Summary
Schizophrenia, a severe psychiatric, neurodevelopmental disorder affecting about 0.29–1 % of the global population, is characterized by hallucinations, delusions, cognitive impairments, disorganized thoughts and speech, leading to significant social withdrawal and emotional blunting. During the 1980s, considerations about diseases that result from complex interactions of genetic background and environmental factors started to appear. One of the critical times of vulnerability is the perinatal period. Concerning schizophrenia, obstetric complications that are associated with hypoxia of the fetus or neonate were identified as a risk. Also, maternal infections during pregnancy were linked to schizophrenia by epidemiological, serologic and genetic studies. Research efforts then led to the development of experimental models testing the impact of perinatal hypoxia or maternal immune activation on neurodevelopmental disorders. These perinatal factors are usually studied separately, but given that the models are now validated, it is feasible to investigate both factors together. Inclusion of additional factors, such as metabolic disturbances or chronic stress, may need to be considered also. Understanding the interplay of perinatal factors in schizophrenia’s etiology is crucial for developing targeted prevention and therapeutic strategies.
Keywords: Schizophrenia, Perinatal hypoxia, Perinatal infection, Microbiota, SARS-CoV-2
Introductory remarks on Professor Jan Herget’s contribution to understanding developmental disorders
In this Special issue of Physiological Research, we celebrate the legacy of Professor Jan Herget (1945–2019) of the Second Faculty of Medicine, Charles University in Prague, Czech Republic. Although this review focuses on perinatal factors in schizophrenia, it is pertinent to remember Jan’s unique contributions to uncovering the long-term effects of perinatal hypoxemia on responses to decreased oxygen in adulthood [1].
In the pioneering experiments, pregnant rats were placed into the hypoxic chambers and were kept there until their offspring were a week old. After placing the animals into the normoxic air, they recovered from hypoxia and had comparable pressure in pulmonary circulation as control mice unexposed to perinatal hypoxia. However, when these animals were re-exposed to acute hypoxia in adulthood, their responses were more severe than in animals born in normal air [1]. The perinatal exposure to hypoxia also blunted humoral immune responses in adult rats [2]. The mechanisms of these intriguing, lifelong-lasting effects are not yet fully understood. [3]
Jan received the Robert F. Grower Prize from the American Thoracic Society for his research work. He was also an astounding teacher and mentor who profoundly impacted his students and research trainees. His vision that the course of a disease processes needs to be studied from a very early time in life took another dimension with models where perinatal hypoxia and maternal immune activation were identified as critical players in neurodevelopmental conditions.
Perinatal exposures and risk for neurodevelopmental conditions
Many perinatal factors influence the fetus during the perinatal period and affect the inherently complex and highly active brain tissue. For example, perinatal exposures that are related to increased risk of schizophrenia include conditions that cause hypoxemia, infections (especially influenza, rubella and toxoplasmosis) [4,5], maternal and offspring psychosocial stress [6], genetic factors [7], advanced paternal age [8], nutritional deficiencies [9], urbanicity [10] and migration status [11].
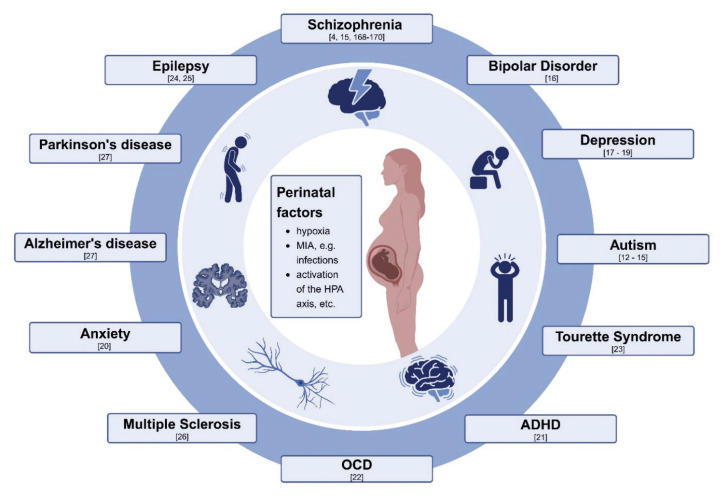
Vice versa, if we look at one group factors, e.g., maternal immune activation (MIA) initiated by viruses, bacteria, fungi, autoimmune conditions, they can play a role in a variety of childhood- or adulthood-onset of disorders, as depicted in Figure 1. They include autism [12–15], schizophrenia [4,15,168–170], bipolar disorder [16], depression [17–19], anxiety disorder [20], attention deficit-hyperreactivity disorder [21], obsessive-compulsive disorder [22], Tourette’s syndrome [23], epilepsy [24,25], multiple sclerosis [26], Parkinson’s disease and Alzheimer disease [27].
Fig. 1.
Multiple factors acting perinatally can increase a risk for neurodevelopmental conditions that may manifest during childhood and adulthood. The picture was made with using a Biorender template.
The precise mechanisms by which individual perinatal factors increase susceptibility to various neuropsychiatric conditions remain incompletely understood. The interplay of genetic predisposition, the timing of exposure, and the intensity of these factors collectively determine the outcomes. Elucidating these intricate details presents a formidable intellectual challenge. Resolving this complexity is crucial for identifying diagnostic biomarkers that enable clinicians to detect individuals at elevated risk for these conditions and to provide care strategies that may mitigate the likelihood of disease development.
In clinical psychiatry, diagnoses are based on symptoms and co-morbidities are a very common finding. In turn, making accurate diagnoses is arduous. Significant efforts are ongoing to define objective biomarkers that reflect distinct pathological processes, though they present by mixed behavioral symptoms. Understanding the pathophysiology is a prerequisite for diagnostic precision and enhancing effectiveness of treatment of schizophrenic patients [28].
We will focus here on hypoxia and perinatal immune activation as risk factors for schizophrenia. These two conditions have a high prevalence globally and often coincide, and the recent SARS-CoV-2 pandemic, or Severe Acute Respiratory Syndrome Coronavirus 2, very likely enhanced MIA in pregnant women during the pandemic.
Introduction to schizophrenia
In this selective review, we will focus on one of the neurodevelopmental disorders: schizophrenia, a severe psychiatric illness affecting about 0.29 % to 1 % of people worldwide. In the age group of 15–24 year-old individuals, it is the third most frequent mental disease, after anxiety and depression [29,30]. Patients experience symptoms such as hallucinations, delusions, cognitive impairment, disorganized speech and thought processes, which are categorized as “positive symptoms” of the disease. Their altered perception of reality often provokes severe anxiety and leaves them in profound loneliness. Patients have reduced expressions of emotions and typically withdraw from social contact. These symptoms are labeled as “negative symptoms”, and are more difficult to control by pharmacological treatment. Besides the impact of the disease on affected individuals, there is also a collateral toll on patient’s caregivers, families, friends, and colleagues [31–35]. In addition, the annual societal cost is high [36].
The pathogenic disturbances leading to schizophrenia syndrome result from interactions of multiple genetic and environmental factors [37] that start affecting brain circuitries during brain development [38], though the symptoms manifest in late adolescence to early adulthood. Most patients are affected between 15–25 years of age, with males having an earlier onset than females. In about 20 % of patients, the onset of schizophrenia occurs after forty and before 60 years, and rarely also, the disorder begins in childhood or adults above 60 years of age [29]. The differences in age of onset may reflect distinct pathogenic pathways or accumulation of causative factors, as suggested by the multiple-hit hypothesis [39].
Diagnosis of schizophrenia is still based on clinical symptoms because no objective, validated biomarkers exist for clinical use. As a result, diagnosis is not based on mechanistic principles; existing treatments are non-curative, and 30 % of patients remain resistant to existing therapeutics [40]. A better understanding of pathogenesis will help us subtyping patients into groups according to distinct pathological processes in individual patients and treat patients with high precision, as it has already been developed for cancer patients [41]. To this end, we focus here on the early events that increase the chance of schizophrenia development. Extensive research suggests that schizophrenia is a neurodevelopmental disorder, with the pathological processes potentially commencing as early as the in-utero stage [42,43], and progressing to a neurodegenerative condition [44].
Pathophysiology of schizohrenia
Reasons for poor understanding of schizophrenia include the high heterogeneity of patients, the inaccessibility of human brain tissue for biopsy sampling, and the involvement of animal models that do not fully capture the polygenic nature and multitude of environmental factors [45–48]. Our understanding of schizophrenia’s pathophysiology has been to a large degree based on postmortem brain studies, imaging studies, effects of pharmacotherapy and genetic studies.
Morphological considerations
Postmortem brain studies
Studies on brain pathology in schizophrenia initially focused on post-mortem brain specimens of individuals with schizophrenia. One major challenge with postmortem studies is the variability in findings due to factors such as the stage of the disease, medication status, and comorbid conditions at the time of death. Additionally, postmortem changes and tissue preservation issues can complicate interpretations. Despite these challenges, a consistent pattern has been identified in patients with chronic schizophrenia. Macroscopic findings include an enlargement of lateral and third ventricles, reduced brain volume, reduced gray matter involving the cortex, especially the prefrontal cortex, as well as changes in subcortical gray matter (decreased volumes of hippocampus and thalamus, and increased basal ganglia volume) [49,50].
At the microscopic level, the altered density of neuronal and glial cells was reported in the prefrontal cortex, as well as reduced size of pyramidal neurons, reduced number of parvalbumin interneurons, decreased dendritic spine density and reduced neuropil (regions in the gray matter with dense, interwoven network of axons, dendrites, and glial cells), which contribute to disruptions in synaptic connectivity and plasticity [51–56]. In the hippocampus, findings in brains of patients with schizophrenia included neuronal disarray, reduced numbers of neurons and interneurons and decreased gene and protein expression of somatostatin-positive and parvalbumin-positive interneurons [57]. In the thalamus, numbers of neurons and parvalbumin positive interneurons were reduced [58]. Basal ganglia in individuals with schizophrenia are characterized by changes in the density of neurons and cholinergic interneurons [59]. In the corpus callosum, reduced myelin, axon density, and gliosis were noted [60].
Brain imaging
The macroscopic structural findings in schizophrenia were later confirmed in in vivo brain imaging studies [61]. Structural changes in the gray matter include reduced volume of frontal, prefrontal and temporal cortices with progressive loss over the course of the disease [62]. With regard to the white matter, a smaller total volume at a later stage of the disease [63] and hypoactive connectivity in major networks were reported [64].
Brain imaging also enabled the study of patients at different stages of the disease. Comparing individuals at prodromal state, first-episode of psychosis (FEP) or chronic schizophrenia revealed the progressive nature of brain pathology.
Ultra-high risk (UHR) individuals at prodromal state are people with personal features (e.g., subthreshold psychotic symptoms or a history of self-limiting brief intermittent psychotic symptoms) or a family history (e.g. first degree relative with a psychotic disorder) that puts them into a risk of developing a full-blown psychotic disorder. UHR individuals show early signs of anatomical and neuropathological abnormalities, e.g., reduction in frontal, prefrontal, and temporal cortices, often subtler than in FEP, affecting cortical and subcortical gray matter [65]. In FEP, prefrontal hypoactivity and hippocampal and subcortical hyperreactivity were described [66]. Gray matter changes are most robust within thalamocortical networks and brain activity is most altered in fronto-parietal circuitries [66]. In chronic schizophrenia, reduced gray matter involving the cortex (frontal, prefrontal, temporal), decreased volumes of hippocampus and thalamus, and increased basal ganglia volume were described [62].
Neurochemical considerations
Understanding of biochemical disturbances in schizophrenia has been evolving since 1950s when it was discovered that chlorpromazine has neuroleptic effects during anesthesia (Laborit et al.), and later shown to improve symptoms of schizophrenia [67]. Chlorpromazine effects include dopamine receptor inhibition [68]. Studies of postmortem brain showed dysregulation of dopamine synthesis, receptor expression or intracellular signaling in prefrontal and cingulate corte [69,70], hippocampus [69], thalamus [71] and basal ganglia [72,73]. Later it became clear that mono-amine theory of schizophrenia is over-simplified and pathophysiology involves also alterations in glutamatergic and GABAergic systems [74]. In addition, the effectiveness of several medications provided further clinical insight into the roles of neurotransmitters in the pathogenesis of schizophrenia, e.g., blockade of dopamine D2 receptors by a typical antipsychotic (e.g. haloperidol), serotonin 5-hydroxytryptamine (5-HT)-2A receptor inhibition, and 5-HT-21A receptor activation by atypical antipsychotics (e.g. risperidone) and partial glutamatergic agents, such as N-methyl D-aspartate (NMDA) receptor modulators [75,76]. Also, adjunctive treatment of schizophrenia has involved medications affecting GABAergic system, including benzodiazepines, though their use may need to be honed [77].
Genetic factors
Genome-wide association studies revealed over three hundred genes that represent risk factors for schizophrenia [78]. Twin and family studies showed that heritable risk for schizophrenia is about 67 %, which may be inflated due to common environmental conditions of the participants in the study, and thus environmental factors play significant role in development of the disease [79]. Concerning the perinatal factors discussed in this review, risk genes in pathways relevant to hypoxia [80,81] immune [82,83] and gut microbioma [84] responses are significantly enriched.
The relation of perinatal hypoxia to schizophrenia
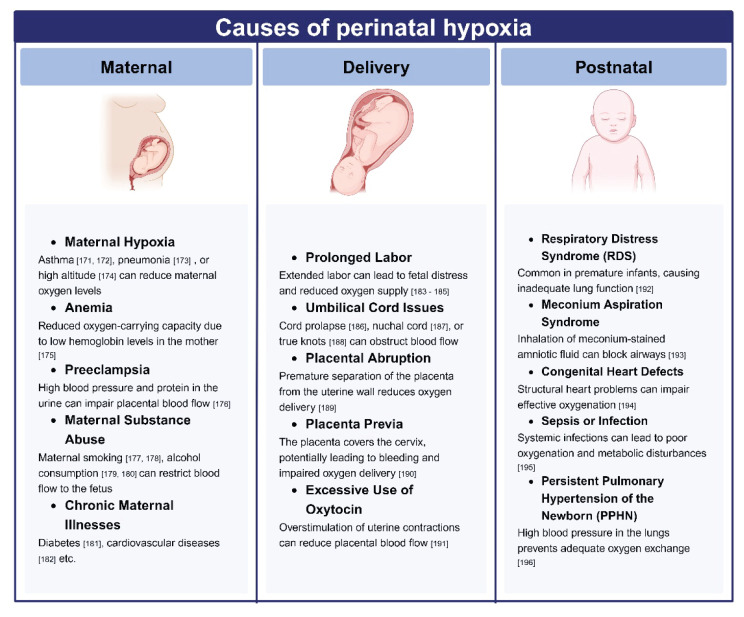
Many conditions are associated with various degrees of maternal and fetal hypoxemia (Fig. 2). Perinatal hypoxia exerts extensive effects on the brain’s histopathology, neurophysiology, and long-term health outcomes. The impact of hypoxemia on brain cells is modulated by the degree and duration of hypoxia. Mild hypoxia may induce reversible cellular changes, leading to adaptations and possibly alterations in transcription programs to cope with reduced ATP synthesis. In contrast, severe or prolonged hypoxia can result in irreversible damage and cell death. Additionally, the effects of hypoxia are influenced by the activity at a specific brain site and time, which is complex in healthy infants and in premature babies.
Fig. 2.
Maternal, fetal and neonatal causes of decreased oxygen content in the blood supplying offspring’s brain. The picture was made with using a Biorender template.
Clinical findings relating perinatal hypoxia to schizophrenia
The hypothesis that the development of psychotic conditions may be associated with brain hypoxia in the perinatal period was formulated in 1975 by a child psychiatrist, H. Allen Handford, based on the clinical history of patients seen in his practice, highlighting at that time that additional factors than genetics are at play [85].
Epidemiological studies then showed that hypoxia-associated obstetric complications significantly increase the risk for schizophrenia with early onset, before the age of 22 years (odds ratio, OR, 2.16) [86], and the degree of hypoxia-associated obstetric complications correlates with risk for developing schizophrenia [87]. After adjusting for other obstetric complications (e.g. maternal history of psychotic illness and social class), the association between signs of asphyxia at birth and schizophrenia reached odds ratio (OR) 4.4 [88].
Concerning the impact of perinatal hypoxia on the brain tissue of newborn, brain imaging studies revealed that the most susceptible areas include injuries within watershed areas, hippocampus, basal ganglia, thalamus, hippocampus and white matter. The impact depends on maturation stages of the brain [89]. Prefrontal cortex that is consistently linked to schizophrenia, is supplied by both anterior and middle cerebral arteries and includes the watershed area between them, making it sensitive to hypoxemia. Prefrontal cortex is also connected to the subcortical nuclei, e.g. thalamus, hippocampus, striatum, and hypoxia-induced white matter injury may affect the connectivity. Also, postmortem brain analysis of the posterior hippocampus in patients with schizophrenia revealed a negative correlation between events associated with hypoxia and the numbers of pyramidal cells in CA4, a deep polymorphic layer of dentate gyrus [90]. Correspondingly, brain imaging studies in patients with psychotic disorder uncovered a decreased hippocampal volume in individuals with schizophrenia as compared to healthy controls, and those volumes were further decreased in patients with hypoxia events in their early life [91]. In summary, hypoxic insults, though highly variable in their strength and timing, appear to impact many areas shown to be affected also in schizophrenia.
It was suggested that the level of susceptibility to hypoxia may depend on genetic background. To this end, Nicodemus et al. tested thirteen hypoxia-regulated genes related to neurovascular functions. Changes in single nucleotide polymorphisms (SNP) in four genes were identified: AKT1 (AKT serine/threonine kinase 1; three SNPs), BDNF (brain-derived neurotrophic factor; two SNPs), DNTBP1 (dystrobrevin-binding protein 1; one SNP) and GRM3 (S-adenosyl-L-methionine-dependent methyltransferases superfamily protein; one SNP). These findings support the gene-environment interactions in schizophrenia [92]. Concerning functional responses to fetal hypoxia, a protein product of one of the factors – BDNF- was increased in cord blood in control subjects without schizophrenia, while in cases with schizophrenia, BDNF levels were decreased by hypoxia [93]. HMGA1 (High mobility group A protein 1a) was found to be a hypoxia-inducible RNA-binding trans-acting factor for aberrant splicing of presenilin-2 pre-mRNA. Morikawa et al. found increased HMGA1a mRNA and protein in patients with schizophrenia [94]. Recently it was also shown that hypoxia-inducible factor induces MIF expression (macrophage migration inhibitory factor, a neuroprotective cytokine at the cross-road of inflammatory and stress responses) by binding to hypoxia-response element at the MIF promoter and that SNP at this site represents a risk factor for schizophrenia by reducing production of MIF in response to hypoxia [95]. Hypoxia also affects the extensively studied pathway of DISC1 (Disrupted in schizophrenia 1, a scaffold protein that interacts with many other proteins and is required for synaptogenesis, neurite outgrowth, and neuronal migration) by reducing the half-life of DISC1 protein [96].
Experimental studies in animal models
Experimental models of schizophrenia induced by perinatal hypoxia typically involve oxygen deprivation during critical developmental periods. For example, a rodent brain at postnatal days 7–8 corresponds to the late gestational period in humans. It represents a critical period for dendritic outgrowth, formation of synapses, and maturation of neuronal tissue [97], neuronal networks with alterations in the glutamatergic receptors (switch subunits of NMDA receptors from GluN2B to GluN2A subtypes) [98] and transformation of GABAergic system from excitatory to inhibitory effects [97]. All the parameters studied during developmental stage can now be linked using 3D eMouse atlas [99] that builds on morphological staging developed by Karl Theiler [100].
The immature brain in this sensitive age is quite vulnerable and depends on the timing and duration of hypoxic insult as it affects dynamic functions, such as neuronal proliferation, migration, and maturation. The models mimic obstetric complications, e.g., C-section, perinatal/postnatal hypoxia, or placental insufficiency [101]. Experimental hypoxia includes several protocols that employ acute or chronic oxygen reduction at sea level barometric pressure or hypobaric conditions where the percentage of oxygen remains the same, but decreased barometric pressure leads to less oxygen delivered to the lungs’ alveolo-capillary membrane.
In relation to schizophrenia, neuregulin-1 (a key factor seen elevated in patients with schizophrenia) was 32 % higher in the frontal cortex of adult rats exposed to 7-day neonatal hypoxemia [102]. In a recent study, perinatal hypoxia was shown to dysregulate spontaneous activity patterns critical for forming functional templates for generating cortical architecture and guidance for establishing thalamocortical and intracortical circuits. These circuits are affected in patients with schizophrenia [103].
Hypoxia-induced behavioral changes resembling schizophrenia
C-section results in greater amphetamine-induced locomotion in adult rats, both in animals born in normoxia or hypoxia as compared to vaginal delivery. Amphetamine increases dopamine levels, and increased locomotion in response to amphetamines indicates heightened sensitivity to dopamine, which models the dopamine dysregulation seen in schizophrenia. The rats also spent more time sniffing than grooming, and hypoxia during C-section was linked to prolonged rearing in adult rats [104]. In animal models, spending more time sniffing the environment and less time grooming can be indicative of increased anxiety or hyperactivity, both of which are observed in individuals with schizophrenia. Prolonged rearing indicates increased exploratory behavior and hyperactivity. In schizophrenia models, this behavior can reflect the hyperdopaminergic state associated with positive symptoms of schizophrenia, such as agitation and hyperactivity.
Adult guinea pigs had also increased amphetamine-induced locomotion and disrupted pre-pulse inhibition (PPI) of acoustic startle, but hypoxia during C-section reduced amphetamine-induced locomotion [105]. PPI is a neurological phenomenon used to measure sensory gating, the brain’s ability to filter out unnecessary information. The test consists of the startle reflex, a rapid, involuntary response to a sudden loud noise or other sensory solid stimulus. When a weak pre-stimulus (pre-pulse) is presented shortly before a strong startling stimulus, PPI occurs, and the startle response to the subsequent more substantial stimulus is reduced. Disrupted PPI in animals indicates impaired sensory gating, a key feature of schizophrenia. In another study, postnatal hypoxia induced by bilateral, continuous occlusion of the common carotid artery in 12-day-old male rats resulted in schizophrenia-like behavior, including locomotor activity in pubertal rats (postnatal day 35) and impaired PPI in post-pubertal males (postnatal day 50) [106].
Hypoxia-induced impact on neurotransmitters and/or their receptors related to schizophrenia
All three central neurotransmitter systems are affected by hypoxia:
Dopamine. Animal models of C-section hypoxia resulted in altered levels of dopamine or dopamine receptors, the key neurotransmitter associated with schizophrenia. Decreased levels of dopamine were found in the prefrontal cortex [107,108], while dopamine release was increased in the nucleus accumbens [108] and amygdala [109];
Glutamatergic system. NMDA receptor binding decreased, and transcription of NR1 subunit increased in frontal and temporal regions, nucleus accumbens, and hippocampus. NR2A subunit expression was downregulated in hippocampal sub-regions. On day 120 postnatally, gene expression of NR1 was still increased in hippocampal, frontal, and temporal sub-regions, as well as nucleus accumbens - a pre-pulse inhibition deficit points to schizophrenia-like behavior in 4-month-old rats. Compensatory upregulation of NR1 expression may occur due to NMDA receptor hypofunction. A subset of glutamate receptors, kainite receptors, increased after exposure to hypoxia [104]. Neuregulin −1, a protein that interacts with glutamate receptors, was elevated after hypoxia in 7-day rats [102];
GABA in the hippocampus increased in 7-day old rats after 1hr ligation of the left carotid artery and exposure to air where the content of oxygen was reduced from 21 to 8 % [110].
In summary, perinatal hypoxia has been linked to many neuropsychiatric conditions. Concerning schizophrenia, the link was established by epidemiological and genetic studies, in vitro experiments on human cells, and in vivo experimental studies in several animal species.
Perinatal immune system activation and the brain
Perinatal infections encompass a range of infectious diseases transmitted from mother to fetus in-utero or during birth or occur shortly after delivery. Any infectious microorganisms, including bacteria, viruses, fungi, or parasites, can cause these infections. Immune responses to invading microorganisms are associated with local and systemic activation of immune cells and the production of soluble molecules, including interleukins, cytokines, complement peptides, and antibodies. The presence of these molecules alters the brain development.
Clinical studies in patients with schizophrenia
Epidemiological evidence
The link between perinatal infection and schizophrenia started to be considered more than three decades ago. In a Finish birth cohort study, Mednick et al. showed that mothers in the second trimester of their pregnancy during the 1957 influenza endemic had children who were much more likely to be admitted by 26 years in an inpatient facility with the diagnosis of schizophrenia [5]. Subsequent birth cohort study employed an improved design by involving pregnant women whose respiratory infection was recorded by a physician and whose offspring had continued follow-up with a diagnosis of schizophrenia established by face-to-face interview [111]. Second-trimester infection represented an increased risk for schizophrenia with a relative rate of 2.13 [111]. Going beyond the in-utero period, a two-fold risk for schizophrenia was found in adults who experienced childhood infections, especially influenza, as a meta-analysis revealed. These findings highlight that the critical developmental period continues in the postnatal period [112]. A recent population-based nationwide cohort study addressed the hazard ratio for neuropsychiatric conditions in children of mothers with autoimmune diseases. The hazard risk with regards to schizophrenia was 1.35, demonstrating increased risk in offspring of women with conditions such as autoimmune diabetes or rheumatoid arthritis [113].
Serologic findings
Other efforts focused on finding infectious microorganisms responsible for these observations. In a nested case-control study, blood samples of mothers of children who turned out to be schizophrenic patients in adulthood were measured. The samples were collected at the end of their pregnancy and were found to have elevated total immunoglobulin (Ig)G and IgM and elevated IgG specific against herpes virus type 2 glycoprotein gG2 [114]. In another nested case-control study, archived blood samples of mothers pregnant between 1959 and 1966 were tested, and offspring were followed for psychiatric disorders for 30–38 years. Influenza infection during the first trimester increased the risk for schizophrenia 7-fold and 3-fold after broadening gestational periods to early to mid-pregnancy [115].
The studies on viral pathogens also expanded to protozoan parasites, toxoplasmosis gondii, and bacterial infections. Xiao et al. developed new antibodies for enzyme-linked immunosorbent assay to distinguish three distinct clonal lineages of toxoplasma and then tested the sera of pregnant mothers whose children developed schizophrenia and schizoaffective disorder with sera of mothers of unaffected children. Serological positivity for Toxoplasma type I, Ukrainian infection, increased risk for the development of psychoses with an odds ratio of 1.94. For affective psychoses, the odds ratio was 5.24 [116]. Bacterial infections during pregnancy also represent a significant risk for the development of schizophrenia (adjusted odds ratio 1.8, primarily when the infection affects multiple systems, which raises the adjusted odds ratio to 2.9 [117].
Neuroanatomic considerations
In patients with schizophrenia, postmortem analyses and brain imaging studies done by the early 1990s established that pathology occurs within fronto-striatal-temporal regions [118]. As further details were learned, more details were identified, and the frontal cortex, hippocampus ([119], cerebellar vermis [120], substantia nigra [121] were added to the neuroanatomical areas related to schizophrenia. A recent review of meta-analyses concluded that schizophrenia is characterized by lower grey matter volumes and cortical thickness, accelerated grey matter loss over time, abnormal gyrification patterns, and lower regional SV2A levels (Synaptic Vesicle Glycoprotein 2A is a protein that plays a crucial role in the regulation of neurotransmitter release at synapses) and metabolic markers in comparison to controls (effect sizes from ~ −0.11 to −1.0), and that critical regions affected include frontal, anterior cingulate and temporal cortices and the hippocampi [42].
Concerning the association between immune system activation and schizophrenia, metanalysis revealed a significant increase in the density of microglia, especially in the temporal cortex, while densities of macroglia (astrocytes and oligodendrocytes) did not differ significantly. On the molecular level, increased expression of proinflammatory genes on transcript and protein levels was seen in schizophrenia, while anti-inflammatory gene expression levels did not differ between schizophrenia and controls [122].
Complex developmental trajectories were detected in the brains of patients with autism and schizophrenia spectrum disorders, which are distinct disorders where autism starts in early childhood and schizophrenia in young adulthood. However, patients with autism are three times more likely to develop schizophrenia later in their life [123]. This association may result from interactions between genetically-defined abnormalities and many environmental factors to which each individual is likely exposed at different times. For a better understanding of the mechanisms of this complex phenomenon, animal models of maternal immune activation (MIA) were developed.
Experimental studies in MIA model
Robert Sidwell’s group established foundations for the MIA by involving C57/BL6 pregnant mice infected with human influenza virus on gestational day 9 and assessing offspring on day 0 after the birth and at 14 weeks. They were the first to report short- and long-lasting impacts both on adult offspring’s behavior and brain neuropathology, including macrocephaly and pyramidal cell atrophy [124]. Paul Patterson and his team then employed the synthetic double-stranded RNA polyinosinic-polycytidylic acid (Poly (I:C)) instead of the influenza virus and demonstrated a similar impact on brain structures and functions [125]. The deficits in pre-pulse inhibition in the acoustic startle response linked the model to autism and schizophrenia spectrum disorders [126]. Using synthetic mimetics of the influenza virus significantly simplified the methodology of this model and facilitated subsequent studies that involved different Toll-Like Receptor (TLR) ligands administered to pregnant dams at different stages of pregnancy [127].
Paul Patterson’s group was pivotal in developing the model’s neuropathology and identifying behavioral abnormalities (e.g., deficits in social interaction, increased anxiety, and cognitive impairments), which helped to draw parallels between the animal model findings and symptoms of patients with autism and schizophrenia [15,128]. They also established essential roles of cytokines, particularly interleukin (IL)-6, in mediating the effects of MIA on neurological abnormalities in exposed offspring [126]. Another critical cytokine, IL-17, is required for elicitation of abnormal cortical and altered behavior in offspring, as discovered by Gloria Choi [13]. Her group further refined the MIA model by establishing neuronal circuitry that is affected by IL-17 [129,130].
Urs Meyer’s group demonstrated the relevance of the model to schizophrenia and the multi-hit hypothesis. In their experiments, MIA-exposed animals received stressful stimuli in the peripubertal period, which resulted in synergistic effects on brain pathology and behavior in adulthood. The MIA exposure significantly increased the vulnerability of the pubescent offspring [131]. Given that the onset of schizophrenia occurs in young adults, this model involving a combination of perinatal and peripubertal challenges likely reflects real-world scenarios.
In summary both preclinical and clinical studies have linked inflammation and maternal immune activation to pathogenesis of schizophrenia. The critical questions that need to be resolved are when the inflammatory processes within the brain are beneficial and when they are detrimental, and how can the injurious events be therapeutically inhibited without impacting the whole immune system and rendering treated individuals more susceptible to infections.
The complexity of interactions in the MIA model is evident from this outline. However, another layer of complexity was added when Sarkis Maznamian’s group reported that MIA alters the gut microbiome, significantly affecting exposed offspring’s neurodevelopment [132].
Gut microbiome and the brain
The gut microbiota comprises over 100 trillion bacteria, viruses, and fungi, which form an essential physiological system. The interactions between the large mass of microorganisms and the gastrointestinal wall are highly regulated by the gut-associated lymphoid tissue, which represents the immune tolerance’s cardinal site. The gut microbiota interacts with other organs through a multidirectional communication network, via which it also influences brain development and functions [133].
The microbiota communicates with the brain via nerves and in an endocrine fashion. Regarding nerves, autonomic parasympathetic, vagal, and splanchnic plexus fibers are directly wired to the central nervous system. The endocrine role of gut microbiota is reflected in the release of many substances that then travel through interstitial fluid or blood to local or distant targets [134].
The most relevant molecules produced or metabolized by the microbiota are short-chain fatty acids (produced by bacteria), bile acids (from the liver and metabolized by the microbiota), and tryptophan (an essential amino acid originating mainly in a diet and then metabolized by the microbiota) [135].
The microbiota can transform tryptophan into indole and other aryl hydrocarbon receptor ligands, critical for maintaining epithelial cell renewal and integrity and controlling intraepithelial leukocyte interactions [135]. Tryptophan is also metabolized by indoleamine-2,3-deoxygenase in epithelial and immune cells to kynurenine and downstream products, which regulate inflammation, adaptive immune responses, and neurotransmission [135]. Another critical role of tryptophan is being a precursor for serotonin. It is produced by two enzymes, tryptophan-hydroxylase 1 and 2, located in the gut and brain. About 95 % of serotonin is found in the gut, produced by enterochromaffin cells [136]. Serotonin acts as a hormone and neurotransmitter in the peripheral and central nervous systems. In the gut, serotonin influences intestinal peristalsis and motility, secretion from gastrointestinal glands, and vasodilation. Serotonin is also a part of the content of platelet and mast cell granules and contributes significantly to inflammatory responses.
Clinical studies on microbiota in schizophrenia
Zheng and his team established the relationship between gut microflora and schizophrenia. They showed that schizophrenia patients exhibited reduced diversity in bacterial species, and revealed a correlation between discriminative microbial markers and the severity of schizophrenia symptoms [137]. A positive correlation was found for Lachnospiraceae OTU (Operational taxonomic unit) 477, Lachnospiraceae OTU629, Ruminococcaceae, Bacteroidaceae OTU 172, and streptococcaceae OTU834, and negative correlation was reported for veillonellaceae OTU191 and Ruminococcaceae OTU725 [137]. The authors then went beyond establishing the clinically obtainable correlative relationships and addressed the pathogenic role of gut microflora in a translational experiment where fecal microbiota from schizophrenic patients versus healthy controls were transplanted into experimental mice. Animal behavior in group recipients of the stool from schizophrenic patients corresponded to behavior considered characteristic in experimental models of schizophrenia. These changes were accompanied by biochemical alterations in the cortex and hippocampus, which are consistently reported to be affected in schizophrenia [137].
In another study, Li et al. show that microbiota in patients with schizophrenia is related to structural changes in their brains. Structural magnetic resonance imaging revealed a reduction in gray matter volume and regional homogeneity in several brain regions in patients. Alpha diversity of the gut microbiota in patients showed a strong linear relationship with the values of both MRI parameters. These results further strengthened the argument that gut microbiome may play a role in the neuropathogenesis of schizophrenia [138].
Evidence for clinical MIA affecting brain development via the impact on microbiota
Whether maternal infection during pregnancy affects the child’s brain development at least partially via gut microbiota has not been documented. However, fragmented clinical evidence suggests that such a pathway exists. First, even a minor infection affects the composition of gut microbiota, as shown by a longitudinal study on patients with mild, asymptomatic SARS-CoV-2 infection. Their stool was collected during the infection, and then after they turned seronegative for the SARS-CoV-2 virus. The microbiota showed more microbial evenness during infection, and Bacteroidetes species were depleted. When seronegativity for the SARS-CoV-2 virus was reached, the microbiota was comparable with healthy controls [139]. Second, maternal microbiota changes affect an infant’s microbiota composition [140]. Third, antibiotics taken during pregnancy alter maternal microbiota and are associated with the development of metabolic and allergic disorders later in childhood, including obesity and asthma [141]. In the context of existing information, maternal microbiota alterations likely affect human offspring’s brain development.
Experimental studies on the impact of MIA on gut microbiome
Mazmanian’s team reported first that MIA-impacted gut flora influences the severity of behavioral and neuropathological phenotypes in offspring by breaking the immune tolerance in the gut and activating the microbiota-gut-brain axis [132]. Oral administration of common commensals, Bacteroides fragilis, corrected gut permeability and microbial composition, improving communicative, stereotypic, anxiety-like, and sensorimotor behaviors [132].
A meta-analysis was performed to assess MIA’s effect on microbiota and neurodevelopmental conditions in rodents. Combining the results of thirteen studies revealed that maternal microbiome disturbances affect the brain, as reflected by a decrease in offspring’s sociability and an increase in stereotypic behaviors [142], which supports the validity of the concept.
Since indigenous spore-forming bacteria from the mouse and human microbiota promote serotonin biosynthesis from colonic enterochromaffin cells [143], MIA’s impact on the serotonin pathway was tested by MacDowell et al. [144]. MIA reduced serotonin content in brain tissue and promoted changes in the expression of serotonin transporter, 5-HT2A, and 5-HT2C receptors. Long-term paliperidone treatment (a dopamine D2 and serotonin 5HT receptor antagonist) counteracted the MIA-induced changes [144]. These findings provide insight into mechanisms by which MIA’s impact on the microbiota can affect the brain.
Microbiota can be altered by antibiotic usage. Except for a few, antibiotics have been considered safe for pregnant women. As we learn more about antibiotics’ effects on microbiota, their safety may need to be re-assessed, and the benefit ratio may need to be considered individually. One of the safest antibiotics is penicillin. Administration at a low dose during the third trimester of mouse pregnancy resulted in behavior changes of adult offspring [145–147]. The behavioral changes were sex-dependent. Female adult mice showed decreased anxiety patterns, while they had abnormal social behavior. The immune system was affected, as evidenced by a decrease in splenic FOXP3+ regulatory T cells, major players in preventing the development of autoimmunity [145].
In similar experimental conditions, Lebovitz et al. reported decreased expression of Cx3cr1 (a chemokine receptor for neuron-derived fractalkine) in the microglia of the prefrontal cortex [148]. In another report, dysbiosis-induced microglial expression of CxC3cr1 was restored by treating mice with oral Lactobacillus species [146].
Other causes of perinatal alterations of maternal or infant’s microbiota
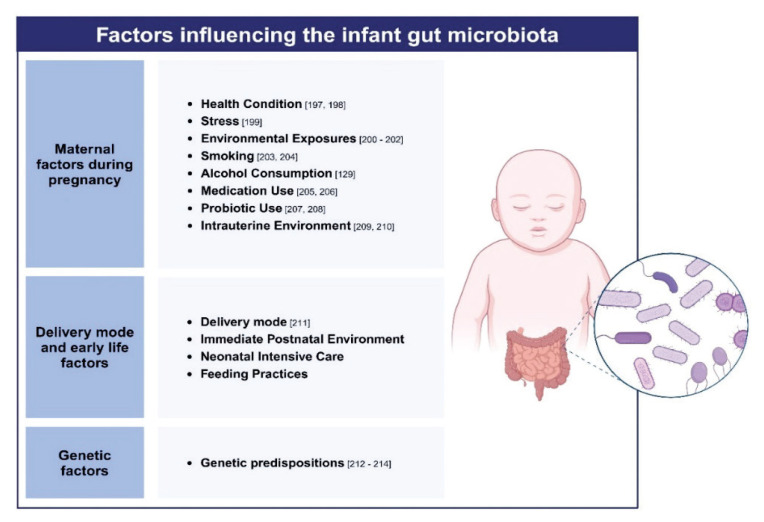
Both maternal and infant microbiota play crucial roles in shaping an infant’s brain development, but their influences are intertwined and impact the infant at different stages. Maternal gut microbiota influences the immune environment and metabolic state during pregnancy, which can impact fetal brain development. During vaginal delivery, infants acquire microbiota from the mother’s vaginal and intestinal flora, which is beneficial for early immune system development. After birth, the infant’s microbiota continues to develop and is influenced by factors such as breastfeeding and environmental exposures. Breast milk contains beneficial bacteria and prebiotics that help shape the infant’s gut microbiota. Figure 3 depicts conditions that can influence maternal or offspring microbiota.
Fig. 3.
Various factors that affect maternal and offspring microbiota, which may alter neurodevelopment in the child through gut-microbiota-brain axis. The picture was made with using a Biorender template.
MIA by SARS-CoV-2
World Health Organization declared global SARS-CoV-2 pandemic in March 2020 and announced its end in May 2023. Statistics vary about the number of women who were pregnant during the COVID-19 pandemic. It is, however, clear that SARS-CoV-2 infection worsened pregnancy outcomes. For example, a recent study reported outcomes of INTERCOVID study infections of the omicron variant of SARS-CoV-2 in pregnant women and their babies. Maternal, Neonatal, and Perinatal Morbidity and Mortality indices (MMI) relative risk were 1.16, 1.23, and 1.21, respectively. In unvaccinated women, Maternal MMI was 1.36; in women with severe COVID-19 symptoms, 2.51; and in unvaccinated women with severe COVID-19 symptoms, 2.88 [149].
Vertical transmission of the virus is believed to occur only rarely [150] and the human placenta has been considered a sound barrier that protects the fetus efficiently [151]. However, in a recent experimental study using mice that express human angiotensin-converting enzyme 2 that allows intracellular entry of SARS-CoV-2, the virus is found in the brain within 48 hours after the infection, indicating direct exposure of brain cells to the virus. All cell types within the brain (endothelium, neurons, glia, and astrocytes) can be infected [152]. Even if direct exposure to human fetuses continues to be refuted, the placenta of infected women (including mild cases of SARS-CoV-2 infection) was reported to have vascular abnormalities (consistent with malperfusion) and villitis [153,154].
SARS-CoV-2 enters cells primarily via binding to Angiotensin Converting Enzyme-2 and Transmembrane Serine Protease 2. The virus also interacts with the host immune system through multiple TLRs, mainly TLR2, TLR4, TLR7, and TLR8, which were all shown before to play a role in MIA [23]. These interactions activate innate immune responses, including producing pro-inflammatory cytokines critical for controlling viral infection and contributing to MIA.
The abnormal fetus oxygenation, local inflammation, and the impact of SARS-CoV-2 on microbiota are all conditions, in which the neural development of the fetus may be affected. These mechanisms may be behind the findings in a recent retrospective study that examined one-year-old children exposed to SARS-CoV-2 in utero (confirmed by polymerase chain reaction test). The study revealed that exposure is associated with a higher rate of neurodevelopmental diagnoses, with an OR of 1.86. When the SARS-CoV-2 occurred in the third trimester, the OR was higher − 2.34 [155]. Another retrospective cohort examined electronic health records and uncovered that males but not females born to SARS-CoV-2 infected mothers were more likely to receive a neurodevelopmental diagnosis in the first 12 months after delivery [156].
While these findings will need additional validations, the existing data already warrant more experimental studies that can help explain histopathological mechanisms involved in the increased vulnerabilities to neurodevelopmental conditions and identify diagnostic markers applicable clinically. Meanwhile, professionals taking care of children exposed to SARS-CoV-2 in utero may closely monitor their development and support formulation of clinical practices where children with vulnerabilities receive more support, as it is the case with individuals at high risk for development of autism or schizophrenia where measures, such as diet, exercise [157,158] showed some positive outcomes.
Interactions between MIA and perinatal hypoxia
The impacts of perinatal hypoxia and MIA on the brain overlap in several key areas. Both conditions can lead to similar neurodevelopmental disruptions and are associated with increased risk for neuropsychiatric disorders, including schizophrenia. They both induce neuroinflammation, which involves microglia activation and release of pro-inflammatory cytokines in the developing brain [45,159]. Both conditions can lead to increased production of oxygen radical species and subsequent oxidative stress, which can cause cellular injury and impair neurodevelopmental processes, including synaptogenesis and myelinization [160,161]. Both perinatal hypoxia and MIA can cause epigenetic changes, such as DNA methylation and histone modification, which impact transcriptional programs involved in various developmental functions and adaptive responses [162–164] and may be dependent on severity of the stimulus [165]. Finally, both perinatal hypoxia and MIA alter the dopaminergic system, which is a crucial feature of schizophrenia [166]. To better understand pathways shared between perinatal hypoxia and MIA, we will need to await experimental evidence where MIA, due to the activation of different TLRs, is tested together with different degrees of perinatal hypoxia (including mild hypoxia). Such efforts can help develop biomarkers for new diagnostic panels, targeted interventions, and preventive strategies for at-risk populations.
Conclusions
This review underscores the roles of perinatal hypoxia, immune system activation, and microbiota in neurodevelopmental conditions, to which also belongs schizophrenia. Clinical and experimental studies demonstrate that these perinatal factors are associated with long-term changes in brain structure and function, as reflected in behavioral and neurotransmitter alterations observed also in schizophrenia. Sophisticated experimental models have been developed during the last two decades to address perinatal hypoxia and MIA but their cumulative effects are rarely studied together.
In light of the SARS-CoV-2 pandemic, these considerations may be needed for children exposed to SARS-CoV-2 in utero, particularly in situations where additional factors contributed to increased risk for asymptomatic brain tissue injury, including obstetric complications causing various degree of hypoxia, additional infection, maternal immune activation or psychosocial stress.
Limitations
One of the significant limitations of translational research on schizophrenia is the heterogeneity of the disease and the existence of subsets of patients that we are not yet able to distinguish objectively. Most existing studies approach schizophrenia as one condition that significantly limits the interpretation of data. The heterogeneity of schizophrenia stems from complex genetic backgrounds that make the susceptibility to environmental factors quite variable. In addition to the heterogeneous nature of schizophrenia, also ethical limitations exist that prevent access to the affected brain tissue.
Future directions
To enhance our understanding of schizophrenia development, using advanced animal models and testing more than one perinatal factor per experiment will be critical. The experimental studies linked to longitudinal clinical studies involving sufficient subjects and assessing patients multimodally will promote the translational value of such work. These efforts should identify objective biomarkers for subsets of patients and hopefully reveal objective biomarkers altered by perinatal factors that are sensitive enough to reveal even pathological processes not immediately evident clinically (e.g., sensory, motor or cognitive deficits) and that are possible to use in longitudinal monitoring during individual’s development as they encounter further hits during their lives. That such goals are feasible is demonstrated by recent advances in other psychiatric conditions, namely Alzheimer disease, where a biomarker detectable in the blood was identified [167]. A better understanding of molecular mechanisms can lead to more effective individualized treatment. In addition, the focus of the research should not only be on the treatment of pathological status but on preventive measures that can limit the prevalence of neurodevelopmental conditions, including schizophrenia.
Acknowledgements
Ivana Kawikova was supported by the PROJECT OP VVV – International mobility of NIMH researchers reg. NO. CZ.02.2.69/0.0/0.0/18_053/0017858 and Merit Fellowship from the Central Bohemian Innovation Center.; Lenka Kleteckova was supported by the Agency for Health Research Czech Republic, No.: NU22J-04-00061; Kristina Hakenova, Lea Jakob, Filip Spaniel and Karel Vales received a grant from Grant Agency of Charles University (SVV/260 648/2024), and were also supported with European Regional Development Fund: Project “PharmaBrain” (no. CZ.CZ.02.1.01/0.0/0.0/16_025/0007444) CZECRIN IV 90249 - MSMT-49/2023, National Institute for Neurological Research (Program EXCELES, No. LX22NPO5107) - Funded by the European Union - Next Generation EU and by Long-term conceptual development of research organization (RVO 00023752), and Specific University Research, Czech Ministry of Education, Youth and Sports (project 260533/SVV/2022), program Cooperatio 38, Neuroscience Charles University, and a grant from Ministry of Health of the Czech Republic, grant nr. NU22-04-00143; Li Wen was supported by National Institute of Health, USA (grants HD097808, DK126809 and DK130318).
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Hampl V, Herget J. Perinatal hypoxia increases hypoxic pulmonary vasoconstriction in adult rats recovering from chronic exposure to hypoxia. Am Rev Respir Dis. 1990;142:619–624. doi: 10.1164/ajrccm/142.3.619. [DOI] [PubMed] [Google Scholar]
- 2.Vizek M, Dostal M, Soukupova D. Perinatal hypoxia suppresses immune response of adult rats. Physiol Res. 1993;42:201–204. [PubMed] [Google Scholar]
- 3.Leckman JF, King RA, Gilbert DL, Coffey BJ, Singer HS, St Dure L, Grantz H, et al. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2011;50:108–18e3. doi: 10.1016/j.jaac.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 6.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey C, Fullard JF, Sleator RD. Unravelling the genetic basis of Schizophrenia. Gene. 2024;902:148198. doi: 10.1016/j.gene.2024.148198. [DOI] [PubMed] [Google Scholar]
- 8.Taylor JL, Debost JPG, Morton SU, Wigdor EM, Heyne HO, Lal D, Howrigan DP, et al. Paternal-age-related de novo mutations and risk for five disorders. Nat Commun. 2019;10:3043. doi: 10.1038/s41467-019-11039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–63. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grover S, Varadharajan N, Venu S. Urbanization and psychosis: an update of recent evidence. Curr Opin Psychiatry. 2024;37:191–201. doi: 10.1097/YCO.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 11.Robinson N, Ploner A, Muller-Eberstein R, Lichtenstein P, Kendler KS, Bergen SE. Migration and risk of schizophrenia and bipolar disorder: A Swedish national study. Schizophr Res. 2023;260:160–7. doi: 10.1016/j.schres.2023.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2018;299:241–51. doi: 10.1016/j.expneurol.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–9. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker-Athill E, Luo D, Bailey A, Giunta B, Tian J, Shytle RD, Murphy T, et al. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J Neuroimmunol. 2009;217:20–7.10. doi: 10.1016/j.jneuroim.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70:677–685. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- 17.Khan D, Fernando P, Cicvaric A, Berger A, Pollak A, Monje FJ, Pollak DD. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl Psychiatry. 2014;4:e363. doi: 10.1038/tp.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronovsky M, Berger S, Molz B, Berger A, Pollak DD. Animal Models of Maternal Immune Activation in Depression Research. Curr Neuropharmacol. 2016;14:688–704. doi: 10.2174/1570159X14666151215095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronovsky M, Berger S, Zambon A, Reisinger SN, Horvath O, Pollak A, Lindtner C, et al. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav Immun. 2017;63:127–136. doi: 10.1016/j.bbi.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Quagliato LA, de Matos U, Nardi AE. Maternal immune activation generates anxiety in offspring: A translational meta-analysis. Transl Psychiatry. 2021;11:245. doi: 10.1038/s41398-021-01361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg JB, Richardt Mollegaard Jepsen J, Mohammadzadeh P, Sevelsted A, Vinding R, Sorensen ME, Horner D, et al. Maternal inflammation during pregnancy is associated with risk of ADHD in children at age 10. Brain Behav Immun. 2024;115:450–457. doi: 10.1016/j.bbi.2023.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Jones HF, Han VX, Patel S, Gloss BS, Soler N, Ho A, Sharma S, et al. Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: Transcriptomic data show common enriched innate immune pathways. Brain Behav Immun. 2021;94:308–317. doi: 10.1016/j.bbi.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Han VX, Patel S, Jones HF, Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. 2021;17:564–579. doi: 10.1038/s41582-021-00530-8. [DOI] [PubMed] [Google Scholar]
- 24.Corradini I, Focchi E, Rasile M, Morini R, Desiato G, Tomasoni R, Lizier M, et al. Maternal Immune Activation Delays Excitatory-to-Inhibitory Gamma-Aminobutyric Acid Switch in Offspring. Biol Psychiatry. 2018;83:680–691. doi: 10.1016/j.biopsych.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Vestergaard M, Christensen J, Nahmias AJ, Olsen J. Prenatal exposure to maternal infections and epilepsy in childhood: a population-based cohort study. Pediatrics. 2008;121:e1100–7. doi: 10.1542/peds.2007-2316. [DOI] [PubMed] [Google Scholar]
- 26.Zager A, Peron JP, Mennecier G, Rodrigues SC, Aloia TP, Palermo-Neto J. Maternal immune activation in late gestation increases neuroinflammation and aggravates experimental autoimmune encephalomyelitis in the offspring. Brain Behav Immun. 2015;43:159–171. doi: 10.1016/j.bbi.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 28.O’Hara R, Beaudreau SA, Gould CE, Froehlich W, Kraemer HC. Handling clinical comorbidity in randomized clinical trials in psychiatry. J Psychiatr Res. 2017;86:26–33. doi: 10.1016/j.jpsychires.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Collaborators GBDMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solmi M, Seitidis G, Mavridis D, Correll CU, Dragioti E, Guimond S, Tuominen L, et al. Incidence, prevalence, and global burden of schizophrenia - data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry. 2023;28:5319–27. doi: 10.1038/s41380-023-02138-4. [DOI] [PubMed] [Google Scholar]
- 31.Kochhar SS, Mishra AK, Chadda RK, Sood M, Bhargava R. Psychosocial correlates of the experience of caregiving among caregivers of patients with schizophrenia. Cureus. 2024;16:e58531. doi: 10.7759/cureus.58531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kretchy IA, Osafo J, Agyemang SA, Appiah B, Nonvignon J. Psychological burden and caregiver-reported non-adherence to psychotropic medications among patients with schizophrenia. Psychiatry Res. 2018;259:289–94. doi: 10.1016/j.psychres.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Carrasco M, Fernandez-Catalina P, Dominguez-Panchon AI, Goncalves-Pereira M, Gonzalez-Fraile E, Munoz-Hermoso P, Ballesteros J, et al. A randomized trial to assess the efficacy of a psychoeducational intervention on caregiver burden in schizophrenia. Eur Psychiatry. 2016;33:9–17. doi: 10.1016/j.eurpsy.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Mittendorfer-Rutz E, Rahman S, Tanskanen A, Majak M, Mehtala J, Hoti F, Jedenius E, et al. Burden for parents of patients with schizophrenia-a nationwide comparative study of parents of offspring with rheumatoid arthritis, multiple sclerosis, epilepsy, and healthy controls. Schizophr Bull. 2019;45:794–803. doi: 10.1093/schbul/sby130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tessier A, Roger K, Gregoire A, Desnavailles P, Misdrahi D. Family psychoeducation to improve outcome in caregivers and patients with schizophrenia: a randomized clinical trial. Front Psychiatry. 2023;14:1171661. doi: 10.3389/fpsyt.2023.1171661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin C, Zhang X, Jin H. The societal cost of schizophrenia: an updated systematic review of cost-of-illness studies. Pharmacoeconomics. 2023;41:139–53. doi: 10.1007/s40273-022-01217-8. [DOI] [PubMed] [Google Scholar]
- 37.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399:473–86. doi: 10.1016/S0140-6736(21)01730-X. [DOI] [PubMed] [Google Scholar]
- 38.Riglin L, Collishaw S, Richards A, Thapar AK, Maughan B, O’Donovan MC, Thapar A. Schizophrenia risk alleles and neurodevelopmental outcomes in childhood: a population-based cohort study. Lancet Psychiatry. 2017;4:57–62. doi: 10.1016/S2215-0366(16)30406-0. [DOI] [PubMed] [Google Scholar]
- 39.Bouet V, Percelay S, Leroux E, Diarra B, Leger M, Delcroix N, Andrieux A, et al. A new 3-hit mouse model of schizophrenia built on genetic, early and late factors. Schizophr Res. 2021;228:519–528. doi: 10.1016/j.schres.2020.11.043. [DOI] [PubMed] [Google Scholar]
- 40.Correll CU, Howes OD. Treatment-resistant schizophrenia: definition, predictors, and therapy options. J Clin Psychiatry. 2021:82. doi: 10.4088/JCP.MY20096AH1C. [DOI] [PubMed] [Google Scholar]
- 41.Messmer MF, Wilhelm EE, Shoulson I. I-SPY 2 breast cancer trial as a model for innovation in Alzheimer disease therapies. JAMA Neurol. 2017;74:1027–8. doi: 10.1001/jamaneurol.2017.1528. [DOI] [PubMed] [Google Scholar]
- 42.Howes OD, Cummings C, Chapman GE, Shatalina E. Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2023;48:151–67. doi: 10.1038/s41386-022-01426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rund BR. The research evidence for schizophrenia as a neurodevelopmental disorder. Scand J Psychol. 2018;59:49–58. doi: 10.1111/sjop.12414. [DOI] [PubMed] [Google Scholar]
- 44.Wen J, Antoniades M, Yang Z, Hwang G, Skampardoni I, Wang R, Davatzikos C. Dimensional neuroimaging endophenotypes: neurobiological representations of disease heterogeneity through machine learning. Biol Psychiatry. 2024;96:p564–584. doi: 10.1016/j.biopsych.2024.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Powell SB, Swerdlow NR. The relevance of animal models of social isolation and social motivation for understanding schizophrenia: review and future directions. Schizophr Bull. 2023;49:1112–1126. doi: 10.1093/schbul/sbad098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uliana DL, Diniz C, da Silva LA, Borges-Assis AB, Lisboa SF, Resstel LBM. Contextual fear expression engages a complex set of interactions between ventromedial prefrontal cortex cholinergic, glutamatergic, nitrergic and cannabinergic signaling. Neuropharmacology. 2023;232:109538. doi: 10.1016/j.neuropharm.2023.109538. [DOI] [PubMed] [Google Scholar]
- 49.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 50.Harrison PJ. Postmortem studies in schizophrenia. Dialogues Clin Neurosci. 2000;2:349–357. doi: 10.31887/DCNS.2000.2.4/pharrison. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glantz LA, Austin MC, Lewis DA. Normal cellular levels of synaptophysin mRNA expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2000;48:389–97. doi: 10.1016/S0006-3223(00)00923-9. [DOI] [PubMed] [Google Scholar]
- 52.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 53.Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 54.Lewis DA, Glantz LA, Pierri JN, Sweet RA. Altered cortical glutamate neurotransmission in schizophrenia: evidence from morphological studies of pyramidal neurons. Ann N Y Acad Sci. 2003;1003:102–12. doi: 10.1196/annals.1300.007. [DOI] [PubMed] [Google Scholar]
- 55.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–24. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 56.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/S0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 57.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danos P, Baumann B, Bernstein HG, Franz M, Stauch R, Northoff G, Krell D, et al. Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin-immunoreactive thalamocortical projection neurons. Psychiatry Res. 1998;82:1–10. doi: 10.1016/S0925-4927(97)00071-1. [DOI] [PubMed] [Google Scholar]
- 59.Holt DJ, Bachus SE, Hyde TM, Wittie M, Herman MM, Vangel M, Saper CB, et al. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: an in situ hybridization study. Biol Psychiatry. 2005;58:408–16. doi: 10.1016/j.biopsych.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Nasrallah HA, McCalley-Whitters M, Bigelow LB, Rauscher FP. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8:251–60. doi: 10.1016/0165-1781(83)90013-6. [DOI] [PubMed] [Google Scholar]
- 61.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 62.Kuo SS, Pogue-Geile MF. Variation in fourteen brain structure volumes in schizophrenia: A comprehensive meta-analysis of 246 studies. Neurosci Biobehav Rev. 2019;98:85–94. doi: 10.1016/j.neubiorev.2018.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cropley VL, Klauser P, Lenroot RK, Bruggemann J, Sundram S, Bousman C, Pereira A, et al. Accelerated Gray and White Matter Deterioration With Age in Schizophrenia. Am J Psychiatry. 2017;174:286–295. doi: 10.1176/appi.ajp.2016.16050610. [DOI] [PubMed] [Google Scholar]
- 64.Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr Bull. 2018;44:168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gong Q, Lui S, Sweeney JA. A Selective Review of Cerebral Abnormalities in Patients With First-Episode Schizophrenia Before and After Treatment. Am J Psychiatry. 2016;173:232–43. doi: 10.1176/appi.ajp.2015.15050641. [DOI] [PubMed] [Google Scholar]
- 67.Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. Int Rec Med Gen Pract Clin. 1955;168:318–326. [PubMed] [Google Scholar]
- 68.Takesada M, Kakimoto Y, Sano I, Kaneko Z. 3,4-Dimethoxyphenylethylamine and Other Amines in the Urine of Schizophrenic Patients. Nature. 1963;199:203–204. doi: 10.1038/199203a0. [DOI] [PubMed] [Google Scholar]
- 69.Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, Watson SJ., Jr Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10:239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- 70.Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 1997;54:1089–1095. doi: 10.1001/archpsyc.1997.01830240045007. [DOI] [PubMed] [Google Scholar]
- 71.Clinton SM, Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res. 2004;69:237–253. doi: 10.1016/j.schres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 72.Benjamin KJM, Chen Q, Jaffe AE, Stolz JM, Collado-Torres L, Huuki-Myers LA, Burke EE, et al. Analysis of the caudate nucleus transcriptome in individuals with schizophrenia highlights effects of antipsychotics and new risk genes. Nat Neurosci. 2022;25:1559–1568. doi: 10.1038/s41593-022-01182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seeman P, Niznik HB. Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J. 1990;4:2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- 74.Dean B, Boer S, Gibbons A, Money T, Scarr E. Recent advances in postmortem pathology and neurochemistry in schizophrenia. Curr Opin Psychiatry. 2009;22:154–160. doi: 10.1097/YCO.0b013e328323d52e. [DOI] [PubMed] [Google Scholar]
- 75.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1–56. [PubMed] [Google Scholar]
- 76.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 77.Stroup TS, Gerhard T, Crystal S, Huang C, Tan Z, Wall MM, Mathai C, et al. Comparative Effectiveness of Adjunctive Psychotropic Medications in Patients With Schizophrenia. JAMA Psychiatry. 2019;76:508–515. doi: 10.1001/jamapsychiatry.2018.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan PF, Yao S, Hjerling-Leffler J. Schizophrenia genomics: genetic complexity and functional insights. Nat Rev Neurosci. 2024;25:611–624. doi: 10.1038/s41583-024-00837-7. [DOI] [PubMed] [Google Scholar]
- 79.Wray NR, Gottesman Using summary data from the danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet. 2012;3:118. doi: 10.3389/fgene.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Du Y, Hu Y, Li XS, Chen Y, Cheng Y. Whole-exome sequencing of individuals from an isolated population under extreme conditions implicates rare risk variants of schizophrenia. Transl Psychiatry. 2024;14:267. doi: 10.1038/s41398-024-02984-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidt-Kastner R, Guloksuz S, Kietzmann T, van Os J, Rutten BPF. Analysis of GWAS-Derived Schizophrenia Genes for Links to Ischemia-Hypoxia Response of the Brain. Front Psychiatry. 2020;11:393. doi: 10.3389/fpsyt.2020.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hervoso JL, Amoah K, Dodson J, Choudhury M, Bhattacharya A, Quinones-Valdez G, Pasaniuc B, et al. Splicing-specific transcriptome-wide association uncovers genetic mechanisms for schizophrenia. Am J Hum Genet. 2024;111(8):1573–1587. doi: 10.1016/j.ajhg.2024.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parker N, Cheng W, Hindley GFL, O’Connell KS, Karthikeyan S, Holen B, Shadrin AA, et al. Genetic overlap between global cortical brain structure, c-reactive protein, and white blood cell counts. Biol Psychiatry. 2024;95:62–71. doi: 10.1016/j.biopsych.2023.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gong W, Guo P, Li Y, Liu L, Yan R, Liu S, Wang S, et al. Role of the gut-brain axis in the shared genetic etiology between gastrointestinal tract diseases and psychiatric disorders: a genome-wide pleiotropic analysis. JAMA Psychiatry. 2023;80:360–370. doi: 10.1001/jamapsychiatry.2022.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Handford HA. Brain hypoxia, minimal brain dysfunction, and schizophrenia. Am J Psychiatry. 1975;132:192–194. doi: 10.1176/ajp.132.2.192. [DOI] [PubMed] [Google Scholar]
- 86.Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lonnqvist J, Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157:801–807. doi: 10.1176/appi.ajp.157.5.801. [DOI] [PubMed] [Google Scholar]
- 87.Cannon TD, Rosso IM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Dev Psychopathol. 1999;11:467–485. doi: 10.1017/S0954579499002163. [DOI] [PubMed] [Google Scholar]
- 88.Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia. Population-based case-control study. Br J Psychiatry. 2001;179:403–408. doi: 10.1192/bjp.179.5.403. [DOI] [PubMed] [Google Scholar]
- 89.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benes FM, Sorensen I, Bird ED. Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull. 1991;17:597–608. doi: 10.1093/schbul/17.4.597. [DOI] [PubMed] [Google Scholar]
- 91.Van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, Salonen O, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- 92.Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, Straub RE, Weinberger DR. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry. 2008;13:873–7. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 93.Cannon TD, Yolken R, Buka S, Torrey EF Collaborative Study Group on the Perinatal Origins of Severe Psychiatric D. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry. 2008;64:797–802. doi: 10.1016/j.biopsych.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morikawa T, Manabe T, Ito Y, Yamada S, Yoshimi A, Nagai T, Ozaki N, et al. The expression of HMGA1a is increased in lymphoblastoid cell lines from schizophrenia patients. Neurochem Int. 2010;56:736–739. doi: 10.1016/j.neuint.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Okazaki S, Boku S, Watanabe Y, Otsuka I, Horai T, Morikawa R, Kimura A, et al. Polymorphisms in the hypoxia inducible factor binding site of the macrophage migration inhibitory factor gene promoter in schizophrenia. PLoS One. 2022;17:e0265738. doi: 10.1371/journal.pone.0265738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barodia SK, Park SK, Ishizuka K, Sawa A, Kamiya A. Half-life of DISC1 protein and its pathological significance under hypoxia stress. Neurosci Res. 2015;97:1–6. doi: 10.1016/j.neures.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 98.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 99.Armit C, Richardson L, Hill B, Yang Y, Baldock RA. eMouseAtlas informatics: embryo atlas and gene expression database. Mamm Genome. 2015;26:431–40. doi: 10.1007/s00335-015-9596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Theiler K. The House Mouse: Atlas of Embryonic Development. Springer; Berlin, Heidelberg: 1989. [DOI] [Google Scholar]
- 101.Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–54. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- 102.Nadri C, Belmaker RH, Agam G. Oxygen restriction of neonate rats elevates neuregulin-1alpha isoform levels: possible relationship to schizophrenia. Neurochem Int. 2007;51:447–450. doi: 10.1016/j.neuint.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 103.Molnar Z, Luhmann HJ, Kanold PO. Transient cortical circuits match spontaneous and sensory-driven activity during development. Science. 2020:370. doi: 10.1126/science.abb2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.El-Khodor BF, Boksa P. Birth insult increases amphetamine-induced behavioral responses in the adult rat. Neuroscience. 1998;87:893–904. doi: 10.1016/S0306-4522(98)00194-8. [DOI] [PubMed] [Google Scholar]
- 105.Vaillancourt C, Boksa P. Birth insult alters dopamine-mediated behavior in a precocial species, the guinea pig. Implications for schizophrenia. Neuropsychopharmacology. 2000;23:654–66. doi: 10.1016/S0893-133X(00)00164-0. [DOI] [PubMed] [Google Scholar]
- 106.Tejkalova H, Kaiser M, Klaschka J, Stastny F. Does neonatal brain ischemia induce schizophrenia-like behavior in young adult rats? Physiol Res. 2007;56:815–23. doi: 10.33549/physiolres.931056. [DOI] [PubMed] [Google Scholar]
- 107.Brake WG, Sullivan RM, Gratton A. Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J Neurosci. 2000;20:5538–43. doi: 10.1523/JNEUROSCI.20-14-05538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.El-Khodor BF, Boksa P. Long-term reciprocal changes in dopamine levels in prefrontal cortex versus nucleus accumbens in rats born by Caesarean section compared to vaginal birth. Exp Neurol. 1997;145:118–29. doi: 10.1006/exnr.1997.6437. [DOI] [PubMed] [Google Scholar]
- 109.Laplante F, Brake WG, Chehab SL, Sullivan RM. Sex differences in the effects of perinatal anoxia on dopamine function in rats. Neurosci Lett. 2012;506:89–93. doi: 10.1016/j.neulet.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 110.Papazisis G, Kallaras K, Kaiki-Astara A, Pourzitaki C, Tzachanis D, Dagklis T, Kouvelas D. Neuroprotection by lamotrigine in a rat model of neonatal hypoxic-ischaemic encephalopathy. Int J Neuropsychopharmacol. 2008;11:321–329. doi: 10.1017/S1461145707008012. [DOI] [PubMed] [Google Scholar]
- 111.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26:287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 112.Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139:161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He H, Yu Y, Liew Z, Gissler M, Laszlo KD, Valdimarsdottir UA, Zhang J, et al. Association of maternal autoimmune diseases with risk of mental disorders in offspring in Denmark. JAMA Netw Open. 2022;5:e227503. doi: 10.1001/jamanetworkopen.2022.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–7. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 115.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 116.Xiao J, Buka SL, Cannon TD, Suzuki Y, Viscidi RP, Torrey EF, Yolken RH. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009;11:1011–1018. doi: 10.1016/j.micinf.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 117.Lee YH, Cherkerzian S, Seidman LJ, Papandonatos GD, Savitz DA, Tsuang MT, Goldstein JM, et al. Maternal bacterial infection during pregnancy and offspring risk of psychotic disorders: Variation by severity of infection and offspring sex. Am J Psychiatry. 2020;177:66–75. doi: 10.1176/appi.ajp.2019.18101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buchsbaum MS. The frontal lobes, basal ganglia, and temporal lobes as sites for schizophrenia. Schizophr Bull. 1990;16:379–389. doi: 10.1093/schbul/16.3.379. [DOI] [PubMed] [Google Scholar]
- 119.Soares JC, Innis RB. Neurochemical brain imaging investigations of schizophrenia. Biol Psychiatry. 1999;46:600–615. doi: 10.1016/S0006-3223(99)00015-3. [DOI] [PubMed] [Google Scholar]
- 120.Supprian T, Ulmar G, Bauer M, Schuler M, Puschel K, Retz-Junginger P, Schmitt HP, et al. Cerebellar vermis area in schizophrenic patients - a post-mortem study. Schizophr Res. 2000;42:19–28. doi: 10.1016/S0920-9964(99)00103-6. [DOI] [PubMed] [Google Scholar]
- 121.van Hooijdonk CFM, van der Pluijm M, Bosch I, van Amelsvoort T, Booij J, de Haan L, Selten JP, et al. The substantia nigra in the pathology of schizophrenia: A review on post-mortem and molecular imaging findings. Eur Neuropsychopharmacol. 2023;68:57–77. doi: 10.1016/j.euroneuro.2022.12.008. [DOI] [PubMed] [Google Scholar]
- 122.van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7:e1075. doi: 10.1038/tp.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jutla A, Foss-Feig J, Veenstra-VanderWeele J. Autism spectrum disorder and schizophrenia: An updated conceptual review. Autism Res. 2022;15:384–412. doi: 10.1002/aur.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han VX, Jones HF, Patel S, Mohammad SS, Hofer MJ, Alshammery S, Maple-Brown E, et al. Emerging evidence of Toll-like receptors as a putative pathway linking maternal inflammation and neurodevelopmental disorders in human offspring: A systematic review. Brain Behav Immun. 2022;99:91–105. doi: 10.1016/j.bbi.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 128.Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72:1317–1326. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
- 129.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–32. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reed MD, Yim YS, Wimmer RD, Kim H, Ryu C, Welch GM, Andina M, et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577:249–53. doi: 10.1038/s41586-019-1843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 132.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perez-Morales M, Bello-Medina PC, Gonzalez-Franco DA, Diaz-Cintra S, Garcia-Mena J, Pacheco-Lopez G, Neuro-Psycho-Biota C. Steering the Microbiota-Gut-Brain Axis by Antibiotics to Model Neuro-Immune-Endocrine Disorders. Neuroimmunomodulation. 2024;31:89–101. doi: 10.1159/000538927. [DOI] [PubMed] [Google Scholar]
- 134.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46:77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 135.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 136.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 137.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5:eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li S, Song J, Ke P, Kong L, Lei B, Zhou J, Huang Y, et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci Rep. 2021;11:9743. doi: 10.1038/s41598-021-89166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim HN, Joo EJ, Lee CW, Ahn KS, Kim HL, Park DI, Park SK. Reversion of gut microbiota during the recovery phase in patients with asymptomatic or mild COVID-19: Longitudinal Study. Microorganisms. 2021;9:1237. doi: 10.3390/microorganisms9061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lundgren SN, Madan JC, Emond JA, Morrison HG, Christensen BC, Karagas MR, Hoen AG. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome. 2018;6:109. doi: 10.1186/s40168-018-0490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Isaevska E, Popovic M, Pizzi C, Fiano V, Rusconi F, Merletti F, Richiardi L, et al. Maternal antibiotic use and vaginal infections in the third trimester of pregnancy and the risk of obesity in preschool children. Pediatr Obes. 2020;15:e12632. doi: 10.1111/ijpo.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hassib L, de Oliveira CL, Rouvier GA, Kanashiro A, Guimaraes FS, Ferreira FR. Maternal microbiome disturbance induces deficits in the offspring’s behaviors: a systematic review and meta-analysis. Gut Microbes. 2023;15:2226282. doi: 10.1080/19490976.2023.2226282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.MacDowell KS, Munarriz-Cuezva E, Meana JJ, Leza JC, Ortega JE. Paliperidone reversion of maternal immune activation-induced changes on brain serotonin and kynurenine pathways. Front Pharmacol. 2021;12:682602. doi: 10.3389/fphar.2021.682602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Champagne-Jorgensen K, Mian MF, Kay S, Hanani H, Ziv O, McVey Neufeld KA, Koren O, et al. Prenatal low-dose penicillin results in long-term sex-specific changes to murine behaviour, immune regulation, and gut microbiota. Brain Behav Immun. 2020;84:154–63. doi: 10.1016/j.bbi.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 146.Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, Koren O, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062. doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tejkalova H, Jakob L, Kvasnova S, Klaschka J, Sechovcova H, Mrazek J, Palenicek T, et al. The influence of antibiotic treatment on the behavior and gut microbiome of adult rats neonatally insulted with lipopolysaccharide. Heliyon. 2023;9:e15417. doi: 10.1016/j.heliyon.2023.e15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lebovitz Y, Kowalski EA, Wang X, Kelly C, Lee M, McDonald V, Ward R, et al. Lactobacillus rescues postnatal neurobehavioral and microglial dysfunction in a model of maternal microbiome dysbiosis. Brain Behav Immun. 2019;81:617–629. doi: 10.1016/j.bbi.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 149.Villar J, Soto Conti CP, Gunier RB, Ariff S, Craik R, Cavoretto PI, Rauch S, et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet. 2023;401:447–57. doi: 10.1016/S0140-6736(22)02467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, Taylor HS, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:35–53e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim EH, Kim YI, Jang SG, Im M, Jeong K, Choi YK, Han HJ. Antiviral effects of human placenta hydrolysate (Laennec((R))) against SARS-CoV-2 in vitro and in the ferret model. J Microbiol. 2021;59:1056–62. doi: 10.1007/s12275-021-1367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McMahon CL, Castro J, Silvas J, Muniz Perez A, Estrada M, Carrion R, Jr, Hsieh J. Fetal brain vulnerability to SARS-CoV-2 infection. Brain Behav Immun. 2023;112:188–205. doi: 10.1016/j.bbi.2023.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Patberg ET, Adams T, Rekawek P, Vahanian SA, Akerman M, Hernandez A, Rapkiewicz AV, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224:382e1–e18. doi: 10.1016/j.ajog.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Edlow AG, Castro VM, Shook LL, Kaimal AJ, Perlis RH. Neurodevelopmental Outcomes at 1 Year in Infants of Mothers Who Tested Positive for SARS-CoV-2 During Pregnancy. JAMA Netw Open. 2022;5:e2215787. doi: 10.1001/jamanetworkopen.2022.15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Edlow AG, Castro VM, Shook LL, Haneuse S, Kaimal AJ, Perlis RH. Sex-specific neurodevelopmental outcomes in offspring of mothers with SARS-CoV-2 in pregnancy: an electronic health records cohort. medRxiv. 2022 doi: 10.1101/2022.11.18.22282448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fernandez-Abascal B, Suarez-Pinilla P, Cobo-Corrales C, Crespo-Facorro B, Suarez-Pinilla M. In- and outpatient lifestyle interventions on diet and exercise and their effect on physical and psychological health: a systematic review and meta-analysis of randomised controlled trials in patients with schizophrenia spectrum disorders and first episode of psychosis. Neurosci Biobehav Rev. 2021;125:535–68. doi: 10.1016/j.neubiorev.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 158.Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45:1343–61. doi: 10.1017/S0033291714003110. [DOI] [PubMed] [Google Scholar]
- 159.Allswede DM, Buka SL, Yolken RH, Torrey EF, Cannon TD. Elevated maternal cytokine levels at birth and risk for psychosis in adult offspring. Schizophr Res. 2016;172:41–5. doi: 10.1016/j.schres.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 160.Swanepoel T, Moller M, Harvey BH. N-acetyl cysteine reverses bio-behavioural changes induced by prenatal inflammation, adolescent methamphetamine exposure and combined challenges. Psychopharmacology (Berl) 2018;235:351–368. doi: 10.1007/s00213-017-4776-5. [DOI] [PubMed] [Google Scholar]
- 161.Thordstein M, Bagenholm R, Thiringer K, Kjellmer I. Scavengers of free oxygen radicals in combination with magnesium ameliorate perinatal hypoxic-ischemic brain damage in the rat. Pediatr Res. 1993;34:23–26. doi: 10.1203/00006450-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 162.Palma-Gudiel H, Eixarch E, Crispi F, Moran S, Zannas AS, Fananas L. Prenatal adverse environment is associated with epigenetic age deceleration at birth and hypomethylation at the hypoxia-responsive EP300 gene. Clin Epigenetics. 2019;11:73. doi: 10.1186/s13148-019-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Su Y, Lian J, Chen S, Zhang W, Deng C. Epigenetic histone acetylation modulating prenatal Poly I:C induced neuroinflammation in the prefrontal cortex of rats: a study in a maternal immune activation model. Front Cell Neurosci. 2022;16:1037105. doi: 10.3389/fncel.2022.1037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Labouesse MA, Dong E, Grayson DR, Guidotti A, Meyer U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics. 2015;10:1143–1155. doi: 10.1080/15592294.2015.1114202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu Y, Tian Y, Wang Y, Xu L, Song G, Wu Q, Wang W, et al. Exosomes derived from astrocytes after oxygen-glucose deprivation promote differentiation and migration of oligodendrocyte precursor cells in vitro. Mol Biol Rep. 2021;48:5473–84. doi: 10.1007/s11033-021-06557-w. [DOI] [PubMed] [Google Scholar]
- 166.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Palmqvist S, Tideman P, Mattsson-Carlgren N, Schindler SE, Smith R, Ossenkoppele R, Calling S, et al. Blood biomarkers to detect alzheimer disease in primary care and secondary care. JAMA. 2024 doi: 10.1001/jama.2024.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wolff AR, Cheyne KR, Bilkey DK. Behavioural deficits associated with maternal immune activation in the rat model of schizophrenia. Behav Brain Res. 2011;225:382–387. doi: 10.1016/j.bbr.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 169.Hemmerle AM, Ahlbrand R, Bronson SL, Lundgren KH, Richtand NM, Seroogy KB. Modulation of schizophrenia-related genes in the forebrain of adolescent and adult rats exposed to maternal immune activation. Schizophr Res. 2015;168:411–420. doi: 10.1016/j.schres.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, Chung S, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4:e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Coleman MT, Rund DA. Nonobstetric conditions causing hypoxia during pregnancy: asthma and epilepsy. Am J Obstet Gynecol. 1997;177:1–7. doi: 10.1016/S0002-9378(97)70429-0. [DOI] [PubMed] [Google Scholar]
- 172.Cousins L. Fetal oxygenation, assessment of fetal well-being, and obstetric management of the pregnant patient with asthma. J Allergy Clin Immunol. 1999;103:S343–9. doi: 10.1016/S0091-6749(99)70260-5. [DOI] [PubMed] [Google Scholar]
- 173.Chen YH, Keller J, Wang IT, Lin CC, Lin HC. Pneumonia and pregnancy outcomes: a nationwide population-based study. Am J Obstet Gynecol. 2012;207:288 e1–7. doi: 10.1016/j.ajog.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Krampl E. Pregnancy at high altitude. Ultrasound Obstet Gynecol. 2002;19:535–539. doi: 10.1046/j.1469-0705.2002.00738.x. [DOI] [PubMed] [Google Scholar]
- 175.Woodman AG, Care AS, Mansour Y, Cherak SJ, Panahi S, Gragasin FS, Bourque SL. Modest and Severe Maternal Iron Deficiency in Pregnancy are Associated with Fetal Anaemia and Organ-Specific Hypoxia in Rats. Sci Rep. 2017;7:46573. doi: 10.1038/srep46573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tong W, Giussani DA. Preeclampsia link to gestational hypoxia. J Dev Orig Health Dis. 2019;10:322–333. doi: 10.1017/S204017441900014X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Habek D, Habek JC, Ivanisevic M, Djelmis J. Fetal tobacco syndrome and perinatal outcome. Fetal Diagn Ther. 2002;17:367–71. doi: 10.1159/000065387. [DOI] [PubMed] [Google Scholar]
- 178.Socol ML, Manning FA, Murata Y, Druzin ML. Maternal smoking causes fetal hypoxia: experimental evidence. Am J Obstet Gynecol. 1982;142:214–8. doi: 10.1016/S0002-9378(16)32339-0. [DOI] [PubMed] [Google Scholar]
- 179.Saha PS, Mayhan WG. Prenatal exposure to alcohol: mechanisms of cerebral vascular damage and lifelong consequences. Adv Drug Alcohol Res. 2022;2:10818. doi: 10.3389/adar.2022.10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bosco C, Diaz E. Placental hypoxia and foetal development versus alcohol exposure in pregnancy. Alcohol Alcohol. 2012;47:109–117. doi: 10.1093/alcalc/agr166. [DOI] [PubMed] [Google Scholar]
- 181.Teramo K, Klemetti M, Tikkanen M, Nuutila M. [Maternal diabetes and fetal hypoxia] Duodecim. 2013;129:228–234. [PubMed] [Google Scholar]
- 182.Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr. 2010;2010:401323. doi: 10.1155/2010/401323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wray S, Alruwaili M, Prendergast C. Hypoxia and reproductive health: Hypoxia and labour. Reproduction. 2021;161:F67–F80. doi: 10.1530/REP-20-0327. [DOI] [PubMed] [Google Scholar]
- 184.Acharya A, Swain B, Pradhan S, Jena PK, Mohakud NK, Swain A, Mohanty N. Clinico-Biochemical Correlation in Birth Asphyxia and Its Effects on Outcome. Cureus. 2020;12:e11407. doi: 10.7759/cureus.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Peebles DM, Spencer JA, Edwards AD, Wyatt JS, Reynolds EO, Cope M, Delpy DT. Relation between frequency of uterine contractions and human fetal cerebral oxygen saturation studied during labour by near infrared spectroscopy. Br J Obstet Gynaecol. 1994;101:44–48. doi: 10.1111/j.1471-0528.1994.tb13008.x. [DOI] [PubMed] [Google Scholar]
- 186.Boushra M, Stone A, Rathbun KM. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2023. 2024. Umbilical Cord Prolapse. [PubMed] [Google Scholar]
- 187.Peesay M. Nuchal cord and its implications. Matern Health Neonatol Perinatol. 2017;3:28. doi: 10.1186/s40748-017-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gaikwad V, Yalla S, Salvi P. True Knot of the Umbilical Cord and Associated Adverse Perinatal Outcomes: A Case Series. Cureus. 2023;15:e35377. doi: 10.7759/cureus.35377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Matsuda Y, Ogawa M, Konno J, Mitani M, Matsui H. Prediction of fetal acidemia in placental abruption. BMC Pregnancy Childbirth. 2013;13:156. doi: 10.1186/1471-2393-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jenabi E, Bashirian S, Khoshravesh S. The association between of placenta previa and congenital abnormalities: a systematic review and network meta-analysis. BMC Pediatr. 2023;23:606. doi: 10.1186/s12887-023-04433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Johnson N, van Oudgaarden E, Montague I, McNamara H. The effect of oxytocin-induced hyperstimulation on fetal oxygen. Br J Obstet Gynaecol. 1994;101:805–7. doi: 10.1111/j.1471-0528.1994.tb11951.x. [DOI] [PubMed] [Google Scholar]
- 192.Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, Te Pas A, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023;120:3–23. doi: 10.1159/000528914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Monfredini C, Cavallin F, Villani PE, Paterlini G, Allais B, Trevisanuto D. Meconium Aspiration Syndrome: A Narrative Review. Children (Basel) 2021;8:230. doi: 10.3390/children8030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ottolenghi S, Milano G, Cas MD, Findley TO, Paroni R, Corno AF. Can Erythropoietin Reduce Hypoxemic Neurological Damages in Neonates With Congenital Heart Defects? Front Pharmacol. 2021;12:770590. doi: 10.3389/fphar.2021.770590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yadav P, Yadav SK. Progress in Diagnosis and Treatment of Neonatal Sepsis: A Review Article. JNMA J Nepal Med Assoc. 2022;60:318–24. doi: 10.31729/jnma.7324. https://doi.org/10.31729/jnma.7324 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nandula PS, Shah SD. StatPearls, editor. Persistent Pulmonary Hypertension of the Newborn. Treasure Island (FL): 2024. [PubMed] [Google Scholar]
- 197.Soderborg TK, Carpenter CM, Janssen RC, Weir TL, Robertson CE, Ir D, Young BE, et al. Gestational Diabetes Is Uniquely Associated With Altered Early Seeding of the Infant Gut Microbiota. Front Endocrinol (Lausanne) 2020;11:603021. doi: 10.3389/fendo.2020.603021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lemas DJ, Klimentidis YC, Aslibekyan S, Wiener HW, O’Brien DM, Hopkins SE, Stanhope KL, et al. Polymorphisms in stearoyl coa desaturase and sterol regulatory element binding protein interact with N-3 polyunsaturated fatty acid intake to modify associations with anthropometric variables and metabolic phenotypes in Yup’ik people. Mol Nutr Food Res. 2016;60:2642–53. doi: 10.1002/mnfr.201600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mepham J, Nelles-McGee T, Andrews K, Gonzalez A. Exploring the effect of prenatal maternal stress on the microbiomes of mothers and infants: A systematic review. Dev Psychobiol. 2023;65:e22424. doi: 10.1002/dev.22424. [DOI] [PubMed] [Google Scholar]
- 200.Naspolini NF, Meyer A, Moreira JC, Sun H, Froes-Asmus CIR, Dominguez-Bello MG. Environmental pollutant exposure associated with altered early-life gut microbiome: Results from a birth cohort study. Environ Res. 2022;205:112545. doi: 10.1016/j.envres.2021.112545. [DOI] [PubMed] [Google Scholar]
- 201.Banerjee S, Suter MA, Aagaard KM. Interactions between Environmental Exposures and the Microbiome: Implications for Fetal Programming. Curr Opin Endocr Metab Res. 2020;13:39–48. doi: 10.1016/j.coemr.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zordao OP, Campolim CM, Yariwake VY, Castro G, Ferreira CKO, Santos A, Norberto S, et al. Maternal exposure to air pollution alters energy balance transiently according to gender and changes gut microbiota. Front Endocrinol (Lausanne) 2023;14:1069243. doi: 10.3389/fendo.2023.1069243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McLean C, Jun S, Kozyrskyj A. Impact of maternal smoking on the infant gut microbiota and its association with child overweight: a scoping review. World J Pediatr. 2019;15:341–9. doi: 10.1007/s12519-019-00278-8. [DOI] [PubMed] [Google Scholar]
- 204.Peng Y, Tun HM, Ng SC, Wai HK, Zhang X, Parks J, Field CJ, et al. Maternal smoking during pregnancy increases the risk of gut microbiome-associated childhood overweight and obesity. Gut Microbes. 2024;16:2323234. doi: 10.1080/19490976.2024.2323234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Huang H, Jiang J, Wang X, Jiang K, Cao H. Exposure to prescribed medication in early life and impacts on gut microbiota and disease development. EClinicalMedicine. 2024;68:102428. doi: 10.1016/j.eclinm.2024.102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Morreale C, Giaroni C, Baj A, Folgori L, Barcellini L, Dhami A, Agosti M, et al. Effects of Perinatal Antibiotic Exposure and Neonatal Gut Microbiota. Antibiotics (Basel) 2023;12:258. doi: 10.3390/antibiotics12020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cuinat C, Stinson SE, Ward WE, Comelli EM. Maternal Intake of Probiotics to Program Offspring Health. Curr Nutr Rep. 2022;11:537–562. doi: 10.1007/s13668-022-00429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sanz Y. Gut microbiota and probiotics in maternal and infant health. Am J Clin Nutr. 2011;94:2000S–5S. doi: 10.3945/ajcn.110.001172. [DOI] [PubMed] [Google Scholar]
- 209.Yang J, Hou L, Wang J, Xiao L, Zhang J, Yin N, Yao S, et al. Unfavourable intrauterine environment contributes to abnormal gut microbiome and metabolome in twins. Gut. 2022;71:2451–2462. doi: 10.1136/gutjnl-2021-326482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jeong S. Factors influencing development of the infant microbiota: from prenatal period to early infancy. Clin Exp Pediatr. 2022;65:439–447. doi: 10.3345/cep.2021.00955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reyman M, van Houten MA, van Baarle D, Bosch A, Man WH, Chu M, Arp K, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10:4997. doi: 10.1038/s41467-019-13014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Palmeira O, Matos LRB, Naslavsky MS, Bueno HMS, Soler JP, Setubal JC, Zatz M. Longitudinal 16S rRNA gut microbiota data of infant triplets show partial susceptibility to host genetics. iScience. 2022;25:103861. doi: 10.1016/j.isci.2022.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Matsuki T, Yahagi K, Mori H, Matsumoto H, Hara T, Tajima S, Ogawa E, et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun. 2016;7:11939. doi: 10.1038/ncomms11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, Sanders JG, et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134–42. doi: 10.1038/s41588-021-00991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]