Summary
Pulmonary hypertension is a complex and heterogeneous condition with five main subtypes (groups). This review focuses on pulmonary hypertension caused by chronic hypoxia (hypoxic pulmonary hypertension, HPH, group 3). It is based mainly on our own experimental work, especially our collaboration with the group of Professor Herget, whose fifth anniversary of death we commemorate. We have found that oxidation and degradation of the extracellular matrix (ECM) in vitro, in either the presence or the absence of pro-inflammatory cells, activate vascular smooth muscle cell (VSMC) proliferation. Significant changes in the ECM of pulmonary arteries also occurred in vivo in hypoxic rats, namely a decrease in collagen VI and an increase in matrix metalloproteinase 9 (MMP-9) in the tunica media, which may also contribute to the growth activation of VSMCs. The proliferation of VSMCs was also enhanced in their co-culture with macrophages, most likely due to the paracrine production of growth factors in these cells. However, hypoxia itself has a dual effect: on the one hand, it can activate VSMC proliferation and hyperplasia, but on the other hand, it can also induce VSMC hypertrophy and increased expression of contractile markers in these cells. The influence of hypoxia-inducible factors, microRNAs and galectin-3 in the initiation and development of HPH, and the role of cell types other than VSMCs (endothelial cells, adventitial fibroblasts) are also discussed.
Keywords: Vasoconstriction, Remodeling, Oxidation, Degradation, Extracellular matrix, Collagen, Proteolytic enzymes, Metalloproteinases, Macrophages, Mast cells, Smooth muscle cells, Endothelial cells, Fibroblasts, Mesenchymal stem cells, Hypoxia-inducible factor, microRNA, Galectins, Hyperplasia, Hypertrophy, Therapy of hypoxic pulmonary hypertension
Introduction
Compared to the systemic circulation, the pulmonary vasculature is a low-pressure system. Pulmonary hypertension (PH) is defined as a mean pulmonary artery pressure (mPAP) greater than 20 mmHg at rest, according to the ESC/ERS 2022 Guidelines for the diagnosis and treatment of pulmonary hypertension [1]. PH is a complex, heterogeneous group of diseases with different etiologies, manifestations, course, prognosis and treatment (for a review, see [2–4]). Common to all of them is not only the elevation of mPAP, but also pathological remodelling of the pulmonary vascular wall. Most often PH leads to right heart ventricle hypertrophy and eventually failure. PH markedly worsens the quality of life of the patients and their prognosis.
The current classification of PH recognizes 5 groups [1]:
Group 1
Pulmonary arterial hypertension (PAH). This is pre-capillary hypertension [4], the primary pathology of which lies primarily in the pulmonary vasculature. Its prevalence and incidence is relatively low [5]. It includes idiopathic and familiar subtypes, as well as various drug-induced and other uncommon forms (for a review, see [1,2,6,7]).
Group 2
PH associated with left heart disease, which is the most common type of pulmonary hypertension, at least in the Western world [5]. The increased blood pressure in the left atrium propagates “backwards” to the pulmonary veins. Thus, initially, post-capillary PH develops [4], but later mPAP may also increase, resulting in combined pre- and post-capillary PH (for a review, see [2,3]).
Group 3
PH associated with lung disease and/or hypoxemia. This is the second most common type of PH [5]. It is a pre-capillary form of PH [4] and occurs mainly in chronic obstructive pulmonary disease (COPD), but it has also been reported in obstructive sleep apnea syndrome and in interstitial pulmonary fibrosis (for a review, see [2,3, 8–10]). Chronic exposure to cigarette smoke can also lead to this type of PH [11,12]. Hypoxia of high altitude also elicits PH (high-altitude pulmonary hypertension, HAPH) [13]. In comparison with PAH (group 1), which is progressive and irreversible, the pulmonary vascular remodeling during hypoxic PH is milder and is largely thought to be reversible, although some patients can also develop severe PH with irreversible vascular remodeling similar to that in the group 1 [14]. In addition, group 3 PH can overlap with group 2 (29.3 % of patients with PH) [5].
Group 4
PH associated with chronic pulmonary artery obstruction (or chronic thromboembolic pulmonary hypertension, CTEPH). This is pre-capillary hypertension [4,15].
Group 5
PH with unclear and/or multifactorial mechanisms; it is either pre-capillary or combined pre- and post-capillary PH (for a review, see [2,4,16]).
In our studies performed in collaboration with Professor Jan Herget’s group, we focused on hypoxic pulmonary hypertension (HPH), which belongs to the group 3. Studies by Professor Jan Herget’s group focused on HPH induced by low oxygen partial pressure in the surrounding atmosphere, e.g. in an experimental hypoxic chamber simulating high altitudes [17,18], during airway obstruction [19], or in lung diseases, such as microbial and sterile inflammation [20], asthma, emphysema [21,22], or silicosis [23].
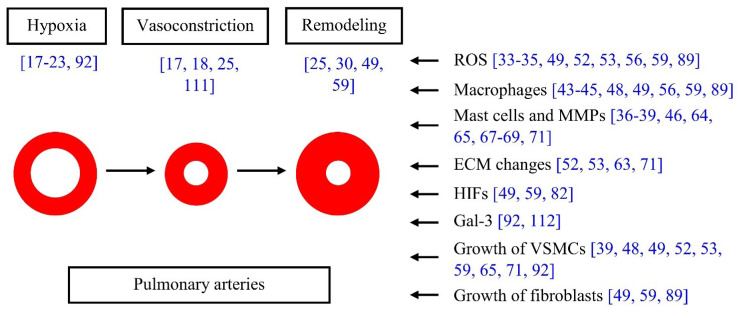
There are two mechanisms involved in the onset and development of HPH: hypoxic pulmonary vasoconstriction and hypoxic pulmonary vascular remodeling (for a review, see [8,24]). Important actors in both these stages are vascular smooth muscle cells (VSMCs). This review focuses on the role and behavior of VSMCs in both of these stages, particularly in the vascular wall remodeling.
VSMCs in hypoxic pulmonary vasoconstriction
The immediate response of pulmonary vessels to a decrease in alveolar oxygen partial pressure is vasoconstriction [13,25]. This differs from systemic circulation, where the main response to hypoxia is vasodilation, caused by the stabilization of hypoxia-inducible factor-1α (HIF-1α), i.e. its avoidance of prolyl hydroxylation [26], and concomitant upregulation of endothelial nitric oxide (NO) synthase. Hypoxic pulmonary vasoconstriction is a physiological mechanism reducing the distribution of blood to hypoventilated areas of the lungs, thereby maintaining optimal blood oxygenation [13,27,28].
Pulmonary vasoconstriction is a functional change in the pulmonary vasculature, mediated by VSMCs, and is completely reversible, at least in health. The mechanism by which VSMCs sense hypoxia has been a subject of considerable research and debate and is beyond the scope of this text; it is reviewed in detail by Archer et al. in this issue. Suffice it to say here that hypoxia-induced redox changes in the VSMCs diminish the open-state probability of several types of potassium channels, resulting in cell membrane depolarization. This, in turn, activates voltage-gated calcium channels, and thus the influx of Ca2+ ions and subsequent activation of the contractile apparatus in VSMCs (for a review, see [8,29,24]). Ca2+ release from the sarcoplasmic reticulum is also an important part of the mechanism, as is Ca2+ influx through other types of Ca2+-conductive channels (see Archer et al. in this issue).
Although hypoxic pulmonary vasoconstriction is intrinsic to VSMCs, it can be modulated by the endothelium, i.e., decreased by endothelium-derived vasodilators, such as NO [25] and prostacyclin, and increased by endothelium-derived vasoconstrictors, such as endothelin and thromboxane [29], and also by inhibition of NO synthase. This inhibition may be caused, at least partly, by the elevation of asymmetric dimethylarginine, an endogenous inhibitor of NO synthesis [27].
VSMCs in structural remodeling of the pulmonary vasculature
With prolonged duration or recurrence of hypoxia, morphological remodeling of pulmonary vessels, particularly peripheral, pre-alveolar vessels, follows [25,30].
The mechanism of remodeling of the vascular wall during hypoxia involves, although it may seem somewhat paradoxical, the production of reactive oxygen species (ROS). On the one hand, it is logical to understand hypoxia as a reducing state. During hypoxia, the limited availability of oxygen prevents the generation of ROS, including superoxide (O2−) and its conversion to hydrogen peroxide, decreases the ratio of oxidized/reduced redox couples (e.g., NAD+/NADH, NADP+/NADPH, FAD2+/FADH2), and reduces sulfhydryl groups on various molecules [24, 29, 31,32]. On the other hand, several authors, including Prof. Herget and his co-workers [33–35], have reported an increased production of ROS in pulmonary blood vessels during chronic hypoxia. This has been attributed to the activity of enzymes such as xanthine oxidase, endothelial NO synthase (eNOS) and NADPH oxidase, all of which are capable of producing superoxide, as well as inducible NO synthase (iNOS), increasing the production of NO, which readily generates peroxynitrite in the presence of superoxide. Further indirect evidence for increased ROS production in hypoxic pulmonary hypertension is that antioxidant treatment ameliorated pulmonary hypertension in rats exposed to chronic hypoxia [33–35].
Another important mechanism of the remodeling of pulmonary blood vessels is the production of proteolytic enzymes, especially by cells of the immune system, infiltrating the vascular wall during hypoxia [36–39]. The effect of both ROS and proteolytic enzymes ultimately stimulates the proliferation of VSMCs, their hyperplasia, increased matrix deposition by these cells, and thickening of the vascular wall in pulmonary hypertension (for a review, see [8, 13, 40]).
Effects of ROS and macrophages
During chronic hypoxia, ROS are produced by cells of the pulmonary vasculature, namely endothelial cells, VSMCs, adventitial fibroblasts [14,41,42], and also cells of the immune system, such as alveolar and interstitial macrophages [33,43–45] and mast cells, infiltrating large and small pulmonary arteries and occurring subpleurally and perivascularly [36,46].
ROS can have a dual effect on cells, including VSMCs. In higher concentrations, they can cause damage to the cell membrane, mitochondria, DNA or alteration of the function of various enzymes in the sense of their inhibition (protein kinase C) or activation (proteases and endonucleases). These effects can then lead to cell growth arrest and cell death. However, dying cells and their surviving neighbors can produce growth factors and other biomolecules that ultimately stimulate the proliferation of the cell population originally damaged by oxidative stress. Moreover, some authors even believe that at lower concentrations, ROS can directly stimulate cell proliferation, acting as signaling molecules. The mechanism of this effect is similar to that of growth factors. ROS can activate the receptor and non-receptor tyrosine kinases, mitogen-activated protein kinase (MAPK), transcription factor NF-κB, the expression of proto-oncogenes c-fos, and c-jun, the apoptosis-regulating Bcl-2 gene, and also autocrine production of growth factors, which plays an important role in activating the growth of the VSMC population. Already in 1996, Burdon formulated the hypothesis that normal production of ROS is necessary for normal transduction of signals regulating cell growth [47]; for a review, see [12,48,49]. Mitochondria and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase are considered to be the major ROS producers in the cardiovascular system. In accordance with this, studies on VSMCs under normobaric hypoxia in both humans and animals have shown that the Nox4 isoform of the NADPH oxidase complex was overexpressed, which contributed to VSMC proliferation leading to pulmonary hypertension [13]. In contrast, studies by Archer and co-workers have shown that ROS produced at physiological levels during mitochondrial electron transport maintain the open state of voltage-gated K channels, which inhibits the influx of Ca2+ ions into cells [29,50]. This keeps the pulmonary circulation in a relaxed state and does not promote VSMC proliferation, because Ca2+ ions are necessary not only for VSMC contraction but also for proliferation [49,51].
However, ROS can also stimulate VSMC proliferation indirectly by modifying their extracellular matrix (ECM). To explore this possibility, we developed a simple model of oxidative damage to the ECM in vitro, i.e., namely collagen irradiated with ultraviolet (UV) light. This was a model of pathological ROS production, not a physiological basal ROS production by electron leakage from mitochondria. We used either type I collagen, which is the major ECM protein of the healthy vessel wall, or type III collagen, another important type of collagen in the blood vessel wall, whose content increases in a pathologically altered vascular wall, such as in hypertension. Although our research was primarily related to PH, we initially used rat aortic smooth muscle cells as a cellular model because of their relatively easy isolation compared to pulmonary vascular cells [52,53].
We found that UV light not only oxidized collagen but also degraded it into low molecular weight fragments. The adhesion of VSMCs to this collagen was weakened, which was reflected not only by their smaller spreading area but also by their higher susceptibility to detachment from the substrate by trypsin. Even molecular markers of cell adhesion differed between cells on irradiated collagen and on non-irradiated collagen. Cells on irradiated collagen had less developed focal adhesion plaques and contained lower concentrations of β1-integrins, which include receptors for collagen, and also lower concentrations of focal adhesion proteins talin and vinculin, contractile protein α-actin (i.e., a marker of VSMC differentiation) and cytoskeletal protein vimentin. At the same time, VSMCs proliferated faster on irradiated collagen, at least at lower population densities - at higher densities these differences disappeared. The faster proliferation of VSMCs was explained by the fact that the cells escaped the growth control provided by cell-matrix contact. In addition, VSMCs contained increased concentrations of immunoglobulin adhesion molecules such as intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which bind the cells of the immune system [52,53]. A similar pro-inflammatory phenotype of VSMCs, expressing ICAM-1 and producing various cytokines and chemokines, e.g. interleukins 1β and 6 (IL1β and IL6), monocyte chemoattractant protein-1 (MCP-1), further stimulating VSMC proliferation, has also been described in the pulmonary arteries of human patients suffering from HPH as well as in animal models of this disease (for a review, see [54,55]).
Our research therefore also focused on immune cells and their influence on the ECM and on the adhesion and proliferation of VSMCs. As the first of these immune cells, we focused on macrophages, as these cells are known to play an important role in the origin and development of vascular diseases, and are an important source of ROS. For example, already in 1996, it was shown by Professor Herget’s group that alveolar macrophages harvested from the lungs of rats exposed to hypoxia produced more ROS than macrophages from normoxic animals [56]. In addition, macrophages can digest the ECM with their proteolytic enzymes and may even proliferate in the pathologically altered vascular wall (for a review, see [14,57]). In our experiments, we therefore grew rat aortic VSMCs either in cocultures with rat alveolar macrophages or on a collagen substrate pre-modified with these macrophages activated by TiO2 dust.
We found that macrophages themselves did not proliferate in co-cultures but activated proliferation of VSMCs. Because this increased proliferative activity of VSMCs became apparent only after several days of coculture, we reasoned that it was induced by paracrine production of cytokines, chemokines and growth factors by macrophages (for a review, see [45,58,59]), rather than being an immediate effect of short-living oxygen radicals produced in these cells. It is known that macrophages exhibit remarkable plasticity in response to the environment and can be polarized into either pro-inflammatory M1 cells, which produce high levels of reactive oxygen and nitrogen species and pro-inflammatory cytokines, such as interferon-gamma and tumor necrosis factor-alpha (TNF-α), or into anti-inflammatory M2 cells, which promote tissue repair by producing immunomodulatory substances, such as interleukin 10 (IL10), and growth factors, such as transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF) [45,60]. A recent study by Professor Herget’s followers has shown that alveolar macrophages exposed to hypoxia were able to produce superoxide only in vivo but not in vitro, although they were able under both conditions to polarize to pro-proliferative M2 macrophages [44].
On collagen modified by activated macrophages, similar to UV light-modified collagen, the VSMCs adhered more weakly, were prone to spontaneous detachment, and showed signs of damage (e.g., vacuolization). The remaining undamaged VSMCs, however, proliferated rapidly and soon compensated for these cell losses [48]. The initial unfavorable response of VSMCs may be due to an adverse effect of activated macrophages on the collagen substrate. The wall of the failing right ventricle in PH contained macrophages with activated nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome, which induced mitochondrial damage, impaired growth and apoptosis of cardiomyocytes [60], and similar cell-damaging substances could also be retained in our macrophage-modified collagen used as a growth substrate for VSMCs.
In the context of ROS, it is also worth mentioning the dual role of NO in the pathogenesis of PH. The effect of NO on VSMCs depends on the amount of NO, the amount of ROS, and the ratio of NO to ROS. At relatively low concentrations, NO exerts vasodilatory and antiproliferative effects on VSMCs through a cyclic guanosine monophosphate (cGMP)-dependent pathway [25,61,62]. However, at high concentrations, NO readily reacts with oxygen and especially with superoxide to form highly reactive substances such as peroxynitrite [25]. At the same time, the production of peroxynitrite is also increased at higher ROS concentrations [12]. Through these mechanisms, NO changes its initial beneficial effect of relieving PH into an effect that further exacerbates PH by contributing to tissue injury [12,13,25,30,33].
Effect of proteolytic enzymes and mast cells
Cells of the immune system that infiltrate the vascular wall during hypoxia also produce proteolytic enzymes, such as chymases, tryptases and metalloproteinases (MMPs). These proteolytic enzymes degrade the ECM of pulmonary vessels, especially collagen [33,63,64], and thus are another important factor that induces VSMC proliferation by releasing these cells from growth inhibition by the physiological ECM (for a review, see [52,53,65]). VSMC proliferation then leads to thickening of the pulmonary vessel walls, increasing their rigidity and peripheral resistance, and thus leads to long-term fixation of PH. The thickening and the rigidity of pulmonary vessels are further promoted by the increased synthesis of ECM molecules, particularly collagen, by the multiplied VSMCs [14,57].
Among the cells of the immune system, in addition to macrophages, we have paid special attention to mast cells. These cells have been relatively little considered in the pathogenesis of HPH, and in the pathogenesis of vascular diseases in general. They have even been referred to as “forgotten cells” in the literature, although in addition to VSMCs, macrophages and fibroblasts, they are another important cell type present in a pathologically altered blood vessel wall (for a review, see [39,46]). These cells also play an important role in various fibrotic diseases, such as renal, pulmonary, hepatic and cardiac fibrosis and the formation of hypertrophic scars (for a review, see [66]).
In our studies on the effect of mast cells on VSMC proliferation, we chose RBL-2H3 cells, i.e. a rat basophilic leukemia (mastocytoma) cell line, as a model of mast cells infiltrating the walls of pulmonary blood vessels. We first compared the production of proteolytic enzymes in these cells and in cell types present in the vascular wall, such as endothelial cells, VSMCs and fibroblasts. We found that the production of chymases, tryptases and metalloproteinases was several times higher in RBL-2H3 cells than in vascular wall cell types. Moreover, the production of these enzymes was usually higher in RBL-2H3 cells cultured in a hypoxic atmosphere (3 % O2 + 5 % CO2) than in a normoxic atmosphere (21 % O2 + 5 % CO2), whereas the production of proteolytic enzymes in vascular wall cell types was not affected by hypoxia or was even reduced. However, immunofluorescence staining of MMP-13 revealed that endothelial cells and VSMCs cultured under hypoxic conditions contained more numerous and more brightly stained granules with this enzyme than the cells cultured in normoxia [37,38]. In a follow-up study, we then monitored the growth of VSMCs on type I collagen pre-exposed to RBL-2H3 cells cultivated for 48 hours in normoxia or hypoxia. On collagen pre-modified by normoxic RBL-2H3 cells, the proliferation activity of VSMCs was similar to that on unmodified collagen. However, on collagen pre-modified by hypoxic RBL-2H3 cells, the cell population doubling time was significantly shorter, and the final cell population density was significantly higher than on unmodified collagen. This behavior of VSMCs was explained by the degradation of collagen by proteases released from RBL-2H3 cells, which was more pronounced in hypoxic RBL-2H3 cells [39].
Increased collagenolysis is known to stimulate the migration and proliferation of mesenchymal cells, including VSMCs, and is an important contributor to vascular remodeling in HPH. Consistent with this, the metalloproteinase inhibitor Batimastat significantly reduced experimental HPH in rats, while reducing the muscularization of peripheral lung vessels and right ventricular hypertrophy [67]. Similarly, disodium cromoglycate (DSCG), an inhibitor of mast cell degranulation, decreased the amount of collagen cleavage fragments in pre-alveolar vessels, inhibited muscularization in peripheral pulmonary arteries, and reduced the development of PH [68]. Interestingly, this effect was only observed when DSCG was applied in the early phase of hypoxia exposure. By contrast, when DSCG was administered to rats with already developed chronic HPH, it delayed the regression of HPH upon return to normoxia by preventing the cleavage of the increased amount of collagen in the blood vessel walls [69]. Another mechanism by which mast cells, including RBL-2H3 cells, can stimulate the growth of VSMCs, is through their production of various chemokines and cytokines, such as TNF-α, interleukins, leukotrienes and MCP-1 (for a review, see [58]).
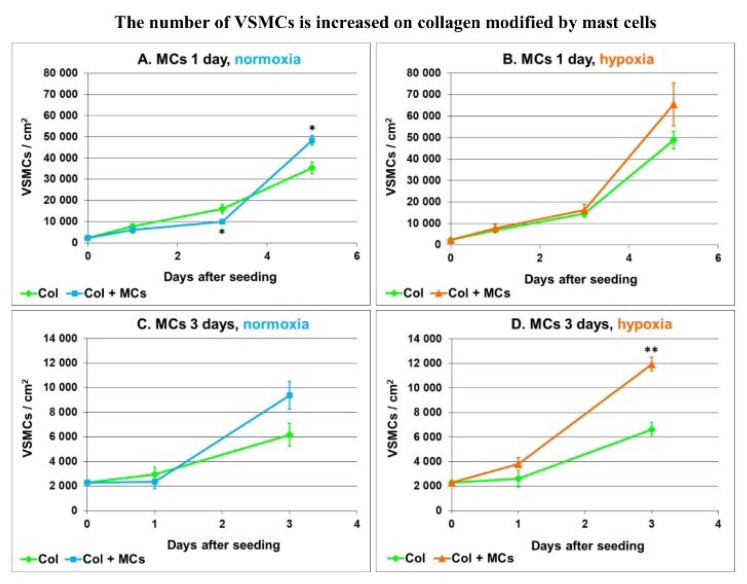
We further focused on the adhesion and growth of VSMCs on collagen modified by primary mast cells isolated from rat lungs. Type I collagen was deposited in polystyrene culture wells and was exposed for 1 or 3 days to mast cells seeded into the inserts above the collagen and cultured in a normoxic or hypoxic atmosphere. The collagen substrate was then rinsed with phosphate-buffered saline (PBS) and was seeded with rat aortic VSMCs (approx. 2150 cells/cm2). The growth curves of VSMCs in Fig. 1 show that collagen pre-modified by mast cells (MCs) promoted the growth of VSMCs more than unmodified collagen. This difference was more apparent on collagen modified by MCs for 3 days than on collagen modified by MCs for only 1 day. On collagen modified by MCs for 1 day, the VSMCs reached a higher population density on day 5 after seeding (Fig. 1 A, B), while on collagen modified for 3 days, a higher cell population density was already reached on day 3 after seeding, and this difference was more striking on collagen modified with hypoxic MCs (Fig. 1 C, D). Overall, the final population densities of VSMCs tended to be higher on collagen modified by hypoxic MCs than modified by normoxic MCs.
Fig 1.
Growth curves of rat aortic VSMCs seeded on unmodified type I collagen (Col) and Col pre-incubated with primary rat lung mast cells (Col + MCs) for 1 day (A, B) or for 3 days (C, D) under normoxic (A, C) or hypoxic (B, D) conditions. Mean ± SEM from 18 samples for each experimental group, Student t-test for unpaired data. Statistical significance: *p≤0.05 and **p≤0.01 in comparison with Col.
However, a disadvantage of primary cultures of mast cells from rat lungs (by bronchoalveolar lavage) was the frequent presence of bacterial contamination. We therefore performed further studies on extracts from normoxic and hypoxic mast cells, containing proteases. The mast cell extracts were prepared using a solution containing 1 part PBS, 1 part 280 mM sorbitol, 1 mM Ca2+ and 0.5 mM Mg2+. Collagen type I, deposited on the bottoms of polystyrene culture wells, was exposed to these extracts for 20 hours in a cell incubator, and was then rinsed with PBS and deionized H2O, dried and seeded with rat aortic VSMCs (approx. 2150 cells/cm2). The cells were cultured either in standard Dulbecco’s Modified Eagle’s Minimum Essential Medium (DMEM) with 10 % of fetal bovine serum (FBS) or in a chemically defined serum-free medium (BD Biocoat, Cat. No. 355160), supplemented with epidermal growth factor (EGF), fibroblast growth factor (FGF) and insulin, already used in our previous study [39]. The reason was to minimize the adsorption of serum proteins, such as vitronectin, fibronectin and albumin, which influence cell adhesion and spreading and can mask the effect of the modified collagen on VSMCs.
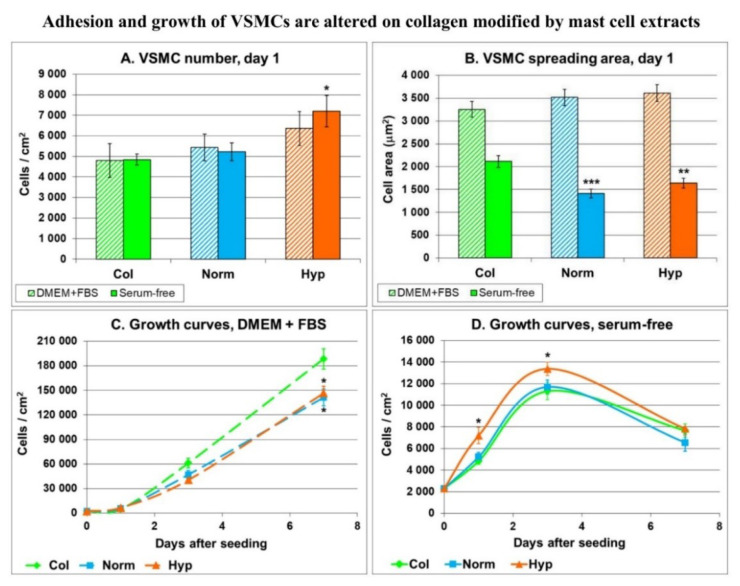
The advantage of using a serum-free medium was evident on day 1 after cell seeding. As shown in Fig. 2A, the number of cells on day 1 after seeding tended to be highest on collagen modified with hypoxic mast cell extract. This was more evident in the serum-free medium than in the standard serum-supplemented medium. In the serum-supplemented medium, the size of the cell spreading area was similar in VSMCs on all collagen layers. However, in the serum-free medium, the cell spreading area was smaller in VSMCs on collagen modified with extracts from mast cells, whether normoxic or hypoxic (Fig. 2B).
Fig. 2.
The number (A) and the spreading area (B) of rat aortic VSMCs on day 1 after seeding on unmodified type I collagen (Col) or with Col exposed to extracts from mast cells cultured under normoxia (Norm) or hypoxia (Hyp). Mean ± SEM from 9–18 samples (A) or from 34–82 cells (B) for each experimental group. Growth curves of these VSMCs from day 1 to day 7 (C, D). The cells were cultured either in a standard serum-supplemented medium (DMEM + FBS) or in a serum-free medium. Student t-test for unpaired data. Statistical significance: **p≤0.01 and ***p≤0.001 in comparison with Col.
In the following days, the number of VSMCs increased faster in cells on unmodified collagen, and on day 7 it became significantly higher than on collagen modified with normoxic or hypoxic mast cell extracts (Fig. 2C). However, in a serum-free medium (where the adsorption of molecules with a potential masking effect was minimized), the highest cell numbers were achieved on collagen modified with hypoxic mast cell extract (Fig. 2D). In this case, there is also an interesting connection between the relatively small adhesion area of the cells and the increased growth of their population. This is in line with the generally accepted fact that cell proliferation is highest at the intermediate level of cell-matrix adhesion [70].
Among all proteolytic enzymes produced by mast cells, we finally turned most attention to MMP-13. This enzyme is an interstitial collagenase, the principal enzyme responsible for the initiation of collagen breakdown in the rat species during the development of HPH [36]. We therefore studied the adhesion, growth and viability of VSMCs in cultures on collagen I exposed to MMP-13. Collagen I was adsorbed on polystyrene culture dishes, digested with MMP-13, seeded with rat aortic VSMC (approx. 2500 cells/cm2) and incubated in DMEM with 10 % of FBS for 1 to 7 days.
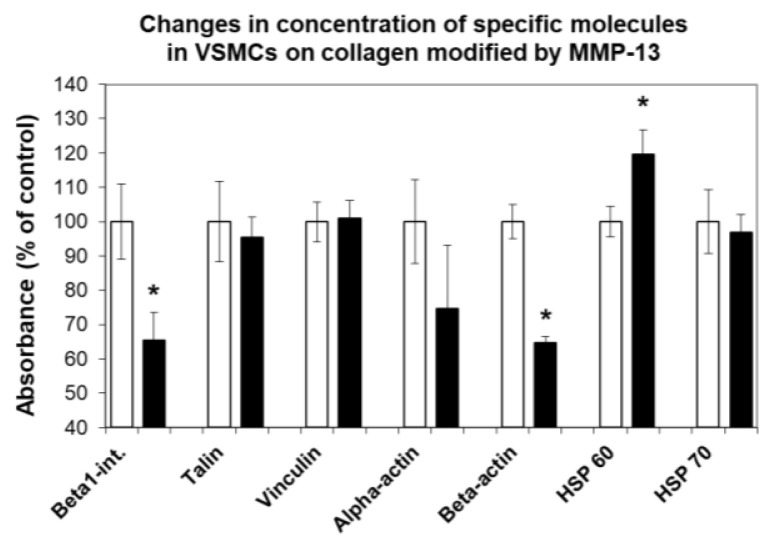
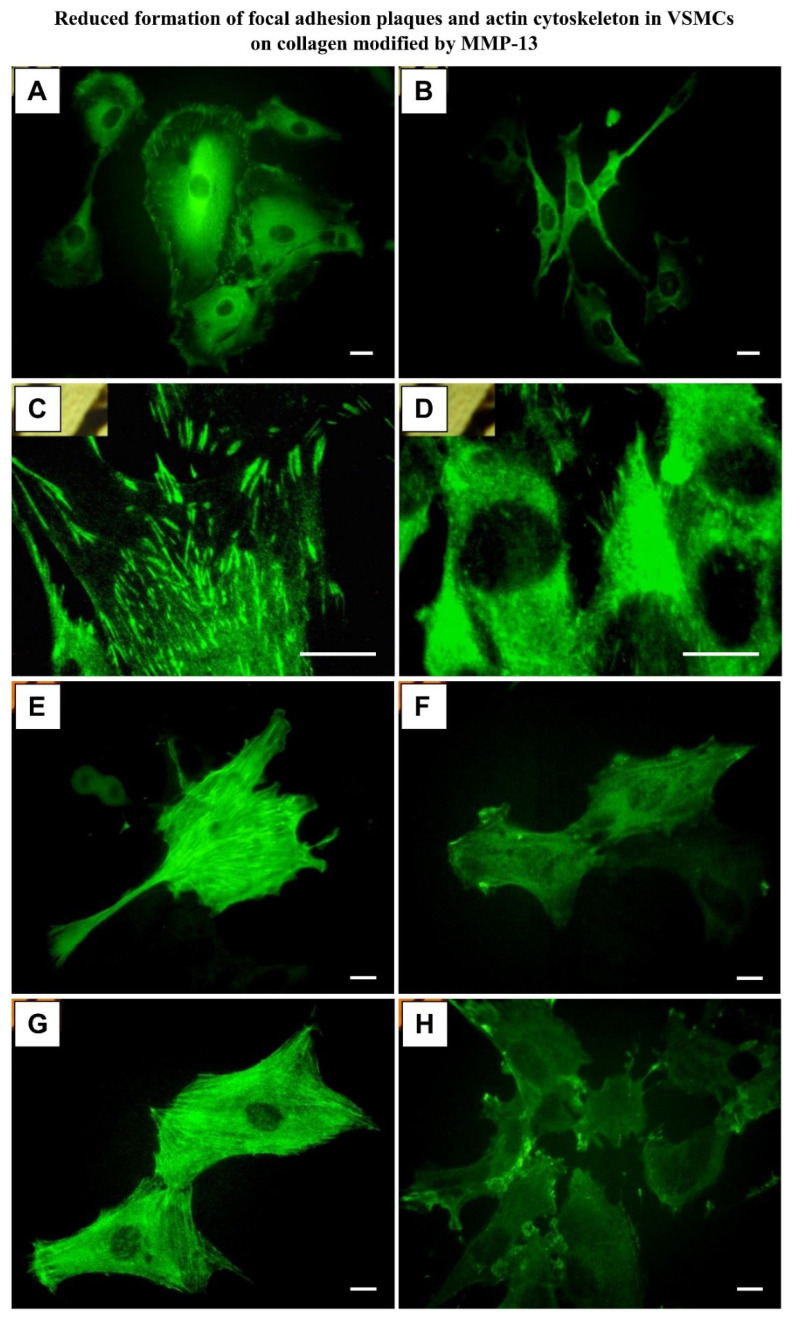
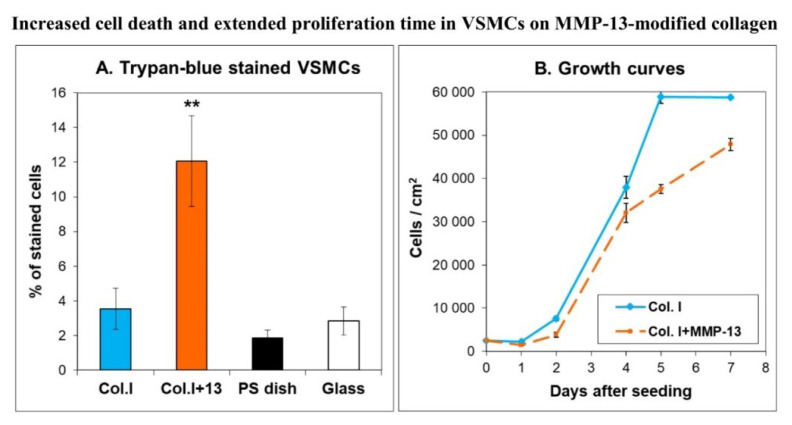
We found that the VSMCs on MMP-13-treated collagen adhered in about 1.5 times lower initial numbers than the cells on unmodified collagen (2200 ± 200 vs. 1490 ± 650 cells/cm2; p<0.01). In addition, these cells were usually less spread, i.e. they contacted the modified collagen by a smaller cell adhesion area. Their shape was often round or spindle-like, whereas the cells on unmodified collagen were mainly polygonal. The concentration of β1-integrin adhesion receptors and β-actin in cells on MMP-13-treated collagen was lower by about 35 % (Fig. 3). The concentrations of focal adhesion proteins talin and vinculin, and a contractile protein α-actin, were unchanged, but the clustering of the first two proteins into focal adhesion plaques, as well as the assembly of α- and β-actin-containing microfilaments, were lower (Fig. 4). The cells on MMP-13-treated collagen contained more heat-shock protein 60 (by 20 ± 7 %), and were more prone to cell death, as indicated by a more than 3 times higher number of trypan blue-stained cells (Fig. 5A). As a result, the growth curves showed that the cells on MMP-13-modified collagen proliferated more slowly than the cells on the control unmodified collagen.
Fig. 3.
Changes in the concentration of adhesion molecules (β1integrins, talin, vinculin), cytoskeletal proteins (α-actin and β-actin) and heat-shock proteins 60 and 70 (HSP 60, HSP 70) in rat aortic VSMCs cultured on unmodified collagen I (white columns) and on MMP-13-degraded collagen I (black columns). Measured by ELISA per mg of protein on day 3 after seeding. The absorbances of cell samples from the degraded collagen are expressed as percentages of the values obtained from control cells on unmodified collagen. Mean ± SEM from 2–4 experiments, Student t-test for unpaired data, *p<0.05 compared to control values.
Fig. 4.
Immunofluorescence staining of vinculin (A, B), talin (C, D), alpha-actin (E, F) and beta-actin (G, H) in 3-day-old cultures of rat aortic VSMCs grown on unmodified collagen I (A, C, E, G) or on collagen I digested with MMP-13 (B, D, F, H). Zeiss Axioplan epifluorescence microscope, scale bar = 10 μm.
Fig. 5.
A) Percentage of trypan blue-stained cells in 2-day-old cultures of rat aortic VSMCs on unmodified collagen I (Col. I), on collagen I digested by MMP-13 (Col. I + 13), on polystyrene tissue culture dishes (PS dish), and on glass coverslips (Glass). Mean ± SEM from 15 measurements. Student t-test for unpaired data, **p<0.01 compared to control values on Col. I. B) Growth curves of VSMCs on Col. I and Col. I+MMP-13. Mean ± SEM from 20 measurements (days 1 to 4) or from 4 measurements (days 5 to 7).
However, the VSMCs on the modified collagen proliferated for a longer period of time, whereas on unmodified collagen the cells reached their maximum population density earlier and entered the stationary phase (Fig. 5B). These results suggested that the cells on MMP-13-degraded collagen escaped the ECM-mediated growth control more easily and increased their turnover [53,65]. Increased turnover of VSMCs, i.e. coexistence of proliferation and apoptosis, has been shown repeatedly in the pulmonary vascular cells of rats with HPH (for a review, see [8]).
Changes in the ECM of pulmonary arteries in vivo
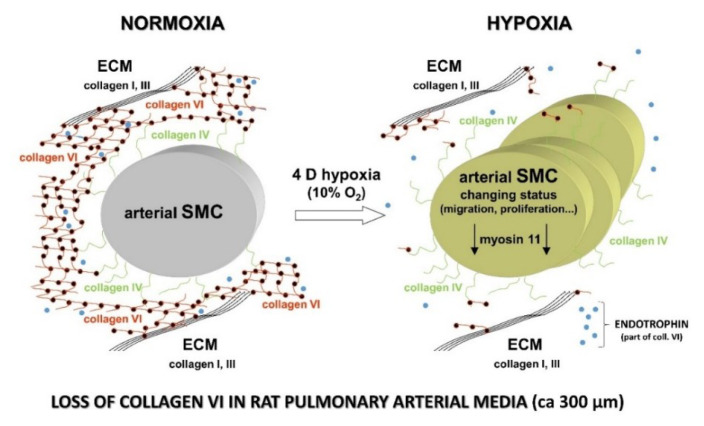
Our further studies on changes in the ECM of pulmonary arteries during hypoxia and their potential influence on the behavior of VSMCs were performed in rats in vivo. The rats were exposed to hypoxia (10 % O2) in a normobaric hypoxic chamber for four days. The rats were then euthanized, and pulmonary arteries (approximately 250–400 μm in diameter, 3rd–5th order, classified as conduit arteries) were subjected to immunohistochemical, proteomic, and real-time PCR analyses, focused particularly on non-fibrillar collagens of type IV and VI [71]. Type IV collagen is located in the basal lamina of cells, including VSMCs, and it helps maintain VSMCs in a differentiated state. Plating VSMCs on type IV collagen induced an increase in contractile proteins, namely α-actin and myosin heavy chain, in these cells. Moreover, various types of stem cells seeded on type IV collagen spontaneously differentiated towards smooth muscle cell phenotype (for a review, see [72]). Type VI collagen is located in the basement membrane and in the interstitial space between cells. It has been reported to induce the differentiation of fibroblasts into myofibroblasts, manifested by their expression of smooth muscle α-actin (for a review, see [72]). Type VI collagen consists of three chains (α1, α2, and α3). The α3(VI) chain is approximately three times longer than the α1(VI) and α2(VI) chains and, unlike the other chains, contains a Kunitz-like terminal domain (C5), with a sequence very similar to that of Kunitz-type A proteinase inhibitors (first described by Bonaldo et al. 1989 [73]). This C5 terminal collagen α3(VI) domain with 58 residues is present as a propeptide in the newly formed type VI collagen microfibrils (composed of α1, α2 and α3 chains). However, immediately after the microfibrils are secreted into the ECM, this C5 propeptide is cleaved off and is no longer present in the mature collagen VI fibrils [74]. This C5 domain, after cleavage from the α3(VI) main chain, represents a biologically active peptide, endotrophin [75–77]. Endotrophin appears to be one of the key players in the signaling effects mediated by collagen VI, including its pro-fibrotic nature and chemoattractant properties for macrophages [77]. Endotrophin was first identified by Park and Scherer (2012) as a pathological signal that promotes breast cancer growth [78], and is an important marker and driver of fibroinflammatory diseases (for a review, see [79]).
We found that the expression of type IV collagen in the tunica media of arteries of hypoxia-exposed rats was not changed at protein and mRNA levels. However, type VI collagen was significantly reduced in the tunica media of hypoxic rats at protein level (Fig. 6), while its expression at mRNA level increased. This phenomenon was described for the first time in our study [71]. At the same time, we detected a significant increase in MMP-9 in the tunica media, while the expression of MMP-2 at both protein and mRNA levels was decreased in the tunica media. We concluded that the loss of collagen VI and increasing concentration of endotrophin could be important factors inducing the phenotypic modulation of VSMCs, i.e., loss of their contractile filaments (e.g., α-actin-containing) and activating their migration and proliferation, which leads to remodeling of the pulmonary arteries during hypoxic pulmonary hypertension. At the same time, collagen VI was retained in the tunica adventitia, which could promote the differentiation of adventitial fibroblasts to myofibroblasts [71].
Fig. 6.
A significant decrease in the content of collagen VI (red lines with black dots: collagen VI forms this type of “beady” fibrils) in the tunica media of pulmonary arteries (about 300 μm in diameter) during hypoxia. At the same time, the amount of type IV collagen (green on the surface of the VSMCs) remained unchanged. Fibrillar collagens (type I and III) are drawn in black, and collagen VI anchors them to collagen IV. A biologically active peptide, endotrophin, cleaved from the α3(VI) is drawn in blue. The status of some VSMCs changes during the development of HPH (decreasing concentration of the protein myosin 11, a marker of the differentiated status of VSMCs). Modified from [71].
A recent comprehensive and systematic proteomic study by the group of K.R. Stenmark shows significant changes in the matrisome (i.e. the ensemble of genes encoding ECM and ECM-associated proteins) in calf pulmonary vessels in response to hypoxia. Major changes included a strong immune response and wound repair signature characterized by increased levels of complement components (mainly membrane attack complex C5 to C9 components), coagulation cascade proteins (fibrinogen and fibrin), and provisional matrix glycoproteins (tenascin C, fibronectin). In addition, the authors observed an upregulation of ECM-modifying enzymes (proteases and protease inhibitors, enzymes involved in collagen biosynthesis and stabilization), growth factors (TGF-β3, insulin-like growth factor-2, IGF-2), and core ECM proteins involved in vascular stiffening, such as collagens (fibrillar, e. g. types I and III, and non-fibrillar, e.g. types IV and VIII), fibronectin, as well as the glycoproteins vitronectin, which promotes cell adhesion spreading and migration, and periostin, which is associated with epithelial-mesenchymal transition [80].
The role of hypoxia-inducible factors in vascular remodeling
In addition to the effects of the changes in ROS and ECM investigated in our previous studies, other important players in the stimulation of VSMC proliferation and vascular remodeling during HPH are hypoxia-inducible factors (HIFs). HIFs are oxygen-sensitive transcription factors governing the metabolic response of cells to low oxygen levels. There are three basic members of the human HIF family, namely HIF-1, HIF-2 and HIF-3. HIFs are heterodimers composed of oxygen-sensitive α subunit (HIF-1α, HIF-2α, HIF-3α) and oxygen-insensitive β subunit (HIF-1β, HIF-2β, HIF-3β) [8]. HIF-1α is primarily expressed in VSMCs, HIF-2α is predominantly found in endothelial cells (although its role in VSMCs should not be underestimated), and HIF-3a is predominantly expressed in pulmonary fibroblasts [51]. The interplay between all members of the HIF family then leads to the onset and development of HPH.
HIFs are responsible for the activation or inhibition of more than 2 % of human genes participating in the cellular adaptive response in order to maintain oxygen homeostasis [8,40,51,81]. Erythropoietin was the first of the genes for which the HIF regulation was described (by HIF-1α), and in this way, pharmacological interference with the HIF system was investigated as a possible therapy for anemia. Professor Herget’s followers focused on roxadustat, a prolyl hydroxylase inhibitor and HIF stabilizer [82]. It is known that HIFs promote VSMC contraction by inhibiting K+ channels, which leads to the influx of Ca2+ ions [8,51]. The application of roxadustat can therefore be associated with a risk of increased pulmonary vascular resistance and vasoconstrictor reactivity. Fortunately, this risk was not confirmed when roxadustat was administered to rats for 14 days. However, this risk should be taken into account, since HIFs and their stabilizers or activators can support blood vessel remodeling [82]. Calcium ions entering the VSMCs can stimulate their proliferation, especially in combination with the shift from oxidative phosphorylation to oxygen-independent glycolysis, which likens the behavior of VSMCs to that of tumor cells [8,49,51,55,59]. In this context, it is interesting to note that gene therapy for oxygen-sensitive Kv1.5 channels, whose expression was downregulated by HIF activation, reduced pulmonary hypertension in chronically hypoxic rats [83].
HIFs also up-regulate growth factors/receptor tyrosine kinases, activate protein kinase B (AKT) and extracellular signal-regulated kinase (ERK), and mammalian target of rapamycin (mTOR), i.e. factors that promote cell proliferation and survival, and suppress growth inhibitory factors such as phosphatase and tensin homolog (PTEN), p53 and the Hippo signaling pathway [8,51].
In endothelial cells, hypoxia and HIF-1α upregulate genes for growth factors such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [95], which stimulate the migration and proliferation of VSMCs [40]. Last but not least, HIF-1α increases the expression of CD146, which is a co-receptor of VEGF receptor 2 (VEGFR2) and PDGF receptor-β (PDGFR-β), and further enhances the expression of HIF-1α [8,51,84].
Ultimately, HIFs in VSMCs activate the migration and proliferation, stimulate a switch from the quiescent contractile phenotype to a synthetic and proliferative phenotype, inhibit VSMC apoptosis, and also induce osteogenic differentiation of VSMCs, which results in blood vessel calcification [8,51,55]. VSMCs also acquire a pro-inflammatory phenotype, characterized by the expression of cell adhesion molecules of the immunoglobulin and selectin families, and by the production of various chemokines, cytokines and growth factors, attracting cells of the immune system (e.g. monocytes, macrophages, lymphocytes) and promoting their proliferation [8,14,54,55,57].
It can be summarized that HIF-1 plays a major role in driving VSMC proliferation, while HIF-2 plays a major role in inflammatory cell recruitment via activation of endothelial cells and VSMCs to a pro-inflammatory phenotype. HIF-2α is therefore considered to play a major role in the initiation of HPH, whereas HIF-1α may play a major role in the progression and perpetuation of the disease [85,86]. Chronic PH is then characterized by the accumulation of persistently activated cell types in the pulmonary vessels, exhibiting aberrant expression of genes involved in proliferation, apoptosis resistance, inflammation and ECM remodeling [55].
The role of miRNA in vascular remodeling
Other important factors in the onset and development of HPH, including vascular remodeling, are microRNAs (miRNAs). These molecules are single-stranded small noncoding RNAs (18–22 nt in length) that can directly degrade or repress the translation of their target mRNAs, thus negatively regulating gene expression at the posttranscriptional level [58,87]. As it is estimated that at least 30 % of genes in the human genome are directly regulated by miRNAs, a growing number of studies are revealing that miRNAs play a unique and pivotal role in the progression of HPH through the phenotypic switch of VSMCs from a contractile to a synthetic phenotype. The non-proliferative differentiated contractile phenotype is characterized by the expression of myocardin, α-actin, SM22α (early markers of VSMC differentiation), h-caldesmon and calponin-1 (intermediate markers of VSMC differentiation) and desmin, meta-vinculin, SM1 and the SM2 isoforms of myosin heavy chain and smoothelin (late markers of VSMC differentiation). During the transition to the synthetic phenotype, these markers are gradually lost, in order from late to early, so that some level of the early markers, e.g. α-actin, is retained by cells in the synthetic phenotype (for a review, see [54,57]). In addition, some isoforms of differentiation markers are replaced by others, e.g. α-actin by β-actin or SM1 and SM2 isoforms of myosin heavy chain by myosin heavy chain embryonic (SMemb). Other markers of synthetic VSMCs include the presence of tropomyosin 4, increased synthesis of ECM proteins and glycoproteins, such as collagen and osteopontin-8, and particularly increased migration and proliferation activity [87].
The transition from the contractile to the synthetic phenotype and vice versa is regulated by specific miRNAs that ultimately either stimulate VSMC proliferation or have an antiproliferative effect (Table 1). Modulation of their expression could therefore be used in the prevention and therapy of HPH.
Table 1.
MicroRNAs which promote or inhibit the switch from contractile to synthetic phenotype and proliferation of VSMCs in HPH.
| Dependence/effect | Pro-proliferative | Anti-proliferative | Reference |
|---|---|---|---|
| Hypoxia-dependent | miRNA-9 | miRNA-17~92 cluster | [87] |
| miRNA-20a | miRNA-30c | ||
| miRNA-23a | miRNA-124 | ||
| miRNA-214 | miRNA-140 | ||
| miRNA-206 | |||
| miRNA-449 | |||
| miRNA-17 | miRNA-26b-5p | [58] | |
| miRNA-18a-5p | miRNA-140-5p | ||
| miRNA-19a | miRNA-150 | ||
| miRNA-92b-3 | miRNA-223 | ||
| miRNA-143 | miRNA-760 | ||
| miRNA-145 | |||
| miRNA-155-5p | |||
| miRNA-214 | |||
| miRNA-1260b | |||
|
| |||
| Growth factor-dependent | miRNA-221 | miRNA-21 | [87] |
| miRNA-15b | miRNA-132 | ||
| miRNA-96 | |||
| miRNA-24 | |||
Cell types other than VSMC in vascular remodeling
During HPH, a metabolically reprogrammed, proliferative and pro-inflammatory phenotype is acquired not only by VSMCs but also by other cell types present in the blood vessel wall. These cells include intimal endothelial cells and adventitial fibroblasts, which can be considered as “sentinel cells” separating VSMCs from the blood and the surrounding vascular environment, and which are activated first in response to various adverse factors [4,14,49,59]. Endothelial cells can produce endothelin-1, a potent vasoconstrictor, which stimulates the expression of HIF-1α in VSMCs, promotes the proliferation of VSMCs and fibroblasts, and facilitates the production of ECM by these cells [11,51]. Moreover, endothelial cells can undergo a so-called endothelial-to-mesenchymal transition and acquire a VSMC-like phenotype, characterized by the presence of smooth muscle α-actin [85].
A similar transformation can be observed in adventitial fibroblasts, which transform into myofibroblasts positive for smooth muscle α-actin, and resemble VSMCs [12,14,40,54]. These fibroblasts are capable of proliferating, stimulating the proliferation of VSMCs, and particularly recruiting monocytes and lymphocytes (e.g., T-cells) and activating them into cells with the pro-inflammatory and pro-remodeling phenotype [14,88,89]. Last but not least, VSMC-like cells capable of proliferation and paracrine secretion of growth factors can arise from progenitor cells, either resident in the vascular wall or circulating in the blood after their release from the bone marrow [12,54]. Importantly, a recent study by Hu et al. (2023) [90] showed that human and bovine pulmonary vascular fibroblasts from patients or animals with PH exhibited even greater expression of cytokines, chemokines and growth factors than VSMCs and endothelial cells from the same vessels, and that HIF inhibition alone was not sufficient to reverse the persistently activated phenotype of these fibroblasts.
Effect of hypoxia on the growth of VSMCs in vitro
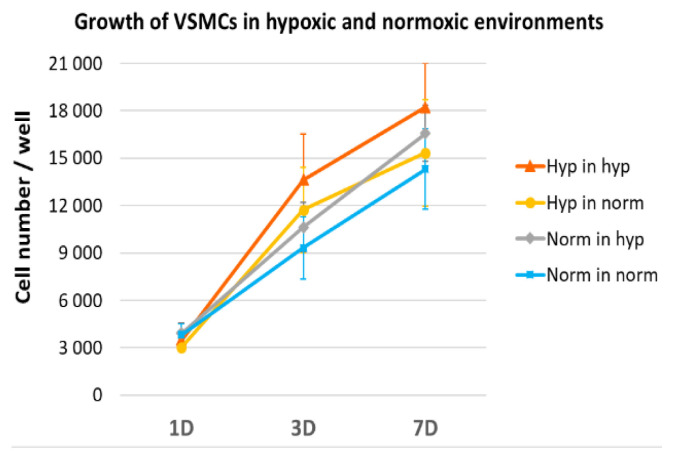
The pulmonary arteries used to study ECM changes in vivo (part “Changes in the ECM of pulmonary arteries in vivo” above) were also used to isolate VSMCs and culture them in vitro for growth studies. The arteries (approx. 250–400 μm in diameter, 3rd–5th order) were dissected from the lungs of normoxic and hypoxic adult male Wistar rats under a microscope, were visually cleared of tunica adventitia, and were minced into fragments (0.5 mm3 or less). These fragments were digested by collagenase, and VSMCs were isolated from them by an explantation method [52,91,92]. The identity of the VSMCs was verified by smooth muscle α-actin detection at the protein level [71]. The cells were then cultured in a humidified air atmosphere either under normoxic conditions (21 % O2 and 5 % CO2) or under hypoxic conditions (2.5 % O2 and 5 % CO2) in a high-glucose DMEM supplemented with 10 % of FBS and gentamicin (40 μg/ml) [92]. The growth of four experimental groups of cells was compared:
VSMCs from hypoxic rats cultured in a hypoxic atmosphere (“hyp in hyp”),
VSMCs from hypoxic rats cultured in a normoxic atmosphere (“hyp in norm”),
VSMCs from normoxic rats cultured in a hypoxic atmosphere, (“norm in hyp”)
VSMCs from normoxic rats cultured in a normoxic atmosphere (“norm in norm”).
We found that the proliferation activity, measured by the increase in cell number from day 1 to day 7 after seeding, tended to be highest in group (a) and lowest in group (d) (Fig. 7). Although these differences were not statistically significant, this result is consistent with our earlier findings of increased VSMC growth on collagen modified by hypoxic mast cells (Figs 1, 2). This result is also in agreement with previous studies by other authors, who described an increased proliferative activity of pulmonary arterial VSMCs in HPH in humans and in various experimental models (for a review, see [8,54]). It is also evident that culturing cells from hypoxic donors in normoxia tended to attenuate the proliferative activity of VSMCs, whereas culturing cells from normoxic donors in hypoxia enhanced this activity, although not to the level of the activity of hypoxic cells cultured in hypoxia (Fig. 7).
Fig. 7.
VSMCs showed a tendency to increase their growth activity under hypoxic conditions. When VSMCs were isolated from pulmonary arteries of hypoxic rats and cultivated for 3 and 7 days (3D, 7D) in a hypoxic atmosphere (Hyp in hyp), they reached on average higher numbers than when cultured in a normoxic atmosphere (Hyp in norm), and higher numbers than VSMCs from normoxic rats grown in a hypoxic atmosphere (Norm in hyp). The lowest average cell numbers were observed for VSMCs from normoxic rats grown in a normoxic atmosphere (Norm in norm). The number of initially adhered cells on 1 day (1D) after seeding was similar in all tested groups. Mean ± SEM, n = 5. One Way ANOVA, Student-Newman-Keuls Method, p ≤ 0.05. No statistically significant difference was detected.
Effect of hypoxia on markers of VSMC differentiation in vitro
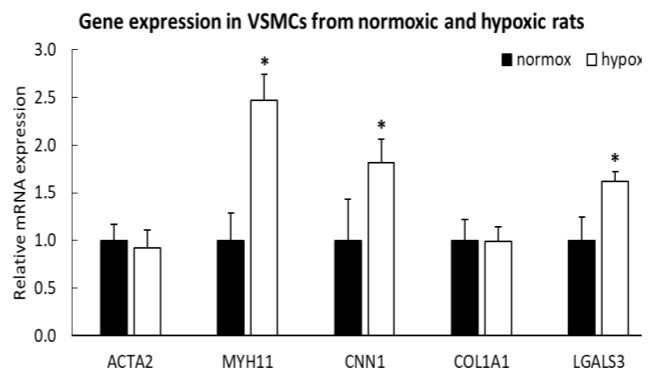
Interesting results were obtained when the expression of genes for markers of VSMC differentiation at the mRNA level was studied by real-time qPCR in cultures of VSMC isolated from pulmonary arteries of normoxic and hypoxic rats. These markers included smooth muscle α-actin, calponin-1 and myosin heavy chain, i.e. early, intermediate and late markers of VSMC differentiation, respectively (for a review, see [54,57,87]). We expected a loss in the mRNA expression of these markers as a sign of phenotypic modulation of VSMCs during HPH. However, the expression of α-actin was unchanged in hypoxic VSMCs, and the expression of calponin-1 and particularly myosin heavy chain was even increased (Fig. 8). At the same time, the expression of type I collagen was unchanged, but the cells from hypoxic rats showed an increased expression of galectin-3. This β-galactosyl-binding protein, considered to be implicated in the pathogenesis of HPH, can have a dual effect on VSMCs. On the one hand, an increased expression of Gal-3 in VSMCs was reported to be associated with increased proliferation activity of these cells and their lower tendency to apoptosis [93]. Moreover, in VSMCs, Gal-3 induced the expression of osteopontin, a marker of phenotypic modulation of VSMCs towards synthetic phenotype, associated with VSMC proliferation and vascular calcification [94]. On the other hand, Gal-3 was reported to stimulate cell differentiation towards contractile VSMC phenotype, e.g. by inducing the expression of α-actin in endothelial cells [95] and by increasing the expression of α-actin and calponin in VSMCs [92,94]. This is probably related to the pro-fibrotic effect of Gal-3, as α-actin and calponin can also be considered as markers of fibrosis [92,96].
Fig. 8.
Expression of genes encoding α-actin (ACTA2), myosin heavy chain (MYH11), calponin-1 (CNN1), type I collagen (COL1A1) and galectin-3 (LGALS3) in VSMCs isolated from pulmonary arteries of normoxic and hypoxic rats (passage 2) and cultivated for 6 days in a normoxic atmosphere and in a hypoxic atmosphere, respectively. Mean + SD, n = 5. Student’s t-test, p ≤ 0.05. * significant difference compared to normoxic cells.
Hypoxia may also have a dual effect on the differentiation status of cells. On the one hand, as already explained, it promotes the dedifferentiation of initially contractile VSMCs into a synthetic phenotype, but on the other hand, it is used to differentiate stem cells into different cell types, such as endothelial cells [97,98], chondrocytes [99], osteoblasts [100], cardiomyocytes (for a review, see [101]), and also smooth muscle cells. For example, in a recent study by Lin et al. (2020) [102], human subcutaneous adipose tissue-derived stem cells (ADSCs), cultured in an induction medium consisting of low-glucose DMEM supplemented with 1 % of FBS, TGF-β1 (5 ng/ml) and bone morphogenetic protein-4 (BMP-4; 2.5 ng/ml) and subjected to hypoxia (1 % of O2), increased the expression of α-actin, SM22α, calponin and myosin heavy chain, i.e. markers of VSMC differentiation, which was mediated by N6-adenosine methyltransferases (Mettl3). At the same time, however, hypoxia and Mettl3 induced paracrine production of VEGF, TGF-β, hepatocyte growth factor (HGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), basic fibroblast growth factor (bFGF), and stromal cell-derived factor-1 (SDF-1) in ADSCs, i.e. factors promoting not only the differentiation but also the proliferation of ADSCs.
Therefore, the described dual effects of hypoxia, i.e., two opposing tendencies of hypoxia to stimulate either growth or differentiation of VSMCs, may have coincided in our studies on HPH. As expected, we observed an increased tendency of hypoxic VSMCs to proliferate, i.e., to increase in number, although this tendency did not reach statistical significance. At the same time, we also observed an increased expression of differentiation markers in VSMCs. This led us to the idea that in addition to hyperplasia, hypertrophy of VSMCs could also occur in our experimental setup.
The question of VSMC hypertrophy in HPH
First, it is important to note that even under basal physiological conditions, pulmonary (and also systemic) VSMCs are a highly heterogeneous population, ranging from less differentiated cells prone to proliferative and synthetic behavior to highly differentiated quiescent cells with contractile function [103]. The representation of individual subpopulations of VSMCs in the pulmonary vascular bed varies transversely and longitudinally in the tunica media of pulmonary arteries. For example, the middle tunica media of the bovine main pulmonary artery contained highly differentiated VSMCs (expressing both α-actin and SM myosin), while the sub-endothelial and outer media also contained less differentiated VSMCs expressing only α-actin [104]. At the same time, the content of highly differentiated cells increased in the proximal-to-distal axis, so that the distal pulmonary arteries contained a relatively homogeneous population of differentiated contractile VSMCs [105]. Obviously, these morphologically different subpopulations of VSMCs also exhibited different growth responses to hypoxic exposure. For example, hypoxia is thought to activate proliferation only in a specific less differentiated subpopulation of VSMCs, rather than directly causing phenotypic modulation and proliferation of originally differentiated contractile VSMCs [103,104]. Another important point is that the growth activation of VSMCs involves not only an increase in their number, but also an increase in their volume and protein content [105]. Moreover, the growth responses of VSMCs can change over time - the proliferative activity of VSMCs was higher in the early stages than in the late stages of HPH [54].
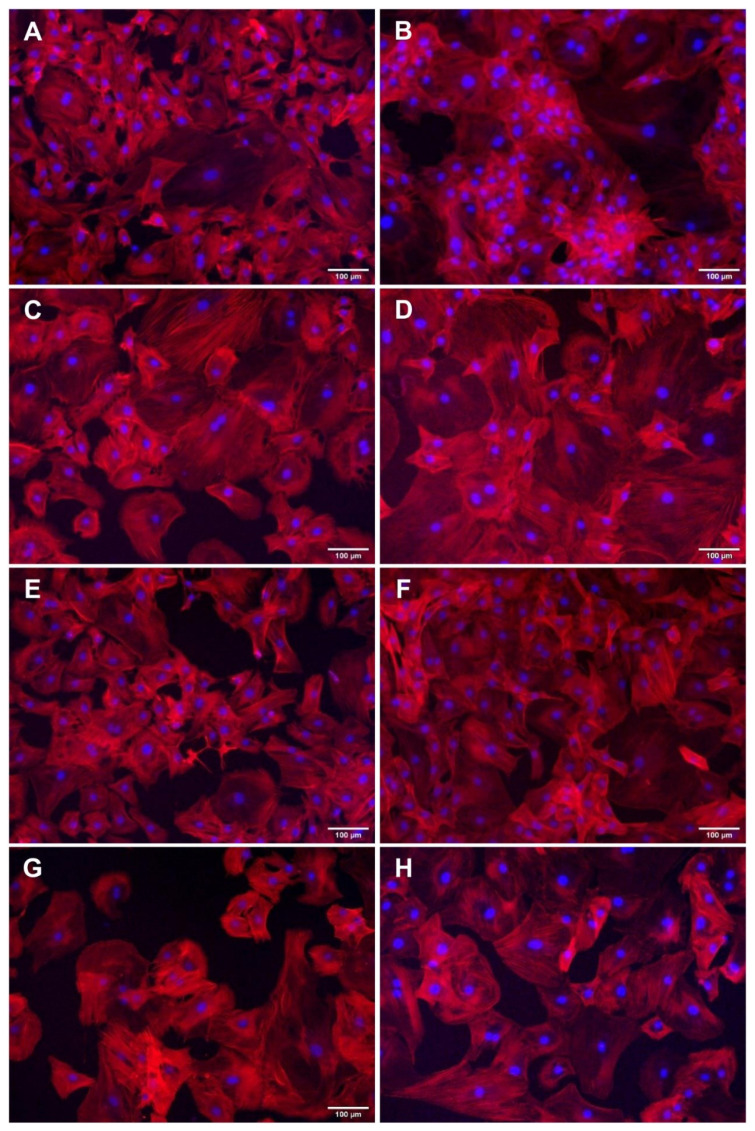
For studies of VSMC morphology, we used the same VSMCs as for the ECM and proliferation studies, i.e., described in the first paragraphs of parts “Changes in the ECM of pulmonary arteries in vivo” and “Effect of hypoxia on the growth of VSMCs in vitro”. As shown in Fig. 9, the population of VSMCs isolated from the pulmonary arteries of normoxic rats and cultured under normoxic conditions was heterogeneous, i.e. containing cells adhering to the substrate with a spreading area of varying size and shape and with a more or less developed filamentous actin (F-actin) cytoskeleton (Fig. 9 A, B). This is consistent with the previously described heterogeneity of VSMCs isolated by an explantation method from bovine pulmonary arteries, containing small rhomboid cells and larger spindle-shaped or cobblestone-like epitheloid cells, which also differed in immunofluorescence staining for SM alpha-actin and SM myosin [104].
Fig. 9.
Morphology of VSMCs isolated from pulmonary arteries of normoxic (A, B, E, F) and hypoxic (C, D, G, H) rats and cultivated for 1 day (A, C, E, G) and for 4 days (B, D, F, G) in DMEM with 10 % of FBS (A–D) or with 0.5 FBS (E–H) in a normoxic atmosphere (A, B, E, F) and in a hypoxic atmosphere (C, D, G, H). F-actin in the cells was stained with phalloidin conjugated with TRITC, cell nuclei were counterstained with Hoechst 33342. Olympus IX 71 microscope, DP 70 digital camera. Scale bar represents 100 μm.
However, in cultures of VSMCs isolated from pulmonary arteries of hypoxic rats, more cells were spread over a larger area on the culture substrate than in the case of VSMCs from normoxic arteries, which may be indicative of their larger volume (Fig. 9 C, D). Furthermore, this larger cell spreading area did not depend on the composition of the culture medium or the cell adhesion substrate. This larger area of hypoxic VSMCs was found both in the standard culture medium with 10 % FBS (Fig. 9 A–D) and in the serum-deprived medium with only 0.5 % FBS (Fig. 9 E–H), and not only on the culture polystyrene (Fig. 9) but also on various ECM proteins present in the vessel wall, such as collagen I, IV, VI, and fibronectin (Fig. 10). In addition, the larger spreading area of hypoxic VSMCs was evident not only when these cells were cultured under hypoxia (Fig. 9), but also when they were cultured under normoxia (“hyp in norm”; Fig. 10). These results suggest that larger cells were not generated or selected by the culture conditions, but were already primarily present in the pulmonary arteries of hypoxic rats. Increased volume and proteosynthesis in VSMCs in response to hypoxia were found in the outer media of the main pulmonary artery and in distal pulmonary arteries (diameter from 1500 μm to 100 μm) [105], i.e. in VSMCs from similar regions to those used in our experiments.
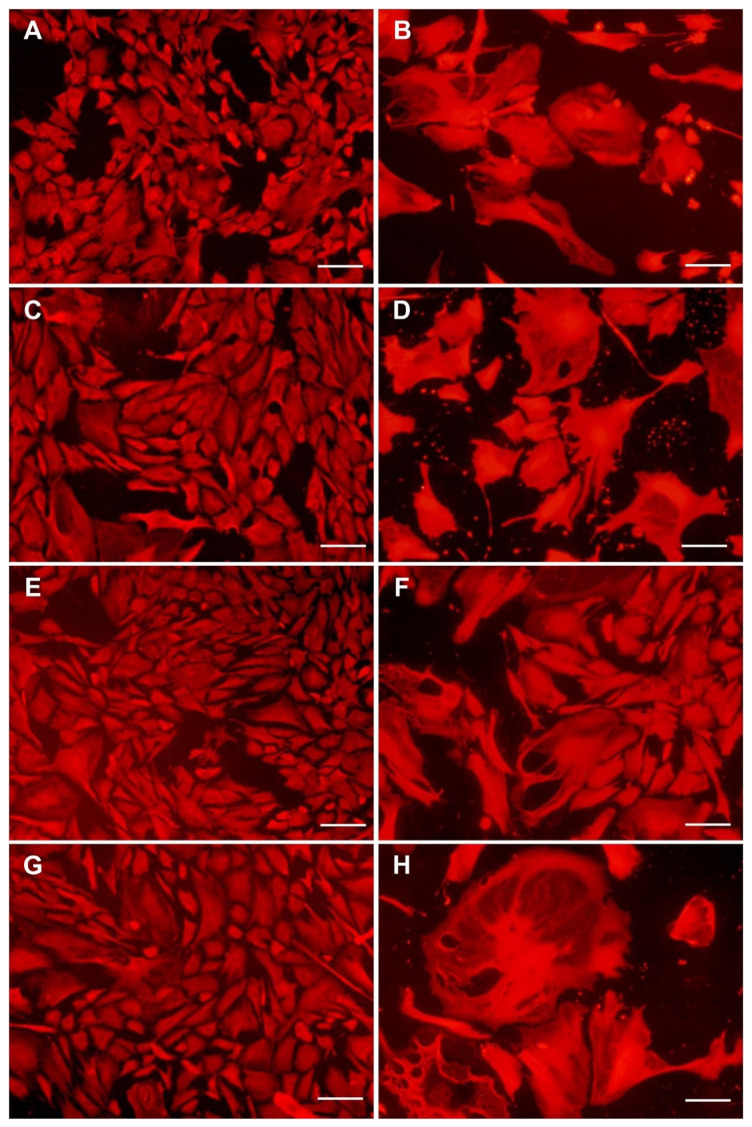
Fig. 10.
Morphology of VSMCs isolated from pulmonary arteries of normoxic (A, C, E, G) and hypoxic (B, D, F, H) rats and cultivated in DMEM with 10 % of FBS for 4 days in pure polystyrene wells (A, B), in wells coated with type I collagen (C, D), with type IV collagen (E, F) or with type VI collagen (G, H). Cells stained with Texas Red C2 maleimide. Olympus IX 51 microscope, DP 70 digital camera, obj. 10x. Scale bar represents 100 μm.
An increased volume of VSMCs of intrapulmonary arteries was also observed in a study by Shimoda et al. (2001) [106] in mice exposed for 21 days to normobaric hypoxia in a hypoxic chamber with 10 ± 0.5 % O2. This increased volume of VSMCs explained, at least partly, the reduction in KV current density in these cells. However, normal HIF-1α levels were a prerequisite for this VSMC hypertrophy, because hypoxic mice with a null allele at the Hif1a locus, i.e. Hif1a(+/−) mice, lacked this hypertrophic response of VSMCs, as well as the reduction in KV current induced by chronic hypoxia.
Hypertrophy and strengthening of the contractile apparatus of VSMCs may result from their increased mechanical stress in hypertension. In the vessels of the systemic circulation, this hypertrophy may also be associated with polyploidy of the cells. This polyploidy is the result of incomplete growth stimulation, which leads to DNA synthesis and mitosis of cells that are not followed by karyokinesis of the cells, or at least their cytokinesis, which also leads to the formation of binucleated cells. This process is also referred to as endoreduplication [107]. VSMCs with duplicated or multiplied chromosomal equipment are then considered to be more efficient in the synthesis of contractile and ECM proteins, and thus better able to withstand an increased mechanical load. In this sense, hypertrophy and polyploidization of cells are considered a kind of differentiation of VSMCs [91]; for a review, see [57]. Another mechanism of possible polyploidy in pulmonary hypertension could be cell fusion initiated by circulating cells that contribute to tissue repair [108]. However, the presence of polyploidy in pulmonary vessels is rather unlikely, as shown by studies based on flow cytometry [109] or based on fluorescent in situ hybridization (FISH; [108]). Consistent with this, VSMCs of the outer media of the bovine main pulmonary artery and distal pulmonary arteries, which increased their volume and photosynthesis in response to hypoxia, exhibited relatively low DNA synthesis [105].
However, cell hypertrophy can also be a sign of cell senescence. A recent study by Born et al. (2023) [110] showed that HPH was associated with the accumulation of senescent VSMC, and also endothelial cells, mainly at sites of vascular hypertrophy. This accumulation coincided with increases in the DNA damage markers gamma H2A histone family member X (γ-H2AX) and tumor suppressor p53 binding protein 1 (53BP1). Senescent VSMCs stimulated the migration and growth of neighboring cells through the secretion of paracrine factors, whereas senescent endothelial cells released pro-inflammatory factors attracting cells of the immune system. Nevertheless, the elimination of senescent cells by senolytic therapies aggravated PH, which resulted mainly from the removal of senescent endothelial cells and further activation of VSMC proliferation and loss of lung capillaries [110]. Senescent cells with reduced proliferative capacity have been demonstrated in pulmonary arteries during HPH in studies by the group of Stenmark et al. Their occurrence depended on the duration and the severity of hypoxia, the duration of hypertension, and the localization in the pulmonary circulation, where they varied in the length and thickness of the vessels (for a review, see [54]).
The work of Prof. Herget’s group and collaborating scientists from the Institute of Physiology of the Czech Academy of Sciences in the field of pulmonary hypertension is schematically shown in Fig. 11.
Fig. 11.
Work of Professor Jan Herget’s group and scientists from the Institute of Physiology of the Czech Academy of Sciences. Resulting publications in brackets.
Treatment options for HPH
Despite decades of research on HPH mechanisms, the practical outcome – effective therapy – still remains limited. As extensive recent reviews on this topic are available, we supplement our review focusing on pulmonary vascular remodeling in hypoxia only with a brief, non-exhaustive overview of the main concepts.
While several options to slow down or even stop the progression of PAH (group 1 PH) are available, they are not necessarily useful and could even be counterproductive in HPH [113]. The main reason is that these drugs (targeting NO/cGMP axis, prostacyclin, or endothelin) [114–116] not only reduce the morphological remodeling of the pulmonary vascular wall, but also inhibit hypoxic pulmonary vasoconstriction. As this physiological mechanism is important for optimization of lung ventilation/perfusion ratio and thus arterial blood oxygenation, its inhibition easily results in further worsening of oxygenation (already compromised in patients with HPH). Currently, the therapy of patients with HPH therefore primarily targets the underlying disease, e.g. COPD with bronchodilators or interstitial lung diseases with anti-inflammatory drugs and antifibrotics (for a review, see [9,113]).
The problem of the inhibition of hypoxic pulmonary vasoconstriction may, in principle, be overcome by using inhaled instead of systemically administered agents. That way, the agent reaches poorly ventilated regions of the lung much less easily that the well ventilated areas. Any vasodilator (and anti-proliferative) effect is thus meager in the poorly ventilated parts and the worsening of the oxygenation is mostly prevented.
A good example of such a substance is inhaled NO. It must be administered in low concentrations (low ppm at most) to prevent lateral diffusion from the ventilated to the non-ventilated alveoli. This also limits the potential toxic effects of NO [117,118]. Inhaled NO has the added advantage of being selective only for pulmonary vessels. After diffusion from the alveoli, it is very rapidly scavenged by hemoglobin in erythrocytes and therefore does not enter the systemic vasculature in large enough quantities to cause vasodilation. However, NO has the major drawback of being severely toxic in higher concentrations, which hinders its usefulness for long-term outpatient treatment.
Prostacyclin analogues can also be administered by inhalation, and they (e.g. treprostinil) were shown to be useful in patients with PH associated with interstitial lung disease, i.e. PH belonging to group 3 [119–121].
Other promising therapeutic interventions against vascular remodeling in HPH could include silencing or enhancing the specific miRNAs mentioned above (Table 1; [58,87]), and also silencing the expression of HIFs by small interfering RNAs (siRNAs) [85,122] antisense oligonucleotides or small molecule inhibitors [86].
Statins, competitive inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductases, are another promising group of drugs for the therapy of HPH. Although they were primarily developed to reduce cardiovascular risks by lowering the level of blood cholesterol, they display a number of health-positive effects unrelated to their primary function (so-called pleiotropic effects). They proved beneficial in several animal models of HPH and some human studies. Moreover, they may amplify the effects of other drugs targeting HPH [8,111].
Conclusion and further perspectives
This review focuses on the history of more than twenty years of collaboration between a group of scientists at the Institute of Physiology of the Czech Academy of Sciences, and Professor Jan Herget’s group at the 2nd Faculty of Medicine of Charles University, and was written to mark the five-year anniversary of Jan’s death. The collaboration concerned HPH, and in particular the changes in pulmonary vascular wall components. With prolonged, continuous or intermittent hypoxia, vascular wall remodeling can occur when migration, proliferation and phenotypic modulation of VSMCs and other cell types present in the vascular wall, such as endothelial cells and adventitial fibroblasts, are activated. In this activation, an important role is played by reactive oxygen and nitrogen species, by the presence of pro-inflammatory cells (macrophages, mast cells), by degradation of the extracellular matrix by proteases and qualitative changes in its composition, by the production of hypoxia-inducible factors, by changes in the expression of specific micro RNAs, and also by the expression of galectins (galectin-3).
Our results show that hypoxia has a dual effect on the growth of VSMCs. On the one hand, it promotes their proliferation and hyperplasia; on the other hand, it also promotes the hypertrophy of VSMCs and the expression of contractile proteins in these cells, such as calponin-1 and myosin heavy chain, which seems to be related to the pro-fibrotic effect of hypoxia. This dual effect of hypoxia on VSMC growth in terms of hyperplasia and hypertrophy needs to be further investigated.
Acknowledgements
This review article, summarizing results from several studies, was supported by the Czech Science Foundation (grant No. 22-00317S) and by the National Institute for Research of Metabolic and Cardiovascular Diseases project (Programme EXCELES, ID Project No. LX22NPO5104) - funded by the European Union - Next Generation EU. Further support was provided by the Czech Academy of Sciences, Praemium Academiae grant No. AP2202, and also by OP JAC Project No. CZ.02.01.01/00/22_008/0004562, of the Ministry of Education, Youth and Sports, co-funded by the European Union. Robin Healey from Czech Technical University, Prague, Czech Republic, is gratefully acknowledged for the language revision of the manuscript.
Abbreviations
- 53BP1
tumor suppressor p53 binding protein 1
- ACTA2
actin alpha 2, an actin protein also referred to as alpha-actin, alpha-actin-2, aortic smooth muscle or alpha-smooth muscle actin (α-SMA, SMactin, alpha-SM-actin, ASMA)
- ADSC(s)
adipose tissue-derived stem cell(s)
- AKT
alpha serine/threonine-protein kinase, protein kinase B
- ANOVA
Analysis of Variance
- Bcl-2
B cell lymphoma-2
- bFGF
basic fibroblast growth factor (also known as FGF-2)
- BMP-4
bone morphogenetic protein-4
- CD146
cluster of differentiation 146, also known as the melanoma cell adhesion molecule (MCAM) or cell surface glycoprotein MUC18
- cGMP
cyclic guanosine monophosphate
- CNN1
gene encoding calponin-1
- Col
collagen
- COL1A1
gene encoding collagen type I alpha 1
- COPD
chronic obstructive pulmonary disease
- CTEPH
chronic thromboembolic pulmonary hypertension
- DMEM
Dulbecco’s Modified Eagle’s Minimum Essential Medium
- DNA
deoxyribonucleic acid
- DSCG
disodium cromoglycate
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ELISA
enzyme-linked immunosorbent assay
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinase
- ERS
European Respiratory Society
- ESC
European Society of Cardiology
- F-actin
filamentous actin
- FAD2+/FADH2
oxidized and reduced form of flavin adenine dinucleotide
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- FISH
fluorescent in situ hybridization
- Gal-3
galectin-3
- γ-H2AX
gamma H2A histone family member X
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HAPH
high-altitude pulmonary hypertension
- HGF
hepatocyte growth factor
- HIF(s)
hypoxia-inducible factor(s)
- HPH
hypoxic pulmonary hypertension
- HSP
heat-shock protein
- Hyp
hypoxia
- Hypox
hypoxia
- ICAM-1
intercellular cell adhesion molecule-1
- IGF
insulin-like growth factor
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LGALS3
gene encoding human galectin-3
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MC(s)
mast cells
- Mettl3
methyltransferase 3 (N6-Adenosine-Methyltransferase Complex Catalytic Subunit)
- miRNA(s)
micro ribonucleic acid(s)
- MMP(s)
metalloproteinase(s)
- mTOR
mammalian target of rapamycin
- mPAP
mean pulmonary artery pressure
- mRNA
messenger ribonucleic acid
- MYH11
Myosin Heavy Chain 11 (a gene encoding myosin-11 protein, also referred to as AAT4, FAA4, SMHC, SMMHC, myosin heavy chain 11, smooth muscle myosin heavy chain 11, VSCM2, SMMS-1)
- NAD+/NADH
oxidized/reduced form of nicotinamide adenine dinucleotide
- NADP+/NADPH
oxidized/reduced form of nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3
- NO
nitric oxide
- Norm
normoxia
- Normox
normoxia
- nt
nucleotide
- p
probability under the null hypothesis of obtaining a real-valued test statistic at least as extreme as the one obtained
- p53
Tumor protein P53, cellular tumor antigen p53 (UniProt name), or transformation-related protein 53 (TRP53), a regulatory protein that is often mutated in human cancers
- PAH
pulmonary arterial hypertension
- PBS
phosphate-uffered saline
- PCR
polymerase chain reaction
- PDGF
platelet-derived growth factor
- PDGFR-β
platelet-derived growth factor receptor-β
- PH
pulmonary hypertension
- PS
polystyrene
- PTEN
phosphatase and tensin homolog
- qPCR
quantitative polymerase chain reaction
- RBL-2H3
a subline of rat basophilic leukemia cells
- RNAs
ribonucleic acid(s)
- ROS
reactive oxygen species
- SEM
Standard Error of the Mean
- SD
Standard Deviation
- SDF-1
stromal cell-derived factor-1
- SM1, SM2
smooth muscle myosin of SM1 and SM2 isoforms
- SM22α
Smooth Muscle 22α, a marker of adult smooth muscle
- SMC(s)
smooth muscle cell(s)
- siRNA(s)
small interfering RNA(s)
- SMemb
smooth muscle myosin heavy chain embryonic
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor-alpha
- TRITC
tetramethylrhodamine isothiocyanate
- UV
ultra-violet
- VCAM-1
and vascular cell adhesion olecule-1
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- vs
versus
- VSMC(s)
vascular smooth muscle cell(s)
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Noordegraaf AV, Delcroix M, Rosenkranz S, Grp EESD. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61:2200879. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 2.Vonk Noordegraaf A, Groeneveldt JA, Bogaard HJ. Pulmonary hypertension. Eur Respir Rev. 2016;25:4–11. doi: 10.1183/16000617.0096-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeper MM, Ghofrani HA, Grünig E, Klose H, Olschewski H, Rosenkranz S. Pulmonary Hypertension. Dtsch Arztebl Int. 2017;114:73–84. doi: 10.3238/arztebl.2016.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson AAR, Lawrie A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol Med. 2017;23:31–45. doi: 10.1016/j.molmed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, Barber D, Johnson A, Doliszny KM, Archer SL. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension A Population-Based Cohort Study in Ontario, Canada. Circ-Cardiovasc Qual. 2018;11:e003973. doi: 10.1161/CIRCOUTCOMES.117.003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai T, Qiu C, Xian Z, Lu Y, Zeng Y, Li J. A narrative review of research advances in hypoxic pulmonary hypertension. Ann Transl Med. 2022;10:230. doi: 10.21037/atm-22-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Dorfmuller P, Shlobin OA, Ventetuolo CE. Group 3 Pulmonary Hypertension: From Bench to Bedside. Circ Res. 2022;130:1404–1422. doi: 10.1161/CIRCRESAHA.121.319970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maimaitiaili N, Zeng YX, Ju PA, Zhakeer G, Guangxi E, Yao HY, Shi YF, Zhai M, Zhuang JH, Peng WH, Zhuoga D, Yu Q. NLRC3 deficiency promotes hypoxia-induced pulmonary hypertension development via IKK/NF-?B p65/HIF-1a pathway. Exp Cell Res. 2023;431:113755. doi: 10.1016/j.yexcr.2023.113755. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YP, Xu CB. The roles of endothelin and its receptors in cigarette smoke-associated pulmonary hypertension with chronic lung disease. Pathol Res Pract. 2020;216:153083. doi: 10.1016/j.prp.2020.153083. [DOI] [PubMed] [Google Scholar]
- 12.Karnati S, Seimetz M, Kleefeldt F, Sonawane A, Madhusudhan T, Bachhuka A, Kosanovic D, Weissmann N, Krüger K, Ergün S. Chronic Obstructive Pulmonary Disease and the Cardiovascular System: Vascular Repair and Regeneration as a Therapeutic Target. Front Cardiovasc Med. 2021;8:649512. doi: 10.3389/fcvm.2021.649512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siques P, Pena E, Brito J, El Alam S. Oxidative stress, kinase activation, and inflammatory pathways involved in effects on smooth muscle cells during pulmonary artery hypertension under hypobaric hypoxia exposure. Front Physiol. 2021;12:690341. doi: 10.3389/fphys.2021.690341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol. 2015;308:L229–L252. doi: 10.1152/ajplung.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandras SA, Mehta HS, Vaidya A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin Proc. 2020;95:1978–1988. doi: 10.1016/j.mayocp.2020.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Suggett AJ, Herget J. Effect of alpha-methyldopa on the pulmonary vascular changes induced by chronic hypoxia in rats. Clin Sci Mol Med. 1977;53:397–400. doi: 10.1042/cs0530397. [DOI] [PubMed] [Google Scholar]
- 18.Herget J, Kuklík V. Perinatal lung injury extends in adults the site of hypoxic pulmonary vasoconstriction upstream. Physiol Res. 1995;44:25–30. [PubMed] [Google Scholar]
- 19.Herget J, Paleček F, Vízek M, Holusa R. Causes of Experimental Pulmonary-Hypertension in Rats. Physiol Bohemoslov. 1976;25:411–418. [PubMed] [Google Scholar]
- 20.Herget J, Paleček F, Preclík P, Čermáková M, Vízek M, Petrovická M. Pulmonary-Hypertension Induced by Repeated Pulmonary Inflammation in the Rat. J Appl Physiol Respir Environ Exerc Physiol. 1981;51:755–761. doi: 10.1152/jappl.1981.51.3.755. [DOI] [PubMed] [Google Scholar]
- 21.Herget J, Holusa R, Palecek F. Pulmonary-Hypertension in Rats with Experimental Emphysema. Physiol Bohemoslov. 1974;23:55–65. [PubMed] [Google Scholar]
- 22.Herget J, Paleček F, Čermáková M, Vízek M. Pulmonary-Hypertension in Rats with Papain Emphysema. Respiration. 1979;38:204–212. doi: 10.1159/000194082. [DOI] [PubMed] [Google Scholar]
- 23.Herget J, Kuncová M, Havránková J, Paleček F. Pulmonary-Hypertension in Silicotic Rats. Arch Environ Health. 1979;34:320–324. doi: 10.1080/00039896.1979.10667424. [DOI] [PubMed] [Google Scholar]
- 24.Weir EK, López-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- 26.Osada-Oka M, Ikeda T, Imaoka S, Akiba S, Sato T. VEGF-enhanced proliferation under hypoxia by an autocrine mechanism in human vascular smooth muscle cells. J Atheroscler Thromb. 2008;15:26–33. doi: 10.5551/jat.E533. [DOI] [PubMed] [Google Scholar]
- 27.Böger R, Hannemann J. Dual role of the L-arginine-ADMA-NO pathway in systemic hypoxic vasodilation and pulmonary hypoxic vasoconstriction. Pulm Circ. 2020;10:2045894020918850. doi: 10.1177/2045894020918850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slobod D, Damia A, Leali M, Spinelli E, Mauri T. Pathophysiology and clinical meaning of ventilation-perfusion mismatch in the acute respiratory distress syndrome. Biology (Basel) 2022;12:67. doi: 10.3390/biology12010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunham-Snary KJ, Wu DC, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, Parlow JL, Archer SL. Hypoxic pulmonary vasoconstriction from molecular mechanisms to medicine. Chest. 2017;151:181–192. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herget J, Bíbová J, Novotná J. [Mechanisms of remodeling of pulmonary blood vessels in chronic hypoxia] Cesk Fysiol. 1999;48:179–184. [PubMed] [Google Scholar]
- 31.Archer SL, Will JA, Weir EK. Redox status in the control of pulmonary vascular tone. Herz. 1986;11:127–141. [PubMed] [Google Scholar]
- 32.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A Redox-Based O2 Sensor in Rat Pulmonary Vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.RES.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 33.Herget J, Wilhelm J, Novotná J, Eckhardt A, Vytášek R, Mrázková L, Ošťádal M. A possible role of the oxidant tissue injury in the development of hypoxic pulmonary hypertension. Physiol Res. 2000;49:493–501. [PubMed] [Google Scholar]
- 34.Lachmanová V, Hnilicková O, Povýsilová V, Hampl V, Herget J. N-acetylcysteine inhibits hypoxic pulmonary hypertension most effectively in the initial phase of chronic hypoxia. Life Sci. 2005;77:175–182. doi: 10.1016/j.lfs.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Hodyc D, Johnson E, Skoumalová A, Tkaczyk J, Maxová H, Vízek M, Herget J. Reactive oxygen species production in the early and later stage of chronic ventilatory hypoxia. Physiol Res. 2012;61:145–151. doi: 10.33549/physiolres.932206. [DOI] [PubMed] [Google Scholar]
- 36.Vajner L, Vytášek R, Lachmanová V, Uhlík J, Konrádová V, Novotná J, Hampl V, Herget J. Acute and chronic hypoxia as well as 7-day recovery from chronic hypoxia affects the distribution of pulmonary mast cells and their MMP-13 expression in rats. Int J Exp Pathol. 2006;87:383–391. doi: 10.1111/j.1365-2613.2006.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maxová H, Novotná J, Vajner L, Tomášová H, Vytášek R, Vízek M, Bačáková L, Valoušková V, Eliášová T, Herget J. In Vitro Hypoxia Increases Production of Matrix Metalloproteinases and Tryptase in Isolated Rat Lung Mast Cells. Physiol Res. 2008;57:903–910. doi: 10.33549/physiolres.931278. [DOI] [PubMed] [Google Scholar]
- 38.Maxová H, Bačáková L, Lisá V, Novotná J, Tomášová H, Vízek M, Herget J. Production of proteolytic enzymes in mast cells, fibroblasts, vascular smooth muscle and endothelial cells cultivated under normoxic or hypoxic conditions. Physiol Res. 2010;59:711–719. doi: 10.33549/physiolres.931909. [DOI] [PubMed] [Google Scholar]
- 39.Maxová H, Bačáková L, Eckhardt A, Mikšík I, Lisá V, Novotná J, Herget J. Growth of vascular smooth muscle cells on Collagen I exposed to RBL-2H3 mastocytoma cells. Cell Physiol Biochem. 2010;25:615–622. doi: 10.1159/000315080. [DOI] [PubMed] [Google Scholar]
- 40.Gallardo-Vara E, Ntokou A, Dave JM, Jovin DG, Saddouk FZ, Greif DM. Vascular pathobiology of pulmonary hypertension. J Heart Lung Transplant. 2023;42:544–552. doi: 10.1016/j.healun.2022.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenmark KR, Bouchey D, Nemenoff R, Dempsey EC, Das M. Hypoxia-induced pulmonary vascular remodeling: contribution of the adventitial fibroblasts. Physiol Res. 2000;49:503–517. [PubMed] [Google Scholar]
- 42.Nozik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol. 2007;618:101–112. doi: 10.1007/978-0-387-75434-5_8. [DOI] [PubMed] [Google Scholar]
- 43.Zaloudíková M, Herget J, Vízek M. The contractile response of isolated small pulmonary arteries induced by activated macrophages. Physiol Res. 2014;63:267–270. doi: 10.33549/physiolres.932698. [DOI] [PubMed] [Google Scholar]
- 44.Žaloudíková M, Vytášek R, Rašková M, Vízek M, Uhlík J, Hampl V. The effect of exposure to hypoxia on superoxide formation by alveolar macrophages is indirect. Life Sci. 2019;236:116864. doi: 10.1016/j.lfs.2019.116864. [DOI] [PubMed] [Google Scholar]
- 45.Žaloudíková M. Mechanisms and Effects of Macrophage Polarization and Its Specifics in Pulmonary Environment. Physiol Res. 2023;72:S137–S156. doi: 10.33549/physiolres.935058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maxová H, Herget J, Vízek M. Lung mast cells and hypoxic pulmonary hypertension. Physiol Res. 2012;61:1–11. doi: 10.33549/physiolres.932221. [DOI] [PubMed] [Google Scholar]
- 47.Burdon RH. Control of cell proliferation by reactive oxygen species. Biochem Soc Trans. 1996;24:1028–1032. doi: 10.1042/bst0241028. https://doi.org/10.1042/bst0241028, https://doi.org/10.1042/bst024521sc. [DOI] [PubMed] [Google Scholar]
- 48.Bačáková L, Herget J, Wilhelm J. Influence of macrophages and macrophage-modified collagen I on the adhesion and proliferation of vascular smooth muscle cells in culture. Physiol Res. 1999;48:341–351. [PubMed] [Google Scholar]
- 49.Plecitá-Hlavatá L, D’Alessandro A, El Kasmi K, Li M, Zhang H, Ježek P, Stenmark KR. Metabolic Reprogramming and Redox Signaling in Pulmonary Hypertension. Adv Exp Med Biol. 2017;967:241–260. doi: 10.1007/978-3-319-63245-2_14. [DOI] [PubMed] [Google Scholar]
- 50.Archer SL, Nelson DP, Weir EK. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol (1985) 1989;67:1903–1911. doi: 10.1152/jappl.1989.67.5.1903. [DOI] [PubMed] [Google Scholar]
- 51.Hu YQ, Zhao YC, Li P, Lu H, Li H, Ge JB. Hypoxia and panvascular diseases: exploring the role of hypoxia-inducible factors in vascular smooth muscle cells under panvascular pathologies. Sci Bull (Beijing) 2023;68:1954–1974. doi: 10.1016/j.scib.2023.07.032. [DOI] [PubMed] [Google Scholar]
- 52.Bačáková L, Wilhelm J, Herget J, Novotná J, Eckhart A. Oxidized collagen stimulates proliferation of vascular smooth muscle cells. Exp Mol Pathol. 1997;64:185–194. doi: 10.1006/exmp.1997.2219. [DOI] [PubMed] [Google Scholar]
- 53.Bačáková L, Lisá V, Kubínová L, Wilhelm J, Novotná J, Eckhart A, Herget J. Ultraviolet light-irradiated collagen III modulates expression of cytoskeletal and surface adhesion molecules in rat aortic smooth muscle cells in vitro. Virchows Arch. 2002;440:50–62. doi: 10.1007/s004280100463. [DOI] [PubMed] [Google Scholar]
- 54.Stenmark KR, Frid MG, Graham BB, Tuder RM. Dynamic and diverse changes in the functional properties of vascular smooth muscle cells in pulmonary hypertension. Cardiovasc Res. 2018;114:551–564. doi: 10.1093/cvr/cvy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu CJ, Zhang H, Laux A, Pullamsetti SS, Stenmark KR. Mechanisms contributing to persistently activated cell phenotypes in pulmonary hypertension. J Physiol. 2019;597:1103–1119. doi: 10.1113/JP275857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilhelm J, Sojková J, Herget J. Production of hydrogen peroxide by alveolar macrophages from rats exposed to subacute and chronic hypoxia. Physiol Res. 1996;45:185–191. [PubMed] [Google Scholar]
- 57.Bacakova L, Travnickova M, Filova E, Matějka R, Stepanovska J, Musilkova J, Zarubova J, Molitor M. The Role of Vascular Smooth Muscle Cells in the Physiology and Pathophysiology of Blood Vessels. In: SAKUMA K, editor. Muscle Cell and Tissue. London, United Kingdom: IntechOpen; 2018. pp. 229–256. [DOI] [Google Scholar]
- 58.Huang XJ, Akguen EE, Mehmood K, Zhang H, Tang ZX, Li Y. Mechanism of hypoxia-mediated smooth muscle cell proliferation leading to vascular remodeling. Biomed Res Int. 2022;2022:3959845. doi: 10.1155/2022/3959845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, Ježek P, Li M, Zhang H, Gupte SA, Stenmark KR. Hallmarks of Pulmonary Hypertension: Mesenchymal and Inflammatory Cell Metabolic Reprogramming. Antioxid Redox Signal. 2018;28:230–250. doi: 10.1089/ars.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Qazazi R, Lima PDA, Prisco SZ, Potus F, Dasgupta A, Chen KH, Tian L, Bentley RET, Mewburn J, Martin AY, Wu DC, Jones O, Maurice DH, Bonnet S, Provencher S, Prins KW, Archer SL. Macrophage-NLRP3 activation promotes right ventricle failure in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2022;206:608–624. doi: 10.1164/rccm.202110-2274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Napoli C, Paolisso G, Casamassimi A, Al-Omran M, Barbieri M, Sommese L, Infante T, Ignarro LJ. Effects of nitric oxide on cell proliferation novel insights. J Am Coll Cardiol. 2013;62:89–95. doi: 10.1016/j.jacc.2013.03.070. [DOI] [PubMed] [Google Scholar]
- 62.Hildebrand S, Ibrahim M, Schlitzer A, Maegdefessel L, Röll W, Pfeifer A. PDGF regulates guanylate cyclase expression and cGMP signaling in vascular smooth muscle. Commun Biol. 2022;5:197. doi: 10.1038/s42003-022-03140-2. https://doi.org/10.1038/s42003-022-03244-9, https://doi.org/10.1038/s42003-022-03140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novotná J, Herget J. Exposure to chronic hypoxia induces qualitative changes of collagen in the walls of peripheral pulmonary arteries. Life Sci. 1998;62:1–12. doi: 10.1016/S0024-3205(97)01032-1. [DOI] [PubMed] [Google Scholar]
- 64.Novotná J, Herget J. Possible role of matrix metalloproteinases in reconstruction of peripheral pulmonary arteries induced by hypoxia. Physiol Res. 2002;51:323–334. doi: 10.33549/physiolres.930238. [DOI] [PubMed] [Google Scholar]
- 65.Bačáková L, Herget J, Novotná J, Eckhart A, Lisá V. Adhesion, growth and stress adaptation of vascular smooth muscle cells in cultures on collagen I degraded by matrix metalloproteinase - 13. Ateroskleróza: metabolizmus, klinika a liečba. 2002;6:155–161. [Google Scholar]
- 66.Roberts ISD, Brenchley PEC. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol. 2000;53:858–862. doi: 10.1136/jcp.53.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herget J, Novotná J, Bíbová J, Povýsilová V, Vanková M, Hampl V. Metalloproteinase inhibition by Batimastat attenuates pulmonary hypertension in chronically hypoxic rats. Am J Physiol-Lung C. 2003;285:L199–L208. doi: 10.1152/ajplung.00167.2002. [DOI] [PubMed] [Google Scholar]
- 68.Banasová A, Maxová H, Hampl V, Vízek M, Povysilová V, Novotná J, Vajnerová O, Hnilicková O, Herget J. Prevention of mast cell degranulation by disodium cromoglycate attenuates the development of hypoxic pulmonary hypertension in rats exposed to chronic hypoxia. Respiration. 2008;76:102–107. doi: 10.1159/000121410. [DOI] [PubMed] [Google Scholar]
- 69.Maxová H, Vasilková M, Novotná J, Vajnerová O, Banasová A, Vízek M, Herget J. Prevention of mast cell degranulation by disodium cromoglycate delayed the regression of hypoxic pulmonary hypertension in rats. Respiration. 2010;80:335–339. doi: 10.1159/000312403. [DOI] [PubMed] [Google Scholar]
- 70.Bacakova L, Filova E, Parizek M, Ruml T, Svorcik V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv. 2011;29:739–767. doi: 10.1016/j.biotechadv.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Žaloudíková M, Eckhardt A, Vytášek R, Uhlík J, Novotný T, Bačáková L, Musílková J, Hampl V. Decreased collagen VI in the tunica media of pulmonary vessels during exposure to hypoxia: a novel step in pulmonary arterial remodeling. Pulm Circ. 2019;9:2045894019860747. doi: 10.1177/2045894019860747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shamhart PE, Meszaros JG. Non-fibrillar collagens: Key mediators of post-infarction cardiac remodeling? J Mol Cell Cardiol. 2010;48:530–537. doi: 10.1016/j.yjmcc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 73.Bonaldo P, Colombatti A. The carboxyl terminus of the chicken Alpha-3 chain of collagen-vi is a unique mosaic structure with glycoprotein Ib-Like, Fibronectin Type-Iii, and kunitz modules. J Biol Chem. 1989;264:20235–20239. doi: 10.1016/S0021-9258(19)47052-X. [DOI] [PubMed] [Google Scholar]
- 74.Aigner T, Hambach L, Söder S, Schlötzer-Schrehardt U, Pöschl E. The C5 domain of Col6A3 is cleaved off from the Col6 fibrils immediately after secretion. Biochem Biophys Res Commun. 2002;290:743–748. doi: 10.1006/bbrc.2001.6227. [DOI] [PubMed] [Google Scholar]
- 75.Cescon M, Gattazzo F, Chen PW, Bonaldo P. Collagen VI at a glance. J Cell Sci. 2015;128:3525–3531. doi: 10.1242/jcs.169748. [DOI] [PubMed] [Google Scholar]
- 76.Wang JY, Pan WS. The biological role of the collagen Alpha-3 (VI) Chain and its cleaved c5 domain fragment endotrophin in cancer. Oncotargets Ther. 2020;13:5779–5793. doi: 10.2147/OTT.S256654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun K, Park J, Kim M, Scherer PE. Endotrophin, a multifaceted player in metabolic dysregulation and cancer progression, is a predictive biomarker for the response to PPARγ agonist treatment. Diabetologia. 2017;60:24–29. doi: 10.1007/s00125-016-4130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henriksen K, Genovese F, Reese-Petersen A, Audoly LP, Sun K, Karsdal MA, Scherer PE. Endotrophin, a Key Marker and Driver for Fibroinflammatory Disease. Endocr Rev. 2024;45:361–378. doi: 10.1210/endrev/bnad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams J, Maroney SP, Schmitt LR, Brown RD, Krafsur G, Frid MG, McCabe MC, Iheagwam FN, Gandjeva A, Williams KJ, Luyendyk JP, Saviola AJ, Tuder RM, Stenmark K, Hansen KC. A bovine model of hypoxia-induced pulmonary hypertension reveals a gradient of immune and matrisome response with a complement signature found in circulation. Am J Pathol. doi: 10.1152/ajpcell.00274.2024. accepted. [DOI] [PubMed] [Google Scholar]
- 81.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JGN, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 82.Novák T, Žaloudíková M, Smolková P, Kaftanová B, Edlmanová J, Krása K, Hampl V. Hypoxia-inducible factors activator, roxadustat, increases pulmonary vascular resistance in rats. Physiol Res. 2023;72:S587–S592. doi: 10.33549/physiolres.935220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JRB, Hashimoto K, Wang SH, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 84.Luo YT, Teng X, Zhang LL, Chen JN, Liu Z, Chen XH, Zhao S, Yang S, Feng J, Yan XY. CD146-HIF-1α hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension. Nat Commun. 2019;10:3551. doi: 10.1038/s41467-019-11500-6. https://doi.org/10.1038/s41467-019-12107-7, https://doi.org/10.1038/s41467-019-11500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pullamsetti SS, Mamazhakypov A, Weissmann N, Seeger W, Savai R. Hypoxia-inducible factor signaling in pulmonary hypertension. J Clin Invest. 2020;130:5638–5651. doi: 10.1172/JCI137558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu CJ, Poth JM, Zhang H, Flockton A, Laux A, Kumar S, McKeon B, Mouradian G, Li M, Riddle S, Pugliese SC, Brown RD, Wallace EM, Graham BB, Frid MG, Stenmark KR. Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. Eur Respir J. 2019;54:1900378. doi: 10.1183/13993003.00378-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang WF, Tao ZY, Xu F, Diao Q, Li J, Zhou L, Miao YX, Xie SS, Wan JJ, Xu RL. An overview of miRNAs involved in PASMC phenotypic switching in pulmonary hypertension. Biomed Res Int. 2021;2021:5765029. doi: 10.1155/2021/5765029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar S, Frid MG, Zhang H, Li M, Riddle S, Brown RD, Yadav SC, Roy MK, Dzieciatkowska ME, D’Alessandro A, Hansen KC, Stenmark KR. Complement-containing small extracellular vesicles from adventitial fibroblasts induce proinflammatory and metabolic reprogramming in macrophages. JCI Insight. 2021;6:e148382. doi: 10.1172/jci.insight.148382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plecitá-Hlavatá L, Brázdová A, Křivonosková M, Hu CJ, Phang T, Tauber J, Li M, Zhang H, Hoetzenecker K, Crnkovic S, Kwapiszewska G, Stenmark KR. Microenvironmental regulation of T-cells in pulmonary hypertension. Front Immunol. 2023;14:1223122. doi: 10.3389/fimmu.2023.1223122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu CJ, Laux A, Gandjeva A, Wang LY, Li M, Brown RD, Riddle S, Kheyfets VO, Tuder RM, Zhang H, Stenmark KR. The effect of hypoxia-inducible factor inhibition on the phenotype of fibroblasts in human and bovine pulmonary hypertension. Am J Respir Cell Mol Biol. 2023;69:73–86. doi: 10.1165/rcmb.2022-0114OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bačáková L, Pellicciari C, Bottone MG, Lisá V, Mareš V. A sex-related difference in the hypertrophic versus hyperplastic response of vascular smooth muscle cells to repeated passaging in culture. Histol Histopathol. 2001;16:675–684. doi: 10.14670/HH-16.675. [DOI] [PubMed] [Google Scholar]
- 92.Sedlář A, Vrbata D, Pokorná K, Holzerová K, Červený J, Kočková O, Hlaváčková M, Doubková M, Musílková J, Křen V, Kolář F, Bačáková L, Bojarová P. Glycopolymer Inhibitors of Galectin-3 Suppress the Markers of Tissue Remodeling in Pulmonary Hypertension. J Med Chem. 2024;67:9214–9226. doi: 10.1021/acs.jmedchem.4c00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barman SA, Li XY, Haigh S, Kondrikov D, Mahboubi K, Bordan Z, Stepp DW, Zhou JL, Wang YS, Weintraub DN, Traber EE, Snider L, Jonigk D, Sullivan ENE, Crislip RY, Butcher JT, Thompson J, Su YC, Chen F, Fulton DJR. Galectin-3 is expressed in vascular smooth muscle cells and promotes pulmonary hypertension through changes in proliferation, apoptosis, and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2019;316:L784–L797. doi: 10.1152/ajplung.00186.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian L, Chen K, Cao J, Han Z, Wang Y, Gao L, Fan Y, Wang C. Galectin-3 induces the phenotype transformation of human vascular smooth muscle cells via the canonical Wnt signaling. Mol Med Rep. 2017;15:3840–3846. doi: 10.3892/mmr.2017.6429. [DOI] [PubMed] [Google Scholar]
- 95.Li TZM, Zha LH, Luo H, Li SQ, Zhao L, He JN, Li XH, Qi QQ, Liu YW, Yu ZX. Galectin-3 mediates endothelial-to-mesenchymal transition in pulmonary arterial hypertension. Aging Dis. 2019;10:731–745. doi: 10.14336/AD.2018.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choo YY, Sakai T, Komatsu S, Ikebe R, Jeffers A, Singh KP, Idell S, Tucker TA, Ikebe M. Calponin 1 contributes to myofibroblast differentiation of human pleural mesothelial cells. Am J Physiol Lung Cell Mol Physiol. 2022;322:L348–L364. doi: 10.1152/ajplung.00289.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bekhite MM, Finkensieper A, Rebhan J, Huse S, Schultze-Mosgau S, Figulla HR, Sauer H, Wartenberg M. Hypoxia, leptin, and vascular endothelial growth factor stimulate vascular endothelial cell differentiation of human adipose tissue-derived stem cells. Stem Cells Dev. 2014;23:333–351. doi: 10.1089/scd.2013.0268. [DOI] [PubMed] [Google Scholar]
- 98.Podkalicka P, Stepniewski J, Mucha O, Kachamakova-Trojanowska N, Dulak J, Loboda A. Hypoxia as a driving force of pluripotent stem cell reprogramming and differentiation to endothelial cells. Biomolecules. 2020;10:1614. doi: 10.3390/biom10121614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shimomura S, Inoue H, Arai Y, Nakagawa S, Fujii Y, Kishida T, Shin-Ya M, Ichimaru S, Tsuchida S, Mazda O, Kubo T. Hypoxia promotes differentiation of pure cartilage from human induced pluripotent stem cells. Mol Med Rep. 2022;26:229. doi: 10.3892/mmr.2022.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu X, Wan QL, Ye XL, Cheng Y, Pathak JL, Li ZB. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell Mol Biol Lett. 2019;24:64. doi: 10.1186/s11658-019-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen W, Zhuo Y, Duan D, Lu M. Effects of hypoxia on differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2020;15:332–339. doi: 10.2174/1574888X14666190823144928. [DOI] [PubMed] [Google Scholar]
- 102.Lin JY, Zhu QQ, Huang JY, Cai RF, Kuang YP. Hypoxia Promotes Vascular Smooth Muscle Cell (VSMC) Differentiation of Adipose-Derived Stem Cell (ADSC) by Regulating Mettl3 and Paracrine Factors. Stem Cells Int. 2020;2020:2830565. doi: 10.1155/2020/2830565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frid MG, Dempsey EC, Durmowicz AG, Stenmark KR. Smooth muscle cell heterogeneity in pulmonary and systemic vessels - Importance in vascular disease. Arterioscler Thromb Vasc Biol. 1997;17:1203–1209. doi: 10.1161/01.ATV.17.7.1203. [DOI] [PubMed] [Google Scholar]
- 104.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res. 1997;81:940–952. doi: 10.1161/01.RES.81.6.940. [DOI] [PubMed] [Google Scholar]
- 105.Stiebellehner L, Frid MG, Reeves JT, Low RB, Gnanasekharan M, Stenmark KR. Bovine distal pulmonary arterial media is composed of a uniform population of well-differentiated smooth muscle cells with low proliferative capabilities. Am J Physiol Lung Cell Mol Physiol. 2003;285:L819–L828. doi: 10.1152/ajplung.00062.2003. [DOI] [PubMed] [Google Scholar]
- 106.Shimoda LA, Manalo DJ, Sham JSK, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 107.Gui Y, Yin H, He JY, Yang SH, Walsh MP, Zheng XL. Endoreduplication of human smooth muscle cells induced by 2-methoxyestradiol: a role for cyclin-dependent kinase 2. Am J Physiol Lung Cell Mol Physiol. 2007;292:H1313–H1320. doi: 10.1152/ajpheart.00867.2006. [DOI] [PubMed] [Google Scholar]
- 108.Majka SM, Skokan M, Wheeler L, Harral J, Gladson S, Burnham E, Loyd JE, Stenmark KR, Varella-Garcia M, West J. Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1028–L1039. doi: 10.1152/ajplung.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orton EC, LaRue SM, Ensley B, Stenmark K. Bromodeoxyuridine labeling and DNA content of pulmonary arterial medial cells from hypoxia-exposed and nonexposed healthy calves. Am J Vet Res. 1992;53:1925–1930. doi: 10.2460/ajvr.1992.53.10.1925. [DOI] [PubMed] [Google Scholar]
- 110.Born E, Lipskaia L, Breau M, Houssaini A, Beaulieu D, Marcos E, Pierre R, Do Cruzeiro M, Lefevre M, Derumeaux G, Bulavin DV, Delcroix M, Quarck R, Reen V, Gil J, Bernard D, Flaman JM, Adnot S, Abid S. Eliminating Senescent Cells Can Promote Pulmonary Hypertension Development and Progression. Circulation. 2023;147:650–666. doi: 10.1161/CIRCULATIONAHA.122.058794. [DOI] [PubMed] [Google Scholar]
- 111.Krása K, Vajnerová O, Ďurišová J, Minaříkova M, Miková D, Srbová M, Chalupský K, Kaftanová B, Hampl V. Simvastatin and dehydroepiandrosterone sulfate effects against hypoxic pulmonary hypertension are not additive. Physiol Res. 2022;71:801–810. doi: 10.33549/physiolres.934913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sedlář A, Trávníčková M, Bojarová P, Vlachová M, Slámová K, Křen V, Bačáková L. Interaction between galectin-3 and integrins mediates cell-matrix adhesion in endothelial cells and mesenchymal Stem Cells. Int J Mol Sci. 2021;22:5144. doi: 10.3390/ijms22105144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mocumbi A, Humbert M, Saxena A, Jing ZC, Sliwa K, Thienemann F, Archer SL, Stewart S. Pulmonary hypertension. Nat Rev Dis Primers. 2024;10:1. doi: 10.1038/s41572-023-00486-7. https://doi.org/10.1038/s41572-023-00486-7, https://doi.org/10.1038/s41572-024-00493-2. [DOI] [PubMed] [Google Scholar]
- 114.Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke-Zaba J, Provencher S, Weissmann N, Seeger W. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53:1801914. doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Otani N, Tomoe T, Kawabe A, Sugiyama T, Horie Y, Sugimura H, Yasu T, Nakamoto T. Recent Advances in the Treatment of Pulmonary Arterial Hypertension. Pharmaceuticals (Basel) 2022;15:1277. doi: 10.3390/ph15101277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rubin LJ. Endothelin receptor antagonists for the treatment of pulmonary artery hypertension. Life Sci. 2012;91:517–521. doi: 10.1016/j.lfs.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 117.Redaelli S, Magliocca A, Malhotra R, Ristagno G, Citerio G, Bellani G, Berra L, Rezoagli E. Nitric oxide: Clinical applications in critically ill patients. Nitric Oxide-Biol Ch. 2022;121:20–33. doi: 10.1016/j.niox.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rawat M, Lakshminrusimha S, Vento M. Pulmonary hypertension and oxidative stress: Where is the link? Semin Fetal Neonatal Med. 2022;27:101347. doi: 10.1016/j.siny.2022.101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waxman A, Restrepo-Jaramillo R, Thenappan T, Ravichandran A, Engel P, Bajwa A, Allen R, Feldman J, Argula R, Smith P, Rollins K, Deng CQ, Peterson L, Bell H, Tapson V, Nathan SD. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N Engl J Med. 2021;384:325–334. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 120.Behr J. Inhaled Treprostinil in Pulmonary Hypertension in the context of interstitial lung disease: a success, finally. Am J Respir Crit Care Med. 2022;205:144–146. doi: 10.1164/rccm.202110-2444ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piccari L, Wort SJ. Use of inhaled treprostinil in patients with interstitial lung disease and pulmonary hypertension: to boldly go where no other pulmonary vasodilator has gone before? Thorax. 2024;79:295–296. doi: 10.1136/thorax-2023-221167. [DOI] [PubMed] [Google Scholar]
- 122.Wan JJ, Yi J, Wang FY, Zhang C, Dai AG. Expression and regulation of HIF-1a in hypoxic pulmonary hypertension: Focus on pathological mechanism and pharmacological treatment. Int J Med Sci. 2024;21:45–60. doi: 10.7150/ijms.88216. [DOI] [PMC free article] [PubMed] [Google Scholar]