Abstract
The visual system compensates for differences between peripheral and foveal vision using different mechanisms. Although peripheral vision is characterized by higher spatial uncertainty and lower resolution than foveal vision, observers reported objects to be less distorted and less blurry in the periphery than the fovea in a visual matching task during fixation (Valsecchi et al., 2018). Here, we asked whether a similar overcompensation could be found across saccadic eye movements and whether it would bias the detection of transsaccadic changes in object regularity. The blur and distortion levels of simple geometric shapes were manipulated in the Eidolons algorithm (Koenderink et al., 2017). In an appearance discrimination task, participants had to judge the appearance of blur (experiment 1) and distortion (experiment 2) separately before and after a saccade. Objects appeared less blurry before a saccade (in the periphery) than after a saccade (in the fovea). No differences were found in the appearance of distortion. In a change discrimination task, participants had to judge if blur (experiment 1) and distortion (experiment 2) either increased or decreased during a saccade. Overall, they showed a tendency to report an increase in both blur and distortion across saccades. The precision of the responses was improved by a 200-ms postsaccadic blank. Results from the change discrimination task of both experiments suggest that a transsaccadic decrease in regularity is more visible, compared to an increase in regularity. In line with the previous study that reported a peripheral overcompensation in the visual matching task, we found a similar mechanism, exhibiting a phenomenological sharpening of blurry edges before a saccade. These results generalize peripheral–foveal differences observed during fixation to the here tested dynamic, transsaccadic conditions where they contribute to biases in transsaccadic change detection.
Keywords: transsaccadic perception, transsaccadic change, peripheral and foveal appearance, object regularity, blur, distortion, blanking
Introduction
Visual processing varies considerably across the visual field. The fovea, characterized by a dense concentration of photoreceptors, is responsible for high-resolution vision. In contrast, peripheral vision offers a broader field of view with lower resolution, contrast sensitivity, and increased spatial distortion (for reviews see Rosenholtz, 2016; Strasburger, Rentschler, & Jüttner, 2011). To create a more coherent perception across the visual field, various mechanisms work to align the visual experiences of the fovea and the periphery (for reviews see Cohen, Dennett, & Kanwisher, 2016; Knotts, Odegaard, Lau, & Rosenthal, 2019; Stewart, Valsecchi, & Schütz, 2020).
One of the outcomes of these alignment mechanisms seems to be a clearer and less blurry peripheral experience during fixation. Galvin and Williams (1992) first found that observers inadvertently failed to discriminate between low-pass filtered and unfiltered edges in the periphery. Interestingly, this confusion was not due to blurring in peripheral processing, but to the fact that both edges were perceived as equally sharp. They investigated this phenomenon further in a subsequent study, showing that edges in the periphery indeed appear sharper than they are, and called it sharpness overconstancy (Galvin, O'Shea, Squire, & Govan, 1997). In addition, they found that the effect was more pronounced at higher eccentricities. They speculated about various explanations, mostly ruling out all low-level explanations and opting for a higher level explanation of a template of sharp edges based on foveal experience. However, in their subsequent study, they could not find any evidence for the flexibility or context dependence of the template (Galvin, O'Shea, Squire, & Hailstone, 1999). A more recent study by Valsecchi, Koenderink, van Doorn, and Gegenfurtner (2018) showed that observers perceive a peripheral stimulus as less blurry and less distorted than a foveal stimulus, resulting in an overall sharper image. These results extend the previous findings on sharpness overconstancy to more complex geometric shapes and patches of cluttered natural images. The authors also suggested that the visual system may use predictive templates based on foveal experience to reduce the discrepancies between the periphery and the fovea.
The perception of blur (e.g., Maiello, Walker, Bex, & Vera-Diaz, 2017) and distortion (Bex, 2010) varies across the visual field. Bex (2010) found that sensitivity to fine spatial distortions decreases with increasing eccentricity, whereas sensitivity to coarse distortions remains constant. The loss of sensitivity in the periphery is often attributed to an increase in spatial pooling (Balas, Nakano, & Rosenholtz, 2009; Freeman & Simoncelli, 2011; Portilla & Simoncelli, 2000). As suggested by the texture synthesis model of Portilla and Simoncelli (2000), spatial pooling leads to an irreversible loss of information, making stimuli indistinguishable at the population level. This notion is well-demonstrated in images, referred to as metamers (Freeman & Simoncelli, 2011; Portilla & Simoncelli, 2000), mongrels (Balas et al., 2009), or eidolons (Koenderink, Valsecchi, van Doorn, Wagemans, & Gegenfurtner, 2017), with significant distortions in the periphery that are not recognized as distortions. However, attributing the sharpening effect solely to lower sensitivity seems to be insufficient. Galvin et al., (1997) demonstrated that the phenomenon is not merely an outcome of diminished peripheral discrimination. They observed its persistence even after adjusting the field sizes of peripheral stimuli based on a cortical magnification factor.
Although existing studies have focused predominantly on perception during fixation, it is important to recognize that our visual experience extends beyond fixations. Due to the limitations of peripheral processing, we constantly make eye movements to bring new information to the fovea (e.g., Rayner, 1998). It remains unclear whether sharpening would occur across a saccade. This question arises because saccades involve additional mechanisms that reconcile differences between the peripheral and foveal views, which could counteract the sharpening effect. For example, presaccadic attention boosts performance at saccade targets (e.g., Deubel & Schneider, 1996) and enhances visual acuity by shifting spatial frequency tuning toward higher spatial frequencies (Kroell & Rolfs, 2021; Kwak, Hanning, & Carrasco, 2023; Li, Barbot, & Carrasco, 2016; Li, Pan, & Carrasco, 2019). In a Bayesian framework, the sharpening effect could result from the combination of an increased uncertainty in the periphery with a prior that favors sharp over blurry edges. It may be that presaccadic mechanisms can generally decrease uncertainty at high spatial frequencies, so that the prior is weighted less than the signal, resulting in a more accurate estimate of the presaccadic information.
Differences in the appearance of a visual feature can indeed bias the perception of changes across saccades: Hübner and Schütz (2021) showed that stimuli appear more circular when viewed peripherally, before a saccade, than when viewed foveally, after a saccade. They also showed that transsaccadic changes that increase stimulus circularity after a saccade are more readily perceived. Their argument suggests that the apparent high circularity in the peripheral view leads to a transsaccadic prediction that circularity will typically decrease after a saccade. The perception of changes that contradict the prediction is facilitated by a larger prediction error. Sharvashidze, Hübner, and Schütz (2024) found a transsaccadic bias to perceive an increase in spatial frequency. In contrast to the study by Hübner and Schütz (2021), this bias could not be attributed to differences in the visual appearance of spatial frequency, but rather to asymmetric interactions between high and low spatial frequencies across saccades. Investigating changes in distortion and blur across saccades may provide further insight into directional biases in change perception and their relationship to appearance across the visual field. The main aim of this study was to investigate apparent blur and distortion (stimulus regularity) and change discrimination across saccades. To investigate the appearance of stimulus regularity, we used a task that involved comparing stimulus regularity with an implicit average regularity representation, allowing us to interpret relative differences in perceived appearance between presaccadic and postsaccadic conditions. For change discrimination, we used a task that measured the tendency to report one change direction over another. Additionally, change discrimination performance during a blank condition was tested. Deubel, Schneider, & Bridgeman (1996) showed that briefly removing the stimulus during saccades improved change detection by disrupting stimulus consistency across eye movements. For blanking periods of 170 ms or longer (50–300 ms tested), participants' ability to judge the direction of target displacement accurately during saccade becomes nearly perfect.
Object blur and distortion can be manipulated effectively with the Eidolons Factory algorithm (Koenderink et al., 2017) (Figure 1). The algorithm is based on a scale-based representation of the image and applies local spatial distortions to the representation. The Eidolons Factory distorts the representation at different scales through a random vector field, which is a combination of a scale-specific field and a shared field. Two parameters control the distortion: reach and coherence. Reach determines the standard deviation of the random fields, which affects the extent of distortion. Coherence defines the balance between the shared and scale-specific fields. Increasing the reach leads to greater distortion, such as edge bending and warping. Decreasing coherence results in independently diffused edges across scales, making them less defined and blurrier. The third parameter, grain represents the standard deviation of the Gaussian spatial filter that interacts with the noise fields, determining the spatial correlation of the warping. Valsecchi et al. (2018) combined the modulation of coherence and reach. Here, we varied coherence while keeping reach fixed and manipulated reach without scale decomposition. This allowed us to control distortion and blur separately in two experiments.
Figure 1.

An example of the blur and distortion applied to a gray circle with Eidolons Factory.
The first experiment examined the appearance of blur before and after a saccade, as well as the discrimination of changes in two directions: blur increase and decrease. We found that stimuli appeared less blurry before a saccade than after a saccade, replicating the sharpening effect (Galvin et al., 1997; Valsecchi et al., 2018). We additionally found that an increase in blur was detected more readily in both conditions, with and without a blank. In the second experiment, we investigated the appearance of distortion before and after a saccade, as well as the discrimination of changes in two directions: distortion increase and decrease. The results indicated no consistent difference in the appearance of distortion across saccades. However, we found that an increase in distortion was more readily detected across saccades, but only in the blank condition. No effect was observed in the no blank condition.
Experiment 1: Blur
Methods
Participants
A total of 25 participants completed both tasks of Experiment 1 consecutively on the same day. We excluded data from four participants because their point of subjective equality (PSE) and/or point of subjective stability (PSS) values were outside our measurement range, meaning they were unable to perform the task, resulting in a final dataset of 21 participants (mean age 26.24 ± 5.48 years; range 18–42 years; 7 males). Participants were unaware of the purpose of the study and gave informed consent before participating. They had either normal or corrected-to-normal vision and were paid 8€ per hour. The study was conducted according to the ethical guidelines of the 1964 Declaration of Helsinki and was approved by the Ethics Committee of the University of Marburg, Department of Psychology (proposal 2015-35k).
Stimuli
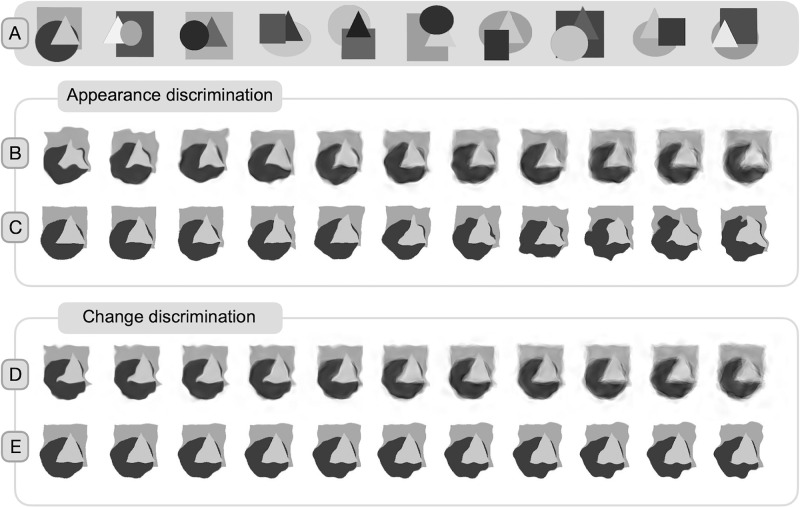
The fixation stimulus, which was a combination of a bull's eye and crosshair, measured 0.6 degrees of visual angle (dva) (Thaler, Schütz, Goodale, & Gegenfurtner, 2013). To prevent afterimage accumulation, the color of the fixation stimulus was selected randomly from a DKL color space array (Derrington, Krauskopf, & Lennie, 1984) with variations in luminance and red–green color channel polarity. The visual base stimuli were 10 images created in Matlab, featuring a rectangle, circle, and triangle superimposed on one another (Figure 2A). The shapes were only partially occluded by the others. The configurations were generated randomly and filtered for compactness, with an average distance from the center of less than 90 pixels. The base stimuli were used solely for generating blur or distortion in the experiments.
Figure 2.
Base stimuli and example of applied blur and distortion in both tasks. (A) Ten base stimuli with a superimposed rectangle, circle, and triangle. (B and C) Appearance discrimination task. (B) Example of 11 blur values applied to the first base stimulus. Coherence values from left to right: 0.9%, 0.82%, 0.74%, 0.66%, 0.58%, 0.50%, 0.42%, 0.34%, 0.26%, 0.18%, and 0.1% with the reach value set to 5 pixels. (C) Example of 11 distortion values applied to the first base stimulus. Reach values from left to right: 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, and 6 pixels. For each trial of the appearance task, stimuli with random noise fields were used. The random displacement field is evident in the random blur/distortion for each stimulus in the example array. (D and E) Change discrimination task. (D) Example of 11 blur values applied to the first base stimulus. Coherence values from left to right: 0.9%, 0.82%, 0.74%, 0.66%, 0.58%, 0.5%, 0.42%, 0.34%, 0.26%, 0.18%, and 0.1% with the reach value set to 5 pixels. (E) Example of 11 distortion values applied to the first base stimulus. Reach values from left to right: 2, 2.3, 2.6, 2.9, 3.2, 3.5, 3.8, 4.1, 4.4, 4.7, and 5 pixels. In the change discrimination task, the noise field of the postsaccadic stimulus was matched to the presaccadic stimulus. In the provided visual example, one of the stimuli could be a presaccadic stimulus and one could be a postsaccadic stimulus, depending on the delta and change direction of the trial. Similar to the appearance task, the noise field of the presaccadic stimulus varied from trial to trial.
The blur manipulation was executed using the ‘remake_eidolon’ function of the Eidolons Factory Matlab Toolbox (Koenderink et al., 2017), adjusting only the coherence parameter while maintaining a constant reach of 5 pixels and grain of 10 pixels. In the change discrimination task, coherence was varied between 0.10% and 0.90%. There were 11 delta steps in this task, ranging from 0.08 to 0.80. A small change of 0.08 delta step was observed when coherence changed from 0.82% to 0.90% (increase in coherence = decrease in blur) or from 0.90% to 0.82% (decrease in coherence = increase in blur). A large delta of 0.80 was implemented when coherence changed from 0.10% to 0.90% (significant decrease in blur) or from 0.90 to 0.10 (significant increase in blur). The presaccadic and postsaccadic stimuli were generated with the same noise field, but the noise fields used varied between trials. In the appearance discrimination task, coherence varied between 0.10% and 0.90% (0.10%, 0.18%, 0.26%, 0.34%, 0.42%, 0.50%, 0.58%, 0.66%, 0.74%, 0.82%, and 0.90%). All eidolons, i.e. blurry objects, were created before the experiment and saved for later use during the experiment (see Figure 2 for a visual example of the blur levels for each task).
Design
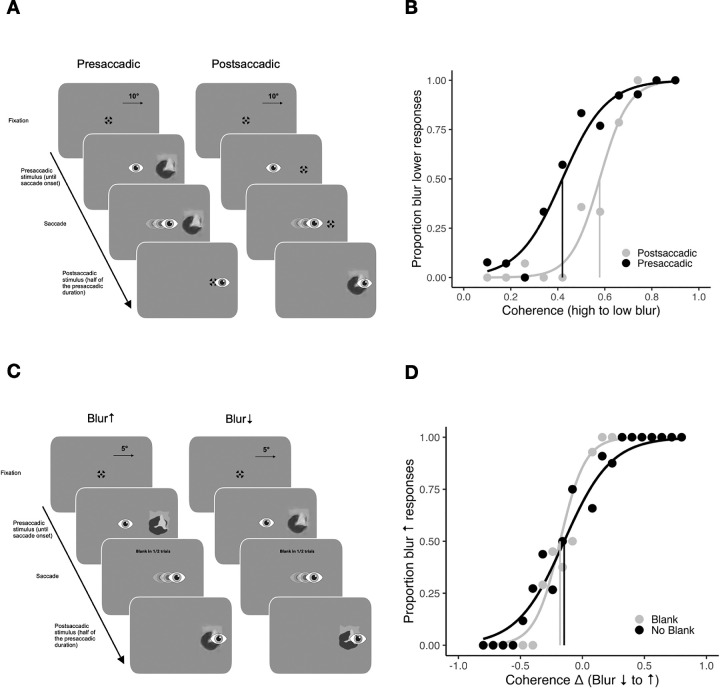
In the appearance discrimination task of the first experiment, we examined presaccadic and postsaccadic blur appearance (Figure 3A). In the presaccadic condition, an eidolon (i.e., blurry object) was presented before a saccade and replaced by a fixation stimulus after the saccade. In the postsaccadic condition, the fixation stimulus was presented before a saccade and replaced by the eidolon after a saccade. Participants were asked to evaluate whether the eidolon in each trial was more or less blurry than the average eidolon seen throughout the experiment using the method of single stimuli (Hübner & Schütz, 2017, Hübner & Schütz, 2021; Morgan, Watamaniuk, & McKee, 2000; Sharvashidze et al., 2024). This task compares participants’ implicit representation of the average stimulus, built up over trials. For the first trials they are instructed to guess. To create an implicit standard, observers have been shown to average over the last 10 to 20 trials (Morgan et al., 2000). This implicit standard could vary between participants and may not correspond to the actual average coherence of the eidolon. Thus, the method allows only for the interpretation of relative differences in PSEs between pre- and postsaccadic conditions, but not for the interpretation of absolute PSEs. The task involved testing 11 coherence values for both conditions, each repeated 15 times, resulting in 330 randomized trials.
Figure 3.
Stimuli and methods of Experiment 1. (A) Trial procedure of the appearance discrimination task. The eidolons were presented either before (presaccadic condition) or after a saccade (postsaccadic condition). Participants had to compare the blur of the eidolon in one trial with the overall average blur of all previous trials. (B) Example psychometric functions for a participant show the proportion of blur-lower responses over coherence from 0.1% to 0.9% (high to low blur), in presaccadic (black) and postsaccadic (gray) conditions. Vertical lines indicate the points of subjective equality (PSE). (C) Trial procedure of the change discrimination task. The blur either increased or decreased during a saccade. Participants had to indicate the direction of change. (D) Example psychometric functions for a participant showing the proportion of blur-increase responses over coherence changes for blank (gray) and no blank (black) conditions. Negative values indicate a decrease in blur, positive values indicate an increase in blur. Vertical lines represent points of subjective stability (PSS). Note that on the y-axis label the proportion blur increase is indicated with an arrow to differentiate it from proportion blur lower (than mean) responses in (B). On the x-axis label, the blur decrease to increase is indicated with arrows to differentiate it from high to low blur in (B).
In the change discrimination task of the first experiment, we investigated transsaccadic change discrimination of the blur (Figure 3C). The blur of the eidolons could either increase or decrease during a saccade. In one half of the trials, a 200-ms blank preceded the onset of the change. In the literature reporting facilitated change detection effects (Table A2) the postsaccadic blank period usually varies between 100 and 300 ms (e.g., Deubel, Schneider, & Bridgeman, 1996; Goktepe & Schütz, 2023; Grzeczkowski, Deubel, & Szinte, 2020; Grzeczkowski, van Leeuwen, et al., 2020; Hübner & Schütz, 2021; Poth & Schneider, 2016; Stewart, Hübner, & Schütz, 2020; Tas, Mordkoff, & Hollingworth, 2021; Weiss, Schneider, & Herwig 2015). For our study, we selected a duration of 200 ms, consistent with previous work by Hübner and Schütz (2021). Participants were asked to discriminate the direction of change and respond whether the eidolon became more or less blurry. Each change direction and blanking condition was assigned two staircases. One staircase started with the smallest possible change magnitude of delta 0.08 and the other with the largest possible change magnitude of delta 0.80. Each staircase was running for 50 trials, resulting in 400 trials in total. The staircases were embedded and all trials were interleaved. Responses with the reported change not matching the actual change direction were classified as a miss, resulting in an increase of change magnitude for the next trial. Conversely, responses were classified as a hit if the reported and actual change directions matched. After achieving two consecutive hits, the change magnitude was decreased. The presaccadic base stimulus was selected randomly. The level of presaccadic blur was determined by the magnitude of change in the trial. Depending on the delta of the trial, there were either one (for the largest magnitude of change) or 10 (for the smallest magnitude of change) options for presaccadic blur. For instance, the largest change could be a change in coherence from 0.10% to 0.90% (blur decrease) or from 0.90% to 0.10% (blur increase).
Pairs were created for each change magnitude to ensure that any change in one direction was also applied in the opposite direction. The order of completion of both tasks was counterbalanced across participants. Before the change discrimination task, participants completed a brief change discrimination training session with accuracy feedback after each trial.
Equipment
Stimuli were displayed on a VIEWPixx monitor (VPixx Technologies Inc., Quebec, Canada; 1920 × 1080 px, 120 Hz) with dimensions 51.5 × 29 cm, at a viewing distance of 60 cm. The monitor was calibrated for linear gamma correction (luminance values: 0.39, 54, and 105 cd/m² for black, gray, and white, respectively). Eye movements were recorded at 1000 Hz using an EyeLink 1000 Plus (SR Research Ltd., Ontario, Canada). MATLAB (R2017a) with Psychophysics Toolbox (3.0.12) (Brainard, 1997; Pelli, 1997) was used for the stimulus display, and the EyeLink Toolbox (Cornelissen, Peters, & Palmer, 2002) was used for controlling the eye tracker. Head movement was constrained by a chinrest. Participants responded using a standard keyboard. For the appearance discrimination task, they used the vertical plus key on the number pad to indicate responses higher than the mean and the horizontal zero key on the number pad to indicate responses lower than the mean. In the change discrimination task, 11 participants were instructed to use the vertical plus key to indicate an increase in blur and the horizontal zero key to indicate a decrease in blur. For the remaining nine participants, the keys were swapped. This was done to reduce potential key-related biases. Data analysis was conducted with MATLAB (R2021a) and RStudio (4.3.0).
Procedure
In both tasks, participants started each trial by pressing the space bar while fixating a central fixation stimulus. In the appearance discrimination task, an eidolon (presaccadic condition) or a fixation stimulus (postsaccadic condition) appeared to the left or right at the 10 dva eccentricity on the horizontal axis after a duration that varied between 750 and 1500 ms. Participants were instructed to execute a saccade to the center of the peripheral eidolon. Online saccade detection was based on boundary criteria. A saccade was detected when the eye position moved 2 dva away from the fixation cross in a horizontal direction. Upon saccade detection, the postsaccadic switch occurred, the presaccadic eidolon was replaced by a fixation stimulus (presaccadic condition), or the fixation stimulus was replaced by the eidolon (postsaccadic condition). The duration of the postsaccadic eidolon presentation was half of the median duration of the presaccadic eidolon presentation, calculated over all completed presaccadic trials (the exact stimulus presentation durations are reported in Table A1; see also Figure A1). After the disappearance of the postsaccadic eidolon, participants had to indicate by keypress whether the blur of the eidolon in that trial was higher or lower compared to the mean blur of eidolons seen up to that point. Excluded from analysis were trials with no saccades, saccade latencies beyond 600 ms or less than 50 ms, and saccades landing beyond 2 dva from the stimulus center.
In the change discrimination task, the presaccadic eidolon appeared at an eccentricity of 5 dva. After saccade detection (same boundary criteria), the stimulus was either replaced immediately (no blank condition) or removed for 200 ms (blank condition) and then replaced by the postsaccadic eidolon, which was either more or less blurry. The postsaccadic eidolon was displayed for one half of the duration of the presaccadic eidolon in that trial. Shortening the postsaccadic stimulus durations in both tasks ensured that the presaccadic and postsaccadic information had comparable reliability. After the stimulus disappeared, participants were asked to indicate by keypress whether the blur increased or decreased.
Eye movement analysis and trial exclusions
Saccades were detected offline using the EyeLink algorithm, with a velocity threshold of 22°/s and an acceleration threshold of 3800°/s². Saccade latency was calculated as the time between the presaccadic stimulus onset and saccade onset. Trials were excluded if saccade latencies were below 50 ms or exceeded 600 ms (6.06 ± 6.68% in the appearance discrimination task and 6.56 ± 6.04% in the change discrimination task), if the gaze position deviated more than 2 dva horizontally or 1.5 dva vertically from the saccade target center in the interval between saccade landing and stimulus offset (10.97 ± 9.02% in the appearance discrimination task and 5.3 ± 4.75% in the change discrimination task). Additionally, trials with blinks between 300 ms before presaccadic stimulus onset and postsaccadic stimulus offset (0.65 ± 0.92% of trials in the appearance discrimination task and 0.85 ± 1.4% in the change discrimination task) were excluded. Also excluded were trials with inaccurate presaccadic to postsaccadic stimulus switches (when the switch did not occur during a saccade) (4.01 ± 4.89% in the appearance discrimination task and 4.36 ± 4.87% in the change discrimination task). This resulted in the overall exclusion of 12.38 ± 10.04% of trials in the appearance discrimination task and 7.63 ± 6.48% in the change discrimination task.
Psychophysical analysis
We used the Quickpsy package in R to model the data using a logistic function (Linares & López-Moliner, 2016). In the appearance discrimination task (Figure 3B), responses were converted into proportions that indicated blur lower than the mean. The PSE represented the coherence level corresponding to 50% blur lower (than mean) responses, with a lower PSE indicating a tendency to perceive objects as less blurry (more coherent). The just-noticeable difference (JND) was half the difference between thresholds at 25% and 75%, reflecting precision in blur discrimination.
In the change discrimination task (Figure 3D), perceptual choices were converted to proportion blur increase responses. To align blur and distortion bias directions, the coherence change magnitude was inverted by multiplying it by −1. The PSS reflected the magnitude and direction of the blur change corresponding to 50% blur-increase responses, with a negative PSS indicating a bias toward reporting an increase in blur. The JNDs in this task indicated an observer's ability to detect changes, calculated as half the difference between thresholds at 25% and 75%, with a lower JND indicating greater precision. We performed t tests, p value calculations, and Bayesian t tests (BF10 indicating evidence against the null hypothesis) in R using the stats (R Core Team, 2023) and BayesFactor (Morey & Rouder, 2024) packages, with an alpha value of 0.05 for two-tailed t tests.
Results
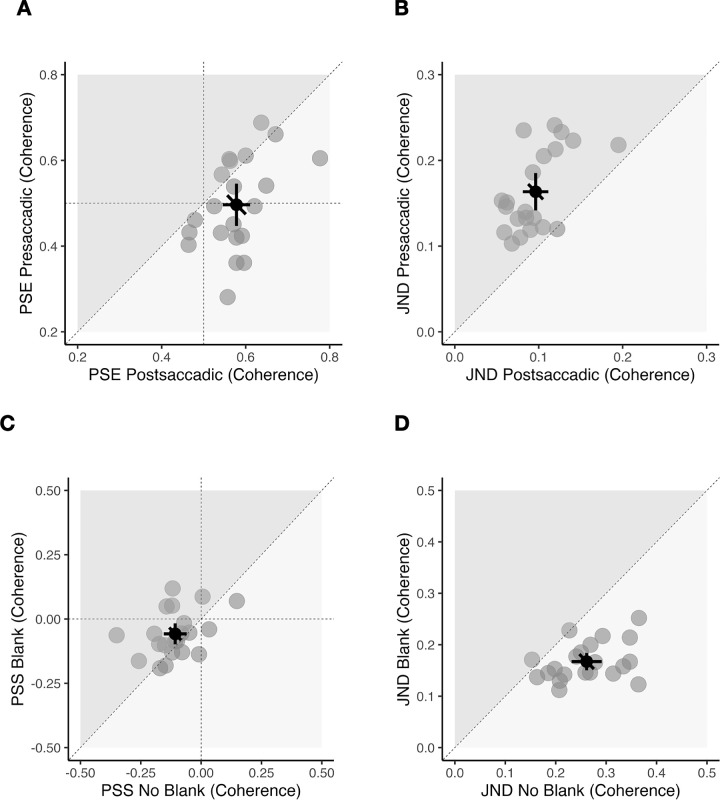
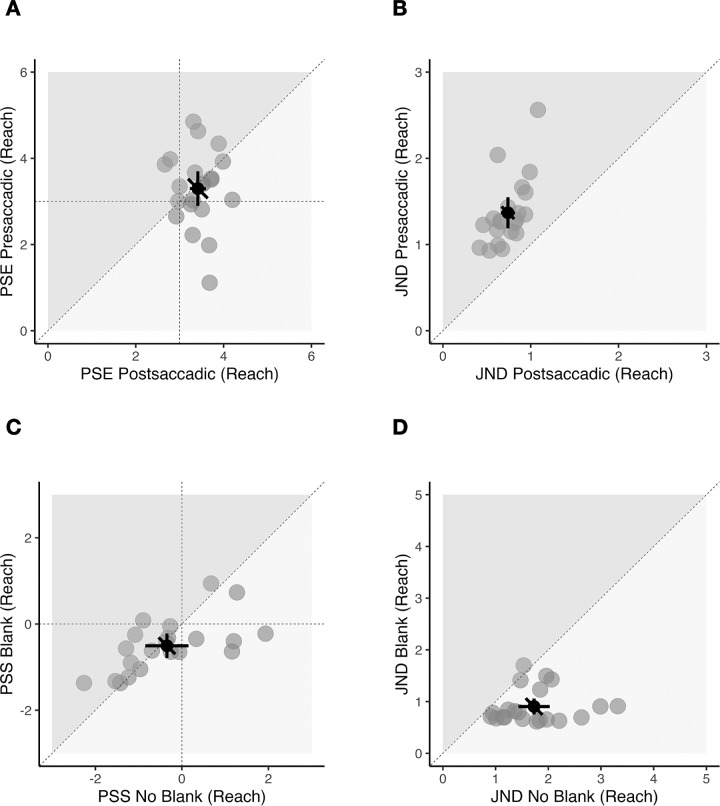
In the appearance discrimination task (Figures 4A and B), the mean PSE was 0.5 ± 0.11 (coherence in %) in the presaccadic condition and 0.58 ± 0.07 (coherence) in the postsaccadic condition. There was a significant difference between the two conditions, t(20) = −3.85, p = 0.001, BF10 = 36.06, very strong evidence for H1, indicating that, in the presaccadic condition, the stimuli were perceived as less blurry compared to the postsaccadic condition. The mean JND in the presaccadic condition was 0.16 ± 0.05 (coherence) and 0.1 ± 0.03 (coherence) in the postsaccadic condition. There was a significant difference between the two conditions, t(20) = 7.87, p < 0.001, BF10 = 101877.9, extreme evidence for H1, indicating a higher precision in the postsaccadic condition.
Figure 4.
Results of Experiment 1. (A and B) Appearance discrimination task. (C and D) Change discrimination task. (A) Scatterplot showing all points of subjective equality (PSE) compared between presaccadic (vertical axis) and postsaccadic (horizontal axis) conditions. Data points below the diagonal line indicate a less blurry appearance in the presaccadic compared to postsaccadic conditions. (B) Scatterplot for all just-noticeable differences (JNDs) compared between presaccadic (vertical axis) and postsaccadic (horizontal axis) conditions. Data points above the diagonal line indicate that participants were more precise in the postsaccadic condition. (C) Scatterplot showing all points of subjective stability (PSS) compared between blank (vertical axis) and no blank (horizontal axis) conditions. Data points in the lower left quadrant (negative PSS) indicate a bias toward blur-increase responses. (D) Scatterplot for all JNDs compared between blank (vertical axis) and no blank (horizontal axis) conditions. Data points below the diagonal line indicate higher precision in the blank condition. (A–D) Gray dots indicate individual participant data, black dot indicates the overall mean. Error bars indicate 95% confidence intervals.
In the change discrimination task (Figures 4C and D), the mean PSS was −0.06 ± 0.09 (coherence) in the blank condition and −0.11 ± 0.1 (coherence) in the no blank condition. Participants showed a bias to perceive changes from low to high blur in both conditions, blank condition, t(20) = −2.96, p = 0.008, BF10 = 6.21, moderate evidence for H1; no blank condition: t(20) = −4.73, p < 0.001, BF10 = 222.81, extreme evidence for H1. There was a significant difference in PSS between blank and no blank conditions, t(20) = 2.12, p = 0.047, BF10 = 1.43, anecdotal evidence for H1.
To assess the retest reliability of the change discrimination measurement, we conducted a PSS correlation analysis between blank and no blank conditions. For blur the correlation between the PSS values in blank and no blank conditions was weak, r = 0.39, t(19) = 1.83, p = 0.082, BF10 = 1.54. Notably, the correlation may underestimate the retest reliability, given the differences between the two conditions. Consequently, the consistency of the measurement is likely higher than suggested by the correlation coefficient. We also investigated the potential correlation between the difference in the PSE values from the appearance discrimination task and the PSS values from the change discrimination task (Hübner & Schütz, 2021). However, no significant correlation was found in either the blank, r = −0.019, t(19) = −0.08, p = 0.94, BF10 = 0.47, or the no blank condition, r = 0.17, t(19) = 0.76, p = 0.46, BF10 = 0.58.
The mean JND in the blank condition was 0.17 ± 0.04 (coherence) and 0.26 ± 0.07 (coherence) in the no blank condition. There was a significant difference between the two groups in JNDs showing a higher precision in the blank condition, t(20) = −6.82, p < 0.001, BF10 = 14757.89, extreme evidence for H1.
Experiment 2: Distortion
Methods
Participants
Another group of 26 participants took part in Experiment 2. Four individuals were excluded due to their PSE and/or PSS values exceeding the measurement range. Additionally, one participant was excluded due to key-related confusion, resulting in a final dataset of 21 participants (mean age, 28.14 ± 4.04 years, range, 19–35 years, 3 males). Two of the remaining participants took part in both experiments. Everything else was identical to Experiment 1.
Stimuli
To apply distortion to the stimuli without introducing any blurring, we produced eidolons using the ‘disarray_image’ function of the Eidolons Factory Matlab Toolbox (Koenderink et al., 2017), which does not use a scale-decomposed representation. The grain parameter was fixed at 10 pixels. In the change discrimination task, we varied reach between 2 and 5 pixels (2.0, 2.3, 2.6, 2.9, 3.2, 3.5, 3.8, 4.1, 4.4, 4.7, and 5.0, the equivalent values in dva: 0.051, 0.059, 0.067, 0.074, 0.082, 0.09, 0.097, 0.105, 0.112, 0.12, and 0.128, respectively). This task involved 11 delta steps ranging from 0.3 to 3.0. For instance, a small change of 0.3 delta step could mean that reach 2.0 changed to 2.3 (resulting in an increase in distortion) or vice versa, from 2.3 to 2.0 (resulting in a decrease in distortion). Conversely, a large delta of 3.0 was implemented when reach 2.0 changed to 5.0 (resulting in a significant increase in distortion) or vice versa, from 5.0 to 2.0 (resulting in a significant decrease in distortion). The random noise field of the presaccadic distortion was used to create the postsaccadic distortion. In the appearance discrimination task, reach was varied between 1 and 6 pixels (1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0, the equivalent values in dva: 0.026, 0.038, 0.051, 0.064, 0.077, 0.09, 0.102, 0.12, 0.128, 0.141, and 0.153, respectively) (see Figure 2 for a visual example of distortion levels for each task).
Design
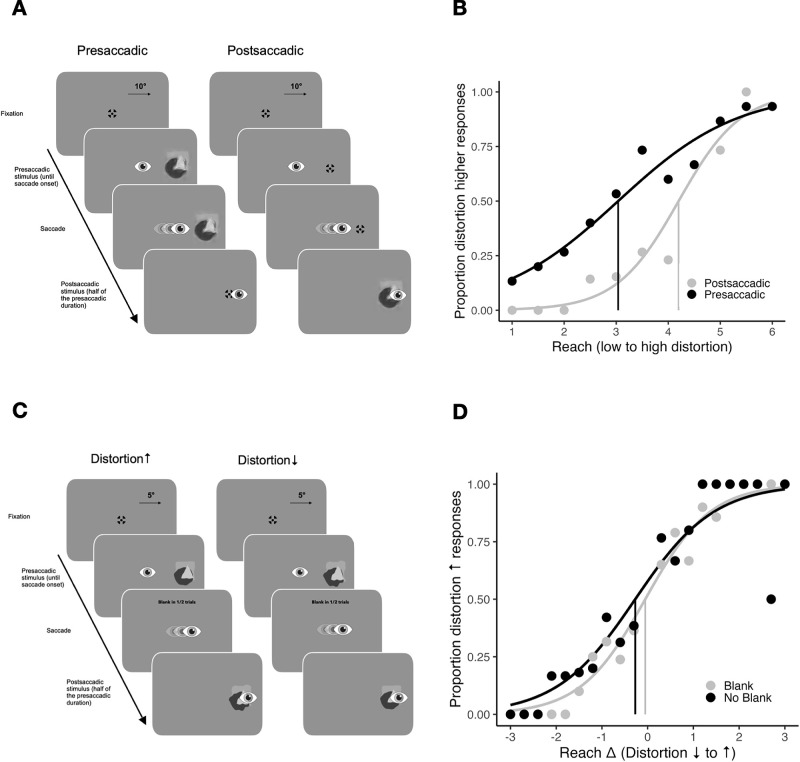
The design of Experiment 2 was identical to that of Experiment 1. In the appearance discrimination task, 11 reach values for presaccadic and postsaccadic conditions were tested in 330 randomized trials using the method of single stimuli (Hübner & Schütz, 2017; Hübner & Schütz, 2021; Morgan et al., 2000; Sharvashidze et al., 2024). In the change discrimination task, two separate staircases were used for each change direction and blanking condition. One staircase started from the smallest possible change of delta 0.3 and the other from the largest possible change of 3.0. Each staircase was repeated for 50 trials, resulting in 400 trials in total. Depending on the delta of the trial, for each change direction, there were one (largest change) or 10 (smallest change) option for the presaccadic distortion value.
Equipment
The equipment used was the same as in Experiment 1. In the change discrimination task, 15 participants used the vertical plus key for distortion increase and horizontal zero for distortion decrease responses, while the remaining six participants followed the reversed instruction.
Procedure
For both tasks, the procedure was the same as in Experiment 1 (Figures 5A and C).
Figure 5.
Stimuli and methods of Experiment 2. (A) Trial procedure of the appearance discrimination task. The distorted eidolons were presented either before (presaccadic condition) or after a saccade (postsaccadic condition). Participants had to compare the distortion of the eidolon in one trial to the overall average distortion across all previous trials. (B) Example psychometric functions for a participant show the proportion of distortion-higher responses over distortion in reach from 1 to 6 pixels, in presaccadic (black) and postsaccadic (gray) conditions. Vertical lines indicate the points of subjective equality (PSE). (C) Trial procedure of the change discrimination task. Distortion either increased or decreased during a saccade. Participants had to indicate the direction of change. (D) Example psychometric functions for a participant show the proportion of distortion-increase responses over changes in distortion for blank (gray) and no blank (black) conditions. Negative values indicate a reduction in distortion, positive values indicate an increase in distortion. Vertical lines represent points of subjective stability (PSS). Note that on the y-axis label the proportion distortion increase is indicated with an arrow to differentiate it from proportion distortion higher (than mean) responses in (B). On the x-axis label, the distortion decrease to increase is indicated with arrows to differentiate it from low to high distortion in (B).
Eye movement analysis and trial exclusions
Eye movement analysis and trial exclusion criteria were the same as in Experiment 1. Trials with latencies of less than 50 ms and greater than 600 ms comprised 8.46 ± 9.42% of the trials in the appearance discrimination task and 6.71 ± 7.29% of the trials in the change discrimination task. In the appearance discrimination task, 12.3 ± 11.12% of the trials were excluded due to position deviations in the interval between saccade offset and stimulus offset. In the change discrimination task, this number was 6.06 ± 3.82%. In the appearance discrimination task, 1.57 ± 4.18% of the trials contained blinks, 3.69 ± 6.16% contained inaccurate presaccadic to postsaccadic stimulus switches, and, in the change discrimination task, the percentages were 1.75 ± 4.93% and 2.96 ± 2.87%, respectively. In total, 14.92 ± 11.64% of the trials in the appearance discrimination task and 8.92 ± 7.78% of the trials in the change discrimination task were excluded in Experiment 2.
Psychophysical analysis
In the appearance discrimination task (Figure 5B), responses were converted to proportions indicating distortion higher (than mean). The PSE represented the distortion level corresponding to 50% distortion higher (than mean) responses, with lower PSE indicating a tendency to perceive less distortion, and a higher PSE indicating a tendency to perceive more distortion.
In the change discrimination task (Figure 5D), perceptual decisions were transformed into the proportion of responses indicating an increase in distortion. So the PSS represented the magnitude and direction of the distortion change corresponding to 50% of responses indicating an increase in distortion, with a negative PSS indicating a tendency to report an increase in distortion. The JNDs were computed using the same method as in Experiment 1.
Results
In the appearance discrimination task (Figures 6A and B), the mean PSE was 3.3 ± 0.88 (reach in pixels) in the presaccadic condition and 3.41 ± 0.40 (reach) in the postsaccadic condition. The mean PSEs did not differ between the two conditions, t(20) = −0.53, p = 0.6, BF10 = 0.26, no evidence. However, the mean JND was 1.37 ± 0.4 (reach) in the presaccadic and 0.74 ± 0.18 (reach) in the postsaccadic condition, indicating a significant precision improvement in the postsaccadic condition, t(20) = 9.07, p < 0.001, BF10 = 825187.3, extreme evidence for H1. In the change discrimination task (Figures 6C and D), the mean PSS was −0.51 ± 0.61 (reach) in the blank condition and −0.35 ± 1.1 (reach) in the no blank condition. Participants showed a bias toward perceiving an increase in distortion in both conditions, but only in the blank condition was the value significantly different from zero, blank condition: t(20) = −3.75, p = 0.001, BF10 = 29.64, strong evidence for H1; no blank condition: t(20) = −1.45, p = 0.163, BF10 = 0.56, anecdotal evidence for H0. There was no difference in PSS between the two conditions, t(20) = −0.86, p = 0.400, BF10 = 0.32, anecdotal evidence for H0.
Figure 6.
Results of Experiment 2. (A and B) Appearance discrimination task. (C and D) Change discrimination task. (A) Scatterplot showing all points of subjective equality (PSE) compared between presaccadic (vertical axis) and postsaccadic (horizontal axis) conditions. Data points on the diagonal line indicate no difference in distortion appearance between the presaccadic and postsaccadic conditions. (B) Scatterplot for all just-noticeable differences (JNDs) compared between presaccadic (vertical axis) and postsaccadic (horizontal axis) conditions. Data points above the diagonal line indicate that participants were more precise in the postsaccadic condition. (C) Scatterplot showing all points of subjective stability (PSS) compared between blank (vertical axis) and no blank (horizontal axis) conditions. Data points in the lower left quadrant (negative PSS) indicate a bias toward distortion-increase responses. (D) Scatterplot for all JNDs compared between blank (vertical axis) and no blank (horizontal axis) conditions. Data points below the diagonal line indicate higher precision in the blank condition. (A–D) Gray dots indicate individual participant data, black dot indicates the overall mean. Error bars indicate 95% confidence intervals.
For distortion, we found a moderate to strong correlation between the PSS values in blank and no blank conditions, r = 0.64, t(19) = 3.61, p = 0.0018, BF10 = 21.14, indicating a high level of measurement consistency. Similar to blur, there was no evidence for a correlation between the PSE differences in peripheral and foveal appearance and the PSS values in the change discrimination task, blank condition: r = 0.20, t(19) = 0.9, p = 0.379, BF10 = 0.63; no blank condition: r = −0.13, t(19) = −0.57, p = 0.573, BF10 = 0.53.
The mean JND in the blank condition was 0.9 ± 0.33 (reach) and 1.73 ± 0.65 (reach) in the no blank condition. Participants were more precise in the blank condition compared to the no blank condition. The JND values in two conditions differed significantly from each other, t(20) = −5.40, p < 0.001, BF10 = 884.89, extreme evidence for H1.
General discussion
This study aimed to examine the appearance of blurry and distorted stimuli viewed presaccadically in the periphery and postsaccadically in the fovea, and to investigate the discrimination of transsaccadic changes in object regularity. Consistent with previous research by Galvin et al. (1997) and Valsecchi et al. (2018), we found that stimuli were perceived to be sharper and less blurry before a saccade than after a saccade. Notably, no appearance differences were observed for distortion. We did not assess image regularity during fixation, as this has been previously demonstrated for similar eidolons (Valsecchi et al., 2018). While the previous study observed sharpening effects at 20 and 30 dva, we demonstrated this effect at 10 dva before saccades. Because we did not compare a fixation and a saccade condition directly, the magnitude of the sharpening effect may still differ between fixation and saccades.
We also found a bias toward perceiving changes from low to high blur and distortion (albeit the distortion effect was significant only in the blank condition). This transsaccadic change discrimination bias for blur may be related to the sharper presaccadic appearance of objects. Furthermore, in the appearance discrimination task, participants showed greater precision in postsaccadic than in presaccadic conditions for both blur and distortion. Additionally, precision was higher in the blank than in the no blank condition in the change discrimination tasks.
Appearance differences
The appearance discrimination task showed that less coherence (higher blur) was needed in the periphery to perceive stimuli as blurry. That is, objects were perceived as sharper before a saccade than after a saccade. How blur is perceived across the visual field has been investigated by making a crucial distinction between blur discrimination and blur detection. In the periphery, blur discrimination is more sensitive than detection. This implies that the participants are better at discriminating changes in the blur of an object than at detecting its initial blur (Ciuffreda, Wang, & Vasudevan, 2007; Maiello, Walker, Bex, & Vera-Diaz, 2017; Wang & Ciuffreda, 2004, Wang & Ciuffreda, 2005; Wang, Ciuffreda, & Irish, 2006). Sharpness overconstancy has been proposed as one of the likely explanations for these threshold differences during fixation (Wang & Ciuffreda, 2004; Wang & Ciuffreda, 2005). Our results suggest that sharpening also occurs across a saccade.
Given the decrease in sensitivity to high spatial frequencies in the periphery (e.g., Rovamo, Virsu, & Näsänen, 1978), it could be expected that edges that require high spatial frequency information become even blurrier. Paradoxically, the opposite happens. Haun (2021) attempts to explain this paradox with a scale-space model of edge sharpness that distinguishes between physical and perceived sharpness. He suggests that foveal and peripheral filters play a role in processing edges at different resolutions. For a high-resolution, sharp edge, foveal filters are engaged fully, capturing the full range of spatial scales. In contrast, for a low-resolution, blurry edge, the foveal filters respond incompletely, possibly because the edge does not elicit a response from the smallest filters. The peripheral filters are coarse scale and do not distinguish between blurry and sharp edges. Both types of edges elicit complete responses from the peripheral filters. This suggests that the apparent sharpness is related to the complete filter response and not to the actual physical resolution of the stimulus. Valsecchi et al. (2018) suggest that sharpening occurs through the detection of edges in visible spatial frequencies, which act as indicators for inferring the presence of edges in spatial frequencies that are invisible in the periphery. The process resembles the filling in of unavailable or uncertain information. Interestingly, Simmers, Bex, and Hess (2003) showed that participants with amblyopia, even those with significant visual impairment, could accurately match blurry edges, including those with the greatest degree of sharpness. This finding suggests that the amblyopic visual system can represent levels of blur determined by spatial frequencies beyond its resolution limit. This result supports the notion that this perceptual effect should be a byproduct of some compensatory mechanism. If, in healthy observers, the veridical information might be coming from foveal experience, in the case of amblyopes it might be coming from the foveal experience of the nonambliopic eye (Simmers et al., 2003).
It has been shown that prior experience, knowledge, and expectations strongly influence the image sharpening effect in general. Rossel, Peyrin, Roux-Sibilon, and Kauffmann (2022) showed that the high-level contextual information can enhance the sharpening of blurry images and this effect seems to be modulated by the reliability of the sensory signal (Rossel, Peyrin, & Kauffmann, 2023; see also Press, Kok, & Yon, 2020). Similar results were found by Abdelhack and Kamitani (2018). They used decoded deep neural network features of functional magnetic resonance imaging activity to show sharpening of the neural representations of blurry images across the visual hierarchy. They also showed that adding a more specific prior increased sharpening. Interestingly, this top-down enhancement of blurry visual input was observed even in the absence of a memory or expectation prior.
Another mechanism that can bias peripheral appearance toward a sharper, regularized appearance is foveal extrapolation (e.g., Otten, Pinto, Paffen, Seth, & Kanai, 2017; for a review see Stewart et al. 2020). Although less relevant in this context where there is no continuity between peripheral and foveal information, it can involve extending the clarity of foveal information into the uncertain peripheral regions. A further common explanation suggests that our perception is often biased by a false, inflated belief that we observe more than we do. This is a type of metacognitive bias, which should be reflected in peripheral overconfidence (e.g., Odegaard, Chang, Lau, & Cheung, 2018; Solovey, Graney, & Lau, 2015). This overconfidence may arise from foveal information being reliably available within an eye movement (O'Regan & Noë, 2001). However, Toscani, Mamassian, and Valsecchi (2021) reported underconfidence in peripheral vision, and Gloriani and Schütz (2019) reported underconfidence even when the peripheral information was accurate and the foveal information was inferred.
It is plausible that metacognition could compensate partially for the limitations of peripheral vision; however, due to our inherent inability to resolve details in the periphery, it remains linked to higher levels of uncertainty. In a Bayesian framework, the greater uncertainty leads to a stronger weighting of the prior. Because the prior should come mostly from foveal experience with sharp edges, the overweighting of a prior could lead to the sharpening effect. This Bayesian explanation is consistent with the original template idea of Galvin and colleagues (Galvin et al., 1997; Galvin et al., 1999) and their results showing increased sharpening at higher eccentricities and shorter presentation durations, i.e., with increasing uncertainty. Perhaps simply changing the context from sharp to fuzzy edges is not enough to counteract the sharp edge prior (Galvin et al., 1999). From this perspective, our results may indicate that our blurry stimuli had more ambiguity and uncertainty than the distorted stimuli.
There are qualitative differences in the manipulation of distortion and blur. Distorting involved only edge bending, whereas blurring included a fixed level of distortion (Figure 2). This could not be avoided, because a certain amount of distortion was necessary to make the blur visible in the first place. Low-pass filtering blurs the image by preserving low spatial frequency information and discarding high spatial frequency information. In the case of the Eidolons Factory, the appearance of blurriness, or fuzziness, is produced by incoherent disarray across spatial scales, i.e., by generating partially independent displacement fields for each scale. Contrary to low-pass filtering, in principle, the edges remain visible at all spatial frequencies, but as a result of the incoherent disarray, they are diffused and appear blurry. The fact that edges in blurry eidolons are both fuzzy and distorted probably resulted in more perceptual uncertainty compared to the case where observers were exposed to eidolons that were entirely sharp and differed only in terms of distortion. Consequently, greater uncertainty may have contributed to the finding of a sharpening effect for blur and lower uncertainty to the absence of a regularization effect for distortion.
In the study of Valsecchi et al. (2018), participants were asked to match the foveal stimulus to a peripheral stimulus by navigating through the Eidolons Factory parameter space in four different directions, which represented slow morphing in both the reach and coherence parameters. Hence, the two parameters did not have an independent influence on the appearance of the eidolons. One could speculate that in their results, coherence may have had a more substantial effect on the overall regularization results.
Transsaccadic change discrimination
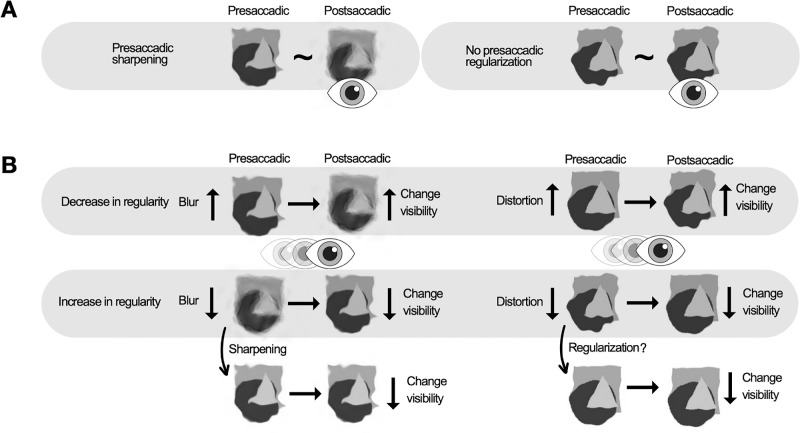
Biases were observed in reporting changes from low to high blur and from low to high distortion (although the distortion bias was significant only in the blank condition). Overall, a decrease in object regularity is detected more readily across saccades (Figure 7). One explanation is that sharpening creates a perceived regularization of the object before a saccade. If apparent regularized input before a saccade switches to actual regularized input after a saccade (decrease in blur and distortion), no change is perceived. Conversely, if the input is regular before a saccade and regularity decreases postsaccadically (increase in blur and distortion), the change becomes more noticeable.
Figure 7.
A schematic illustration of the results. (A) Results of the appearance discrimination. We found presaccadic sharpening effect for the blurry eidolons (left), but no significant presaccadic regularization for the distorted eidolons (right). (B) Results of the change discrimination. The top row shows the result that an increase in both blur (left) and distortion (right) is more noticeable, making a decrease in regularity more visible. The bottom row shows the result that a reduction in blur (left) and distortion (right) is less noticeable, making an increase in regularity more difficult to detect. This effect may be due to sharpening (effect found in appearance task) and regularization (no effect found in appearance task), which increase the perceived regularity of peripheral stimuli before a saccade, making it difficult to detect changes that increase regularity.
Hübner and Schütz (2021) observed that the perceived shape of triangle–circle morphs tends to be more circular before a saccade, aligning with the sharpening effect reported in this study. Hence, presaccadically, the shape appears less distorted. Their results indicated that the transsaccadic change increasing object circularity was more noticeable. Although one might expect that a change reducing circularity would be more noticeable, because the shape is already perceived as more circular in the periphery, their findings suggest otherwise. However, they also observed that the more pronounced the circularity appearance bias was, the less pronounced the circularity increase discrimination bias was.
We, in contrast, showed that a decrease in object regularity is easier to perceive; objects appear more regular before a saccade, and a physical increase in regularity does not produce a noticeable change. Thus, it does not seem to be enough to produce a pronounced separation of presaccadic and postsaccadic information (Tas, Moore, & Hollingworth, 2012). In contrast to Hübner and Schütz (2021), we could not find evidence for a correlation between appearance differences and biases in transsaccadic change discrimination. This lack of correlation may be attributed to the additional variability introduced by employing different eccentricities in the tasks (10 and 5 dva). Nevertheless, it is important to note that the absence of evidence for a correlation does not demonstrate its absence conclusively. For both tasks to be completed successfully, we needed stimulus regularity to be visible in the periphery. First, the performance accuracy of the two tasks differs and is eccentricity dependent. Comparing the regularity of a presaccadic stimulus with a changed postsaccadic stimulus at the same spatial location is more challenging than comparing the internal mean regularity of an unchanged stimulus within a single trial. In addition, the accuracy of change and appearance discrimination varies between the features and stimuli studied. While Hübner and Schütz (2021) and Sharvashidze et al. (2024) investigated both shape and spatial frequency discrimination at 15 dva, regularity change discrimination in eidolons (with the size of 2.56 dva) was not feasible until eidolons were presented at 5 dva, whereas appearance discrimination was possible at 10 dva (using the maximum range of coherence in the first experiment). Regarding the influence of eccentricity on biases in both tasks, it has been shown previously that appearance and change discrimination biases are unaffected by eccentricity (2AFC [15 dva] and spatial 2AFC [5 dva] tasks used by Hübner and Schütz, 2021). However, the present study investigated presaccadic sharpening, which is known to be an eccentricity-dependent effect and is expected to be less pronounced at lower eccentricities and with less uncertainty. Considering these inherent differences between the tasks and specificities of the effects investigated, we selected different eccentricities for each task, while maintaining a constant feature range across both.
Both the distortion and the blur contribute to our regularity decrease bias. If we look at the spatial frequency information that the stimuli carry along the change perception biases, distorted stimuli have higher spatial frequency information, whereas blurry stimuli have lower spatial frequency information. Despite the systematic differences in spatial frequency content in our stimuli across both experiments, we observed facilitated change discrimination for both blur and distortion increases. This finding suggests that spatial frequency alone is less relevant for the eidolons used in this study, probably indicating the importance of the overall appearance, the sum of different features. The circular compared to the triangular shape from Hübner and Schütz (2021) seems to be somewhere in between blur and distortion, being less distorted but having low spatial frequency information. On the other hand, Sharvashidze et al. (2024) reported a bias to perceive an increase of spatial frequency across saccades, while spatial frequency decrease perception seems to be hindered. Taken together, these findings display considerable heterogeneity, posing a challenge for a unified explanation. It should be noted that the effect of distortion as applied in the Eidolons Factory does not correspond directly to the continuum of the triangular–circular transformation (Hübner & Schütz, 2021), making a direct comparison of the results difficult. Also, when comparing the spatial frequency content of the stimuli, it is important to note that, in the Sharvashidze et al. (2024) study, the participants' task was to discriminate the spatial frequency changes of Gabors across saccades explicitly, distinguishing that particular task from the others. Looking at the cortical correlates, there seems to be a distributed network involved in feature discrimination across saccades. However, this system also seems to contain specific modules dedicated to different visual features (Baltaretu, Dunkley, Stevens, & Crawford, 2021; Baltaretu, Stevens, Freud, & Crawford, 2023). It is plausible that peripheral and foveal appearance and transsaccadic change discrimination exhibit large differences between different visual features.
Greater precision was found in the blank conditions of change discrimination for both distortion and blur. When the stimulus is blanked, presaccadic information becomes available and may not be integrated directly with (e.g., Ganmor, Landy, & Simoncelli, 2015; Stewart & Schütz, 2019a; Wijdenes, Marshall, & Bays, 2015; Wolf & Schütz, 2015) or overwritten by (e.g., Grzeczkowski, Deubel et al, 2020; Tas et al., 2021) the postsaccadic information. This finding means that, even if the presaccadic information was sharpened before a saccade and the change from high to low blur/distortion was difficult to notice, making the presaccadic information available through blank improved discrimination precision. These results are consistent with previous blanking findings (e.g., Deubel et al., 1996; Goktepe & Schütz, 2023; Grzeczkowski, Deubel et al., 2020; Grzeczkowski, van Leeuwen, et al., 2020; Hübner & Schütz, 2021; Poth & Schneider, 2016; Stewart, Hübner, & Schütz, 2020; Tas et al., 2021; Weiss et al., 2015). We observed a bias toward perceiving an increase in blur in both the blank and no blank conditions. However, the bias was smaller in the blank condition. This finding is consistent with our explanation for the bias. If the blank condition increases the availability of presaccadic information, it should prevent the sharpened presaccadic input from being immediately overwritten, thereby improving both the precision and accuracy of discrimination. In contrast, no difference was observed between the blank and no blank conditions for distortion. This result means that, although participants in the blank condition were more precise during distortion discrimination, they were not more accurate in their judgments.
One methodological specificity of the studies, including Hübner and Schütz, (2021), Sharvashidze et al. (2024) and the current study, is the presentation duration of the postsaccadic stimulus. In the change discrimination task, the postsaccadic stimulus durations were shorter compared to other studies investigating change detection with the blanking paradigm (Table A2). This was done to compensate for the lower reliability of the presaccadic information and to simplify the task. In particular, it is the only method that does not alter additional stimulus features (e.g., contrast, size) and does not provide an additional cue for a transsaccadic change (e.g., Tas et al., 2012). If the reliabilities are mismatched, the comparison of presaccadic and postsaccadic information becomes difficult because the more reliable postsaccadic information can completely dominate the transsaccadic percept (Ganmor et al., 2015; Tas et al., 2021; Wolf & Schütz, 2015). The use of shorter postsaccadic presentation durations is further justified by findings showing that even brief presaccadic and postsaccadic exposures (as short as 42 ms) are sufficient for transsaccadic integration to occur (Stewart & Schütz, 2019b). However, both presaccadic and postsaccadic foveal information processing periods may have some influence on change discrimination across saccades. In general, foveal information at the fixation location before a saccade contributes significantly to the processing of presaccadic target information. The delayed presentation of incongruent stimuli at the fovea can disrupt peripheral object detection (Fan, Wang, Shao, Kersten, & He, 2016; Goktepe & Schütz, 2024; Weldon, Rich, Woolgar, & Williams, 2016; for reviews see Oletto, Contemori, Bertamini, & Battaglini, 2023; Stewart et al., 2020). In addition, the sensitivity to target congruent features increases in the fovea before a saccade (Kroell & Rolfs, 2022b, Kroell & Rolfs, 2023). Furthermore, the spatial frequency content of foveally predicted information seems to follow the decrease in presaccadic target resolution as a function of increasing target eccentricity (Kroell & Rolfs, 2022a).
For the appearance discrimination task, we adopted the same presentation duration logic as for the change discrimination task. The sharpening bias has been demonstrated previously in fixation studies, thus with relatively long peripheral stimulus durations, because the matching tasks were self-terminated (Galvin et al., 1997; Valsecchi et al., 2018). In our study, presaccadic stimulus durations were determined by the typical saccade latencies, but postsaccadic stimulus durations were reduced (Table A1). Importantly, despite shorter postsaccadic viewing times, postsaccadic precision was still higher compared to presaccadic precision, for both blur and distortion discrimination (Figures 4B and 6B). However, because no other postsaccadic durations were tested and transsaccadic foveal processing varies in sensitivity throughout the oculomotor cycle (Boi, Poletti, Victor, & Rucci, 2017; Hanning & Deubel, 2022; Kroell & Rolfs, 2022b), it cannot be ruled out that the observed presaccadic sharpening bias is specific to the tested durations. Furthermore, although, as discussed elsewhere in this article, blur detection and discrimination differ in sensitivity and are not directly comparable; for blur detection, it has been shown that foveal presentation durations have some influence on detection thresholds (Westheimer, 1991). Also, unlike our experimental conditions, the duration of postsaccadic content in natural settings is not typically constrained. It is, therefore, unclear whether our findings can be generalized to other stimulus conditions or more naturalistic environments.
Conclusions
We manipulated blur and distortion of simple objects as two separate components of regularity with the Eidolons Factory (Koenderink et al., 2017). Using a paradigm that matched presaccadic and postsaccadic reliabilities by decreasing postsaccadic compared to presaccadic stimulus durations, we found that objects appeared less blurry, but not less distorted, in the periphery before a saccade compared to the fovea after a saccade. We speculate that either we were not able to match the perceptual certainty of the tasks perfectly, or that blur may have contributed more to Valsecchi et al., (2018) results. In their study, they simultaneously manipulated distortion and blur, revealing a regularization effect for both parameters.
Additionally, we found a bias toward perceiving a decrease in object regularity across saccades, specifically an increase in blur and distortion. If the object appears less regular in the periphery, this may affect the perception of changes from low to high regularity. The sharpening effect can increase apparent regularity in the periphery, minimizing the visibility of changes that contribute to an increase in regularity. This could help to hide the increase in (perceived) regularity when an object is brought from peripheral to foveal vision by a saccade.
Acknowledgments
The authors thank Joni Blume and Ala Alsaleh for their help with the data collection.
NS and ACS were supported by Deutsche Forschungsgemeinschaft (DFG), Project number 290878970-GRK 2271/Project 5. ACS was supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No. 676786) and by “The Adaptive Mind”, funded by the Excellence Program of the Hessian Ministry of Higher Education, Science, Research and Art. MV was supported by the Italian Ministry of University and Research under the PRIN 2022 programme, project number 2022HEKCWH.
Data availability: Psychophysical data and analysis scripts of the experiments are available at https://doi.org/10.5281/zenodo.10782962.
Commercial relationships: none.
Corresponding author: Nino Sharvashidze.
Email: nino_sh@hotmail.com.
Address: Sensomotoric Learning, Experimental & Biological Psychology, Department of Psychology, Philipps-Universität Marburg, Gutenbergstraße 18, 35032 Marburg, Germany.
Appendix
Table A1.
Stimulus eccentricity, saccade latency and duration, presaccadic and postsaccadic stimulus presentation times for all experimental conditions of both tasks and both experiments.
| Condition | Stimulus horizontal eccentricity (dva) | Saccade latency (ms) | Saccade duration (ms) | Presaccadic stimulus duration (ms) | Postsaccadic stimulus duration (ms) |
|---|---|---|---|---|---|
| Experiment 1 (blur) appearance discrimination | |||||
| All | 10 | 199 ± 49 | 54 ± 4 | 222 ± 49 | 105 ± 24 |
| Presaccadic | 10 | 179 ± 43 | 54 ± 4 | 202 ± 42 | 104 ± 24 |
| Postsaccadic | 10 | 219 ± 58 | 54 ± 4 | 243 ± 58 | 105 ± 25 |
| Experiment 1 (blur) change discrimination | |||||
| All | 5 | 201 ± 71 | 41 ± 3 | 225 ± 71 | 119 ± 36 |
| Blank | 5 | 200 ± 70 | 41 ± 3 | 223 ± 70 | 118 ± 36 |
| No blank | 5 | 203 ± 73 | 41 ± 3 | 227 ± 73 | 120 ± 36 |
| Experiment 2 (distortion) appearance discrimination | |||||
| All | 10 | 211 ± 66 | 55 ± 3 | 234 ± 66 | 107 ± 31 |
| Presaccadic | 10 | 189 ± 58 | 55 ± 3 | 212 ± 58 | 107 ± 31 |
| Postsaccadic | 10 | 234 ± 77 | 54 ± 3 | 258 ± 77 | 107 ± 32 |
| Experiment 2 (distortion) change discrimination | |||||
| All | 5 | 201 ± 65 | 40 ± 3 | 225 ± 65 | 119 ± 33 |
| Blank | 5 | 201 ± 66 | 40 ± 3 | 224 ± 66 | 118 ± 33 |
| No blank | 5 | 201 ± 64 | 40 ± 3 | 226 ± 65 | 120 ± 32 |
Notes: Values are means ± standard deviations rounded to zero decimal values, calculated across all trials for each participant and then averaged across all participants.
Table A2.
A selection of studies including change discrimination task with blanking condition.
| Stimulus | |||||
|---|---|---|---|---|---|
| Reference | Change discrimination feature | Type | Eccentricity (dva) | Blank duration (ms) | Postsaccadic duration (ms) |
| Deubel et al. (1996) | Displacement | Cross | 6 or 8 | 50–300 | Until response |
| Goktepe and Schütz (2023) | Object identity | Greebles | 10 | 300 | 300 |
| Grzeczkowski, Deubel, and Szinte (2020) | Orientation | Gratings | 8 | 200 | 300 |
| Grzeczkowski, van Leeuwen, et al. (2020) | Change within stimulus | Checkerboard-like patterns | 7 | 200 | Until response |
| Hübner and Schütz (2021) | Shape | Triangle—circular morphs | 15 | 200 | Half of the presaccadic duration |
| Irwin and Robinson (2018) | Displacement | Cross | 6 or 8 | 300 | Until response |
| Stewart, Hübner, and Schütz (2020) | Displacement | Cartoon animals | 8 or 10 | 300 | 400 |
| Takano et al. (2020) | Displacement | Disk | 8.9 | 100 | 300 |
| Tas et al. (2012) | Displacement | Disk | 6 to 8 | 250 | Until response |
| Tas et al. (2012) | Displacement | Objects | 6 to 8 | 250 | Until response |
| Tas et al. (2021) | Color | Disk | 5 to 7 | 250 | Presaccadic duration |
| Tas and Parker (2023) | Displacement | Disk | 5 to 7 | 250 | Until response |
| Weiss et al. (2015) | Spatial frequency | Gratings | 6 | 250 | 250 |
| Wexler and Collins (2014) | Displacement | Squares | 6 to 8 | 200 | 600 |
| Current study | Blur and distortion | Eidolons | 5 | 200 | Half of the presaccadic duration |
Figure A1.
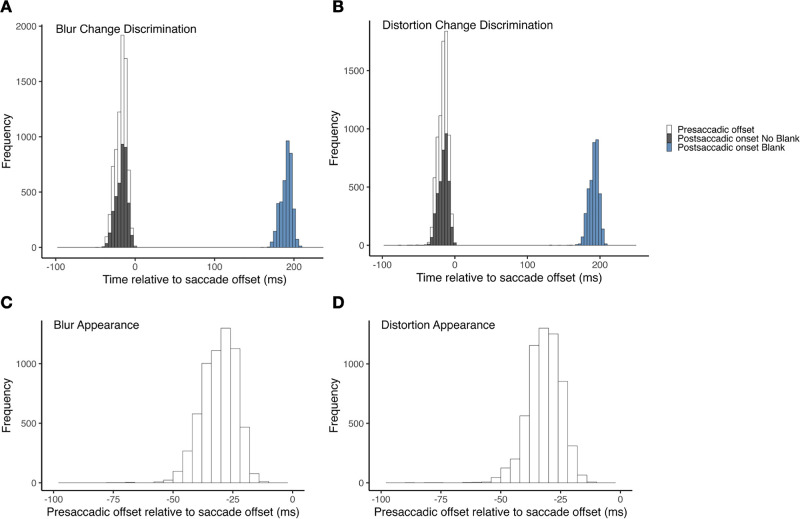
Illustration of stimulus timings. (A) Presaccadic stimulus offset and postsaccadic stimulus onset (blank and no blank conditions) relative to saccade offset (ms) in the blur change discrimination task. (B) Presaccadic stimulus offset and postsaccadic stimulus onset (blank and no blank conditions) relative to saccade offset (ms) in the distortion change discrimination task. (C) Presaccadic stimulus offset (= postsaccadic stimulus onset in appearance discrimination task) in the blur change discrimination task. (D) Presaccadic stimulus offset (= postsaccadic stimulus onset in the appearance discrimination task) in the blur change discrimination task.
References
- Abdelhack, M., & Kamitani, Y. (2018). Sharpening of hierarchical visual feature representations of blurred images. Eneuro, 5(3), ENEURO.0443–0417.2018, 10.1523/eneuro.0443-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas, B., Nakano, L., & Rosenholtz, R. (2009). A summary-statistic representation in peripheral vision explains visual crowding. Journal of Vision, 9(12), 13, 10.1167/9.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltaretu, B. R., Dunkley, B. T., Stevens, W. D., & Crawford, J. D. (2021). Occipital cortex is modulated by transsaccadic changes in spatial frequency: An fMRI study. Scientific Reports, 11(1), 8611, 10.1038/s41598-021-87506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltaretu, B. R., Stevens, W. D., Freud, E., & Crawford, J. D. (2023). Occipital and parietal cortex participate in a cortical network for transsaccadic discrimination of object shape and orientation. Scientific Reports, 13(1), 11628, 10.1038/s41598-023-38554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bex, P. J. (2010). (In) Sensitivity to spatial distortion in natural scenes. Journal of Vision, 10(2), 23, 10.1167/10.2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boi, M., Poletti, M., Victor, J. D., & Rucci, M. (2017). Consequences of the oculomotor cycle for the dynamics of perception. Current Biology, 27(9), 1268–1277, 10.1016/j.cub.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard, D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Ciuffreda, K. J., Wang, B., & Vasudevan, B. (2007). Conceptual model of human blur perception. Vision Research, 47(9), 1245–1252, 10.1016/j.visres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Cohen, M. A., Dennett, D. C., & Kanwisher, N. (2016). What is the bandwidth of perceptual experience? Trends in Cognitive Sciences, 20(5), 324–335, 10.1016/j.tics.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen, F. W., Peters, E. M., & Palmer, J. (2002). The Eyelink Toolbox: Eye tracking with MATLAB and the Psychophysics Toolbox. Behavior Research Methods, Instruments, & Computers, 34(4), 613–617, 10.3758/BF03195489. [DOI] [PubMed] [Google Scholar]
- Derrington, A. M., Krauskopf, J., & Lennie, P. (1984). Chromatic mechanisms in lateral geniculate nucleus of macaque. Journal of Physiology, 357, 241–265, 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel, H., & Schneider, W. X. (1996). Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research, 36(12), 1827–1837, 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Deubel, H., Schneider, W. X., & Bridgeman, B. (1996). Postsaccadic target blanking prevents saccadic suppression of image displacement. Vision Research, 36(7), 985–996, 10.1016/0042-6989(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Fan, X., Wang, L., Shao, H., Kersten, D., & He, S. (2016). Temporally flexible feedback signal to foveal cortex for peripheral object recognition. Proceedings of the National Academy of Sciences of the United States of America, 113(41), 11627–11632, 10.1073/pnas.1606137113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, J., & Simoncelli, E. P. (2011). Metamers of the ventral stream. Nature Neuroscience, 14(9), 1195–1201, 10.1038/nn.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin, S. J., O'Shea, R. P., Squire, A. M., & Govan, D. G. (1997). Sharpness overconstancy in peripheral vision. Vision Research, 37(15), 2035–2039, 10.1016/S0042-6989(97)00016-3. [DOI] [PubMed] [Google Scholar]
- Galvin, S. J., O'Shea, R. P., Squire, A. M., & Hailstone, D. S. (1999). Sharpness overconstancy: The roles of visibility and current context. Vision Research, 39(16), 2649–2657, 10.1016/s0042-6989(98)00306-x. [DOI] [PubMed] [Google Scholar]
- Galvin, S. J., & Williams, D. R. (1992). No aliasing at edges in normal viewing. Vision Research, 32(12), 2251–2259, 10.1016/0042-6989(92)90089-2. [DOI] [PubMed] [Google Scholar]
- Ganmor, E., Landy, M. S., & Simoncelli, E. P. (2015). Near-optimal integration of orientation information across saccades. Journal of Vision, 15(16), 8, 10.1167/15.16.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloriani, A. H., & Schütz, A. C. (2019). Humans trust central vision more than peripheral vision even in the dark. Current Biology, 29(7), 1206–1210.e1204, 10.1016/j.cub.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goktepe, N., & Schütz, A. C. (2023). Familiar objects benefit more from transsaccadic feature predictions. Attention Perception Psychophysics, 85(6), 1949–1961, 10.3758/s13414-022-02651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goktepe, N., & Schütz, A. C. (2024). Frequency-specific and periodic masking of peripheral characters by delayed foveal input. Scientific Reports, 14(1), 4642, 10.1038/s41598-024-51710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeczkowski, L., Deubel, H., & Szinte, M. (2020). Stimulus blanking reveals contrast-dependent transsaccadic feature transfer. Scientific Reports, 10(1), 18656, 10.1038/s41598-020-75717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeczkowski, L., van Leeuwen, J., Belopolsky, A. V., & Deubel, H. (2020). Spatiotopic and saccade-specific transsaccadic memory for object detail. Journal of Vision, 20(7), Article 2, 10.1167/jov.20.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning, N. M., & Deubel, H. (2022). The effect of spatial structure on presaccadic attention costs and benefits assessed with dynamic 1/f noise. Journal of Neurophysiology, 127(6), 1586–1592, 10.1152/jn.00084.2022. [DOI] [PubMed] [Google Scholar]
- Haun, A. M. (2021). What is visible across the visual field? Neuroscience of Consciousness, 2021(1): naib006, 10.1093/nc/niab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner, C., & Schütz, A. C. (2017). Numerosity estimation benefits from transsaccadic information integration. Journal of Vision, 17(13), 12, 10.1167/17.13.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner, C., & Schütz, A. C. (2021). A bias in saccadic suppression of shape change. Vision Res, 186, 112–123, 10.1016/j.visres.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin, D. E., & Robinson, M. M. (2018). How post-saccadic target blanking affects the detection of stimulus displacements across saccades. Vision Research, 142, 11–19, 10.1016/j.visres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Knotts, J. D., Odegaard, B., Lau, H., & Rosenthal, D. (2019). Subjective inflation: Phenomenology's get-rich-quick scheme. Current Opinion in Psychology, 29, 49–55, 10.1016/j.copsyc.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderink, J., Valsecchi, M., van Doorn, A., Wagemans, J., & Gegenfurtner, K. (2017). Eidolons: Novel stimuli for vision research. Journal of Vision, 17(2), 7, 10.1167/17.2.7. [DOI] [PubMed] [Google Scholar]
- Kroell, L. M., & Rolfs, M. (2021). The peripheral sensitivity profile at the saccade target reshapes during saccade preparation. Cortex, 139, 12–26, 10.1016/j.cortex.2021.02.021. [DOI] [PubMed] [Google Scholar]
- Kroell, L. M., & Rolfs, M. (2022a). Foveal prediction of saccade target features alters visual resolution in the center of gaze. Journal of Vision, 22(14), 3337, 10.1167/jov.22.14.3337. [DOI] [Google Scholar]
- Kroell, L. M., & Rolfs, M. (2022b). Foveal vision anticipates defining features of eye movement targets. eLife, 11, e78106, 10.7554/eLife.78106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroell, L. M., & Rolfs, M. (2023). The magnitude and time course of pre-saccadic foveal prediction depend on the conspicuity of the saccade target. Laurel Hollow, NJ: Cold Spring Harbor Laboratory. [Google Scholar]
- Kwak, Y., Hanning, N. M., & Carrasco, M. (2023). Presaccadic attention sharpens visual acuity. Scientific Reports, 13(1), 2981, 10.1038/s41598-023-29990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H.-H., Barbot, A., & Carrasco, M. (2016). Saccade preparation reshapes sensory tuning. Current Biology, 26(12), 1564–1570, 10.1016/j.cub.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H.-H., Pan, J., & Carrasco, M. (2019). Presaccadic attention improves or impairs performance by enhancing sensitivity to higher spatial frequencies. Scientific Reports, 9(1), 2659, 10.1038/s41598-018-38262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares, D., & López-Moliner, J. (2016). quickpsy: An R package to fit psychometric functions for multiple groups. R Journal, 8, 122. [Google Scholar]
- Maiello, G., Walker, L., Bex, P. J., & Vera-Diaz, F. A. (2017). Blur perception throughout the visual field in myopia and emmetropia. Journal of Vision, 17(5), 3, 10.1167/17.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey, R. D., & Rouder, J. N. (2024). BayesFactor: Computation of bayes factors for common designs. (R package version 0.9.12-4.7), https://CRAN.R-project.org/package=BayesFactor.
- Morgan, M. J., Watamaniuk, S. N. J., & McKee, S. P. (2000). The use of an implicit standard for measuring discrimination thresholds. Vision Research, 40(17), 2341–2349, 10.1016/S0042-6989(00)00093-6. [DOI] [PubMed] [Google Scholar]
- O'Regan, J. K., & Noë, A. (2001). A sensorimotor account of vision and visual consciousness. Behavioral and Brain Sciences, 24(5), 939, 10.1017/s0140525x01000115. [DOI] [PubMed] [Google Scholar]
- Odegaard, B., Chang, M. Y., Lau, H., & Cheung, S.-H. (2018). Inflation versus filling-in: Why we feel we see more than we actually do in peripheral vision. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1755), 20170345, 10.1098/rstb.2017.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oletto, C. M., Contemori, G., Bertamini, M., & Battaglini, L. (2023). The role of foveal cortex in discriminating peripheral stimuli: The sketchpad hypothesis. NeuroSci, 4(1), 9–17. https://www.mdpi.com/2673-4087/4/1/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten, M., Pinto, Y., Paffen, C. L. E., Seth, A. K., & Kanai, R. (2017). The uniformity illusion: Central stimuli can determine peripheral perception. Psychological Science, 28(1), 56–68, 10.1177/0956797616672270. [DOI] [PubMed] [Google Scholar]
- Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442. [PubMed] [Google Scholar]
- Portilla, J., & Simoncelli, E. P. (2000). A parametric texture model based on joint statistics of complex wavelet coefficients. International Journal of Computer Vision, 40(1), 49–71, 10.1023/a:1026553619983. [DOI] [Google Scholar]
- Poth, C. H., & Schneider, W. X. (2016). Breaking object correspondence across saccades impairs object recognition: The role of color and luminance. Journal of Vision, 16(11), 1, 10.1167/16.11.1. [DOI] [PubMed] [Google Scholar]
- Press, C., Kok, P., & Yon, D. (2020). The perceptual prediction paradox. Trends in Cognitive Sciences, 24(1), 13–24, 10.1016/j.tics.2019.11.003. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2023). R: A language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing. https://www.r-project.org. [Google Scholar]
- Rayner, K. (1998). Eye movements in reading and information processing: 20 years of research. Psychological Bulletin, 124(3), 372–422, 10.1037/0033-2909.124.3.372. [DOI] [PubMed] [Google Scholar]
- Rosenholtz, R. (2016). Capabilities and limitations of peripheral vision. Annual Review of Vision Science, 2(1), 437–457, 10.1146/annurev-vision-082114-035733. [DOI] [PubMed] [Google Scholar]
- Rossel, P., Peyrin, C., & Kauffmann, L. (2023). Subjective perception of objects depends on the interaction between the validity of context-based expectations and signal reliability. Vision Research, 206, 108191, 10.1016/j.visres.2023.108191. [DOI] [PubMed] [Google Scholar]
- Rossel, P., Peyrin, C., Roux-Sibilon, A., & Kauffmann, L. (2022). It makes sense, so I see it better! Contextual information about the visual environment increases its perceived sharpness. Journal of Experimental Psychology: Human Perception and Performance, 48(4), 331–350, 10.1037/xhp0000993. [DOI] [PubMed] [Google Scholar]
- Rovamo, J., Virsu, V., & Näsänen, R. (1978). Cortical magnification factor predicts the photopic contrast sensitivity of peripheral vision. Nature, 271(5640), 54–56, 10.1038/271054a0. [DOI] [PubMed] [Google Scholar]
- Sharvashidze, N., Hübner, C., & Schütz, A. C. (2024). A bias in transsaccadic perception of spatial frequency changes. Vision Research, 222, 108453, 10.1016/j.visres.2024.108453. [DOI] [PubMed] [Google Scholar]
- Simmers, A. J., Bex, P. J., & Hess, R. F. (2003). Perceived blur in amblyopia. Investigative Ophthalmology & Visual Science, 44(3), 1395–1400, 10.1167/iovs.02-0625. [DOI] [PubMed] [Google Scholar]
- Solovey, G., Graney, G. G., & Lau, H. (2015). A decisional account of subjective inflation of visual perception at the periphery. Attention, Perception Psychophysics, 77(1), 258–271, 10.3758/s13414-014-0769-1. [DOI] [PubMed] [Google Scholar]
- Stewart, E. E. M., Hübner, C., & Schütz, A. C. (2020). Stronger saccadic suppression of displacement and blanking effect in children. Journal of Vision, 20(10), 13, 10.1167/jov.20.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, E. E. M., & Schütz, A. C. (2019a). Transsaccadic integration benefits are not limited to the saccade target. Journal of Neurophysiology, 122(4), 1491–1501, 10.1152/jn.00420.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, E. E. M., & Schütz, A. C. (2019b). Transsaccadic integration is dominated by early, independent noise. Journal of Vision, 19(6), 17, 10.1167/19.6.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, E. E. M., Valsecchi, M., & Schütz, A. C. (2020). A review of interactions between peripheral and foveal vision. Journal of Vision, 20(12), 2, 10.1167/jov.20.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger, H., Rentschler, I., & Jüttner, M. (2011). Peripheral vision and pattern recognition: A review. Journal of Vision, 11(5), 13, 10.1167/11.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, S., Matsumiya, K., Tseng, C.-h., Kuriki, I., Deubel, H., & Shioiri, S. (2020). Displacement detection is suppressed by the post-saccadic stimulus. Scientific Reports, 10(1), 9273, 10.1038/s41598-020-66216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas, A. C., Moore, C. M., & Hollingworth, A. (2012). An object-mediated updating account of insensitivity to transsaccadic change. Journal of Vision, 12(11), 18, 10.1167/12.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas, A. C., Mordkoff, J. T., & Hollingworth, A. (2021). Object-mediated overwriting across saccades. Journal of Vision, 21(2), 3, 10.1167/jov.21.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas, A. C., & Parker, J. L. (2023). The role of color in transsaccadic object correspondence. Journal of Vision, 23(8), 5, 10.1167/jov.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler, L., Schütz, A. C., Goodale, M. A., & Gegenfurtner, K. R. (2013). What is the best fixation target? The effect of target shape on stability of fixational eye movements. Vision Res, 76, 31–42, 10.1016/j.visres.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Toscani, M., Mamassian, P., & Valsecchi, M. (2021). Underconfidence in peripheral vision. Journal of Vision, 21(6), 2, 10.1167/jov.21.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi, M., Koenderink, J., van Doorn, A., & Gegenfurtner, K. R. (2018). Prediction shapes peripheral appearance. Journal of Vision, 18(13), 21, 10.1167/18.13.21. [DOI] [PubMed] [Google Scholar]
- Wang, B., & Ciuffreda, K. J. (2004). Depth-of-focus of the human eye in the near retinal periphery. Vision Research, 44(11), 1115–1125, 10.1016/j.visres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wang, B., & Ciuffreda, K. J. (2005). Blur discrimination of the human eye in the near retinal periphery. Optometry and Vision Science, 82(1), 52–58. [PubMed] [Google Scholar]
- Wang, B., Ciuffreda, K. J., & Irish, T. (2006). Equiblur zones at the fovea and near retinal periphery. Vision Research, 46(21), 3690–3698, 10.1016/j.visres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Weiss, K., Schneider, W. X., & Herwig, A. (2015). A “blanking effect” for surface features: Transsaccadic spatial-frequency discrimination is improved by postsaccadic blanking. Attention Perception Psychophysics, 77(5), 1500–1506, 10.3758/s13414-015-0926-1. [DOI] [PubMed] [Google Scholar]
- Weldon, K. B., Rich, A. N., Woolgar, A., & Williams, M. A. (2016). Disruption of foveal space impairs discrimination of peripheral objects. Frontiers in Psychology, 7, Article 699, 10.3389/fpsyg.2015.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer, G. (1991). Sharpness discrimination for foveal targets. Journal of the Optical Society of America A, 8(4), 681–685, 10.1364/JOSAA.8.000681. [DOI] [PubMed] [Google Scholar]
- Wexler, M., & Collins, T. (2014). Orthogonal steps relieve saccadic suppression. Journal of Vision, 14(2), 13, 10.1167/14.2.13. [DOI] [PubMed] [Google Scholar]
- Wijdenes, L. O., Marshall, L., & Bays, P. M. (2015). Evidence for Optimal Integration of Visual Feature Representations across Saccades. Journal of Neuroscience, 35(28), 10146–10153, 10.1523/jneurosci.1040-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, C., & Schütz, A. C. (2015). Transsaccadic integration of peripheral and foveal feature information is close to optimal. Journal of Vision, 15(16), 1, 10.1167/15.16.1. [DOI] [PubMed] [Google Scholar]