry 20 (1994): 323–333. [Google Scholar]
- 7. Kollmannsberger M., Rurack K., Resch‐Genger U., and Daub J., “Ultrafast Charge Transfer in Amino‐Substituted Boron Dipyrromethene Dyes and Its Inhibition by Cation Complexation: A New Design Concept for Highly Sensitive Fluorescent Probes,” Journal of Physical Chemistry A 102 (1998): 10211–10220. [Google Scholar]
- 8. Panchenko P. A., Fedorov Y. V., Fedorova O. A., and Jonusauskas G., “Comparative Analysis of the PET and ICT Sensor Properties of 1,8‐Naphthalimides Containing Aza‐15‐Crown‐5 Ether Moiety,” Dyes and Pigments 98 (2013): 347–357. [Google Scholar]
- 9. Sprenger T., Schwarze T., Müller H., et al., “BODIPY‐Equipped Benzo‐Crown‐Ethers as Fluorescent Sensors for pH Independent Detection of Sodium and Potassium Ions,” ChemPhotoChem 7 (2022): e202200270. [Google Scholar]
- 10. Li J., Yim D., Jang W. D., and Yoon J., “Recent Progress in the Design and Applications of Fluorescence Probes Containing Crown Ethers,” Chemical Society Reviews 46 (2017): 2437–2458. [DOI] [PubMed] [Google Scholar]
- 11. Pearson A. J. and Xiao W., “Fluorescence and NMR Binding Studies of N‐aryl‐N′‐(9‐methylanthryl)diaza‐18‐Crown‐6 Derivatives,” Journal of Organic Chemistry 68 (2003): 5369–5376. [DOI] [PubMed] [Google Scholar]
- 12. Qin W., Baruah M., Sliwa M., et al., “Ratiometric, Fluorescent BODIPY Dye With Aza Crown Ether Functionality: Synthesis, Solvatochromism, and Metal Ion Complex Formation,” Journal of Physical Chemistry A 112 (2008): 6104–6114. [DOI] [PubMed] [Google Scholar]
- 13. Morgan M. T., Sumalekshmy S., Sarwar M., Beck H., Crooke S., and Fahrni C. J., “Probing Ternary Complex Equilibria of Crown Ether Ligands by Time‐Resolved Fluorescence Spectroscopy,” Journal of Physical Chemistry B 118 (2014): 14196–14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashokkumar P., Ramakrishnan V. T., and Ramamurthy P., “Specific Ca2+ Fluorescent Sensor: Signaling by Conformationally Induced PET Suppression in a Bichromophoric Acridinedione,” European Journal of Organic Chemistry 2009 (2009): 5941–5947. [Google Scholar]
- 15. Jóźwiak M., Trzmielak M. A., Wasiak M., and Łudzik‐Dychto K., “Composition of the Solvation Shell of the Selected Cyclic Ethers (1,4‐Dioxane, 12‐Crown‐4, 15‐Crown‐5 and 18‐Crown‐6) in a Mixture of Formamide With Water at Four Temperatures,” Molecules 28 (2023): 2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pan X., Wang Q., Ma Z., et al., “Assisting Role of Water Molecules in Ionic Recognition by 18‐Crown‐6 Ether in Aqueous Solutions,” Journal of Molecular Liquids 371 (2023): 121127. [Google Scholar]
- 17. Qi Z., Qin Y., Wang J., et al., “The Aqueous Supramolecular Chemistry of Crown Ethers,” Frontiers in Chemistry 11 (2023): 1119240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morozumi T., Anada T., and Nakamura H., “New Fluorescent “off‐on” Behavior of 9‐anthryl Aromatic Amides Through Controlling the Twisted Intramolecular Charge Transfer Relaxation Process by Complexation With Metal Ions,” Journal of Physical Chemistry B 105 (2001): 2923–2931. [Google Scholar]
- 19. Kim J., Oka Y., Morozumi T., Choi E. W., and Nakamura H., “Steric Effects on Controlling of Photoinduced Electron Transfer Action of Anthracene Modified Benzo‐15‐Crown‐5 by Complexation With Mg2+ and Ca2+ ,” Tetrahedron 67 (2011): 4814–4819. [Google Scholar]
- 20. Aoki Y., Umezawa N., Asano Y., et al., “A Versatile Strategy for the Synthesis of Crown Ether‐Bearing Heterocycles: Discovery of Calcium‐Selective Fluoroionophore,” Bioorganic and Medicinal Chemistry 15 (2007): 7108–7115. [DOI] [PubMed] [Google Scholar]
- 21. Cox R. P., Sandanayake S., Scarborough D. L. A., Izgorodina E. I., Langford S. J., and Bell T. D. M., “Investigation of Cation Binding and Sensing by New Crown Ether Core Substituted Naphthalene Diimide Systems,” New Journal of Chemistry 43 (2019): 2011–2018. [Google Scholar]
- 22. Taziaux D., Soumillion J. P., and Habib Jiwan J. L., “Photophysical and Complexing Properties of New Fluoroionophores Based on Coumarin 343 Linked to Rigidified Crown‐Ethers,” Journal of Photochemistry and Photobiology A: Chemistry 162 (2004): 599–607. [Google Scholar]
- 23. Maton L., Taziaux D., Soumillion J. P., and Habib Jiwan J. L., “About the Use of an Amide Group as a Linker in Fluoroionophores: Competition Between Linker and Ionophore Acting as Chelating Groups,” Journal of Materials Chemistry 15 (2005): 2928–2937. [Google Scholar]
- 24. Gomes L. J., Outis M., Gomes C. S. B., Tomé A. C., and Moro A. J., “Development of Fluorescent Chemosensors for Calcium and Lead Detection,” Molecules 29 (2024): 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gatto V. J. and Gokel G. W., “Syntheses of Calcium‐Selective, Substituted Diaza‐Crown Ethers: A Novel, One‐Step Formation of Bibracchial Lariat Ethers (BiBLEs),” Journal of the American Chemical Society 106 (1984): 8240–8244. [Google Scholar]
- 26. Suzuki K., Watanabe K., Matsumoto Y., et al., “Design and Synthesis of Calcium and Magnesium Ionophores Based on Double‐Armed Diazacrown Ether Compounds and Their Application to an Ion Sensing Component for an Ion‐Selective Electrode,” Analytical Chemistry 67 (1995): 324–334. [Google Scholar]
- 27. Capel‐Cuevas S., de Orbe‐Payá I., Santoyo‐González F., and Capitán‐Vallvey L. F., “Double‐Armed Crown Ethers for Calcium Optical Sensors,” Talanta 78 (2009): 1484–1488. [DOI] [PubMed] [Google Scholar]
- 28. Basok S. S., Schepetkin I. A., Khlebnikov A. I., et al., “Synthesis, Biological Evaluation, and Molecular Modeling of Aza‐Crown Ethers,” Molecules 26 (2021): 2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong H. Q., Wei T. B., Ma X. Q., et al., “1,8‐Naphthalimide‐Based Fluorescent Chemosensors: Recent Advances and Perspectives,” Journal of Materials Chemistry C 8 (2020): 13501–13529. [Google Scholar]
- 30. Hoang M. D., Bodin J. B., Savina F., et al., “CinNapht Dyes: A New Cinnoline/Naphthalimide Fused Hybrid Fluorophore. Synthesis, Photo‐Physical Study and Use for Bio‐Imaging,” RSC Advances 11 (2021): 30088–30092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alexiou M. S., Tychopoulos V., Ghorbanian S., Tyman J. H. P., Brown R. G., and Brittain P. I., “The UV–Visible Absorption and Fluorescence of Some Substituted 1,8‐Naphthalimides and Naphthalic Anhydrides,” Journal of the Chemical Society, Perkin Transactions 2 (1990): 837–842. [Google Scholar]
- 32. Xu Z., Yoon J., and Spring D. R., “A Selective and Ratiometric Cu2+ Fluorescent Probe Based on Naphthalimide Excimer–Monomer Switching,” Chemical Communications 46 (2010): 2563. [DOI] [PubMed] [Google Scholar]
- 33. Martin E., Weigand R., and Pardo A., “Solvent Dependence of the Inhibition of Intramolecular Charge‐Transfer in N‐Substituted 1,8‐Naphthalimide Derivatives as Dye Lasers,” Journal of Luminescence 68 (1996): 157–164. [Google Scholar]
- 34. Karaman M., Demir N., Bilgili H., et al., “Synthesis, Characterization, Aggregation‐Induced Enhanced Emission and Solvatochromic Behavior of Dimethyl 4′‐(diphenylamino)biphenyl‐3,5‐Dicarboxylate: Experimental and Theoretical Studies,” New Journal of Chemistry 44 (2020): 11498–11506. [Google Scholar]
- 35. Cesaretti A., Bianconi T., Coccimiglio M., et al., “Aggregation‐Induced Emission in Phenothiazine‐Based Fluorophores: An Insight Into the Excited State and Aggregate Formation Mechanism,” Journal of Physical Chemistry C 126 (2022): 10429–10440. [Google Scholar]
- 36. Du X., Zhang Y., and Xu D., “A 1,8‐Naphthimide‐Based Fluorescent Probe for Analyzing DMF/H2O Composition,” Journal of Fluorescence 34 (2023): 169–178. [DOI] [PubMed] [Google Scholar]
- 37. Patel D. A., Anand T., Selvam P., and Sahoo S. K., “Aggregation‐Induced Emission Active Naphthalimide Derived Schiff Base for Detecting Cu2+ and Its Applications,” Journal of Fluorescence 34 (2023): 359–366. [DOI] [PubMed] [Google Scholar]
- 38. Junk P. C., Louis L. M., and Smith M. K., “Solid‐State Binding Studies of Group 2 Metal Ions With 12‐Crown‐4,” Zeitschrift Für Anorganische Und Allgemeine Chemie 628 (2002): 1196–1209. [Google Scholar]
- 39. Lee Y., Kim J. R., and Lee E., “A Benzothiazole‐Coupled NS4‐donor Macrocycle and Its Complexation‐Based Dual‐Channel Sensing for Hg2+ : The Influence of Anions and Structure–Function Relationship,” New Journal of Chemistry 47 (2023): 19897–19905. [Google Scholar]
- 40. Brynn Hibbert D. and Thordarson P., “The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and Other Developments in Supramolecular Chemistry Data Analysis,” Chemical Communications 52 (2016): 12792–12805. [DOI] [PubMed] [Google Scholar]
- 41. Junk P. C., “Crown Ethers as Stabilising Ligands for Oxonium Ions,” New Journal of Chemistry 32 (2008): 762–773. [Google Scholar]
- 42. Hurtado P., Gámez F., Hamad S., Martínez‐Haya B., Steill J. D., and Oomens J., “Crown Ether Complexes With H3O+ and NH 4+: Proton Localization and Proton Bridge Formation,” Journal of Physical Chemistry A 115 (2011): 7275–7282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


