Summary
The necessity of oxygen for metabolic processes means that hypoxia can lead to serious cell and tissue damage. On the other hand, in some situations, hypoxia occurs under physiological conditions and serves as an important regulation factor. The airway epithelium is specific in that it gains oxygen not only from the blood supply but also directly from the luminal air. Many respiratory diseases are associated with airway obstruction or excessive mucus production thus leading to luminal hypoxia. The main goal of this review is to point out how the airway epithelium reacts to hypoxic conditions. Cells detect low oxygen levels using molecular mechanisms involving hypoxia-inducible factors (HIFs). In addition, the cells of the airway epithelium appear to overexpress HIFs in hypoxic conditions. HIFs then regulate many aspects of epithelial cell functions. The effects of hypoxia include secretory cell stimulation and hyperplasia, epithelial barrier changes, and ciliogenesis impairment. All the changes can impair mucociliary clearance, exacerbate infection, and promote inflammation leading to damage of airway epithelium and subsequent airway wall remodeling. The modulation of hypoxia regulatory mechanisms may be one of the strategies for the treatment of obstructive respiratory diseases or diseases with mucus hyperproduction.
Keywords: Secretory cells, Motile cilia, Epithelial barrier, Oxygenation, Obstructive respiratory diseases
Introduction
The continuous cellular need for oxygen requires the maintenance of oxygen homeostasis. While in very simple microscopic organisms diffusion is enough to distribute oxygen sufficiently, in vertebrates, including humans, the increase of body mass led to the development of a complicated system for oxygen distribution [1,2]. Lack of oxygen in the environment or failure of the oxygen distribution system can lead to hypoxia.
Hypoxia is defined as deprivation of oxygen level in a tissue. The term was probably used for the first time in 1938 [3]. In his recent review, Sargon divided hypoxia into four groups: hypoxemic hypoxia, ischemic hypoxia, anemic hypoxia and histotoxic hypoxia [4]. The relationship between the terms hypoxia and ischemia should be mentioned, too. Whereas hypoxia is the result of disproportion between oxygen supply and demand, regardless of the cause, ischemia is defined as reduction or interruption of the blood flow leading to hypoxia of perfused tissue [5]. The necessity of oxygen for metabolic processes means that hypoxia, due to its severity and duration, can lead to serious cell and tissue damage. On the other hand, in some situations, hypoxia occurs under physiological conditions and in this case, hypoxia serves as an important regulation factor, e.g. in the intrauterine period [6,7], in the intestinal epithelium [8] or in renal medulla, bone marrow, lymphoid tissue and placenta [9]. General hypoxia affecting the whole organism can be caused by high altitudes, by various pathological conditions including respiratory, circulatory, hematological or infectious disorders, or by artificial conditions in animal experiments. Local hypoxia can develop in many situations of local failure of natural oxygenation [10].
In the respiratory system, hypoxia influences many processes. They include pulmonary vascular remodeling, shifts in immune response and changes in the airway and alveolar wall. However, the airway epithelium is specific in that it gains oxygen not only from the blood supply but that the majority of oxygen transported into epithelial cells is provided directly by the luminal flowing air [11–13]. Many respiratory diseases are associated with airway obstruction or excessive mucus production and thus with epithelial hypoxia. The main goal of this review is to point out how the airway epithelium reacts to hypoxic conditions.
Cellular hypoxia
The need for oxygen homeostasis led to the evolution of many regulative mechanisms. The quick adaptation involves increasing respiration, blood flow, and survival responses. Longer hypoxia activates mechanisms either increasing the oxygen supply or allowing the adaptation to hypoxia. The effect of hypoxia on cells is an important research field. In 2019 Nobel Prize in Physiology or Medicine “for their discoveries of how cells sense and adapt to oxygen availability” [14] was awarded to G. Semenza, P. J. Ratcliffe and W. G. Kaelin.
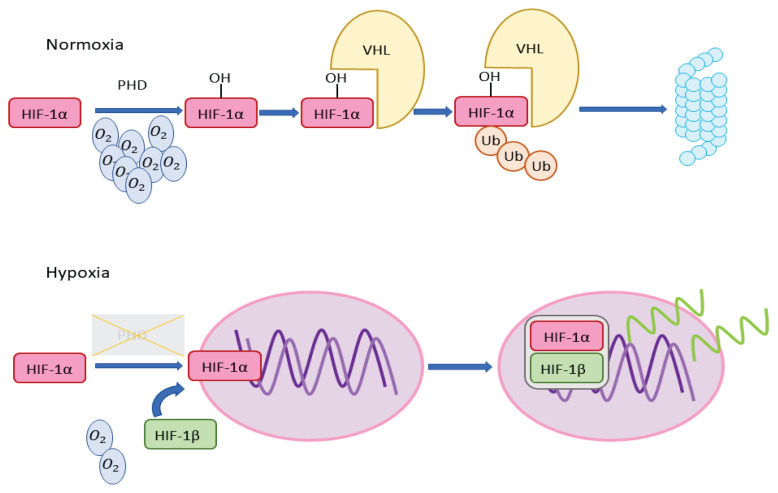
Cells detect low oxygen levels using molecular mechanisms involving hypoxia-inducible factors (HIFs) [15]. These mechanisms are linked to oxygen-sensing prolyl hydroxylase domain proteins (PHDs), which hydroxylate proline in α subunits of HIFs [16], and are summarized in Fig. 1. HIFs are heterodimeric transcription factors, consisting of α (1α or 2α) and β subunits [1]. Under normoxic conditions, the α subunit is hydroxylated by PHDs using oxygen as a substrate. Hydroxylated HIFα interacts with Von Hippel-Lindau protein (VHL) and this complex undergoes ubiquitination. Under hypoxic conditions, the hydroxylation is arrested. This leads to stabilization of the α subunit, transport to the nucleus, and dimerization with the β subunit. This HIF complex then acts as a transcription factor for many genes [17], with VEGF, leptin and TGF-β3 among the regulated genes. The number of genes known to be directly or indirectly regulated by HIF-1 gradually increases - more than 800 genes had been described by 2015 [18], and they accounted for over 2 % of known genes in 2017, respectively [19]. Main HIF-2 targets are EPO (erythropoietin) and EDN1 (endothelin 1). HIF-2 also plays a role in NO metabolism [17]. Currently, in the HIF family, three HIFs are described - HIF-1, HIF-2 and HIF-3. HIF-3 was for some time considered to be only a negative mediator of HIF-regulated genes [20] but later findings have shown different functions of HIF-3 [21]. HIF-3 is particularly important in chronic hypoxia [22,23].
Fig. 1.
In normoxia, prolyl hydroxylase domain proteins with O2 as a substrate hydroxylate HIF-1α prolines. Hydroxylated HIF-1α subunit forms a complex with VHL and this complex is subjected to ubiquitination and proteasome digestion. In hypoxia, HIF-1α gets not hydroxylated and it leads to stabilization of this subunit and its transport to the nucleus. In the nucleus, the α subunit dimerizes with the β subunit and this HIF complex then functions as a transcription factor. HIF-1α - α-subunit of the hypoxia-inducible factor 1; PHD - prolyl hydroxylase domain proteins; VHL - Von Hippel-Lindau protein; Ub - ubiquitin; HIF-1β - β-subunit of the hypoxia-inducible factor 1
Stabilization of HIFs is the main way cells detect low oxygen levels. However, low oxygen levels can be also detected in other stress pathways as well as changes in metabolite levels and the generation of reactive oxygen species by mitochondria [10]. Some genes are influenced by hypoxia without the HIFs [24].
Under normoxic conditions, HIF-1α is the target of VHL-mediated degradation [25]. VHL protein is the main regulation factor of HIFs in normoxia [26]. The importance of VHL for this regulation can be shown in a genetic condition called Von Hippel-Lindau disease. Von Hippel-Lindau disease is a tumor predisposition characterized by the occurrence of highly vascularized tumors in the CNS, retina and visceral organs. The VHL protein (pVHL), is the product of the VHL gene. Protein pVHL influences many cellular processes, especially the cellular adaptive response to hypoxia [27]. Highly vascularized tumors in Von Hippel-Lindau disease overproduce angiogenic polypeptides such as VEGF. The situation when the pVHL protein is non-functional (as here due to genetic condition in Von Hippel-Lindau disease) has analogous consequences as physiological hypoxia [18]. And vice versa, hypoxia can show tumorigenic effects [28–30].
VHL also interferes with other processes. It has an impact on extracellular matrix organization [31–33]. It also affects microtubules [34–36] and through the control of microtubule growth orientation and stabilization influences the ciliogenesis of primary cilia [37,38]. Hypoxia, which stabilizes HIFs, weakens the primary cilia [39] and induces their elongation [40]. The mechanism connecting hypoxia and primary cilia formation is probably VHL regulation of primary cilia formation [41]. Primary cilia are important for embryogenesis, too, because they are deeply involved in the mediation of intercellular [42] and intracellular signaling [43]. A relationship exists between the ciliogenesis of primary cilia and that of motile cilia, as seen in some types of ciliopathies where the gene mutation affects both primary and motile cilia [44].
The activity of HIF-1 is regulated by many genes [45,46]. Besides hypoxia, HIF-1 can be activated by other mechanisms, e.g. by lipopolysaccharide [47,48]. In tumor tissue, HIF-1 activation can occur due to mutations in oncogenes and tumor suppressor genes [49]. Rohwer and her co-workers described increased levels of HIF-1 even in cells without hypoxia in intestinal tumorigenesis, showing that non-canonical HIF-1 stabilization through oncogenes exists [50].
Genetic selection as an adaptation to hypoxia at high altitudes was described. The principles of adaptation are lower levels of hemoglobin and the absence of polyglobulia (which otherwise are linked to chronic mountain sickness - Monge’s disease) [51]. This adaptation in Tibet is represented by single nucleotide polymorphisms in the endothelial Per-Arndt-Sim domain protein 1 (EPAS1) gene, coding for the HIF-2α subunit, involved then in the stimulation of red blood cell production [52]. Chronic mountain sickness occurs more frequently in Andeans than in Tibetans and the search for this genetic adaptation in Andeans was unsuccessful at first [51]. It may be a consequence of the fact that the population lives in the Andes for a shorter period than in Tibet [53]. However, later the EPAS1 adaptation was found in Andeans as well. Furthermore, a genetic adaptation affecting the G protein-coupled receptor 126 (GPR126) gene was revealed in the Andean population [54]. The Andean EPAS1 adaptation results in a hypomorphic allele [17]. Another genetic adaptation localized in the PHD2 gene was later discovered in both Tibetans and Andeans [55,56]. The relationship between HIF-2α and erythropoietin levels can be also illustrated by inherited HIF-2α mutation in a large pedigree, accompanied by erythrocytosis and an increase of erythropoietin in serum [57].
The relationship between hypoxia and inflammation exists [58–60]. Indirectly, in people with mountain sickness, an increased level of inflammation markers was observed [16,61]. Hypoxia activates HIFs influencing many aspects of immune cells’ function [9,62]. HIFs can be stabilized also under normoxic conditions during inflammation and regulate then the metabolism and expression of immune genes, thus HIFs can be seen not only as homeostasis regulators under hypoxic conditions but as well as specific regulators of immune and inflammatory genes [63]. Hypoxia induces airway epithelium to produce compounds influencing innate immunity in surrounding areas and this mechanism can then contribute to chronic pulmonary disease pathogenesis [64,65]. This process might be related to HIF stabilization in myeloid cells [62].
Hypoxia and airway epithelium
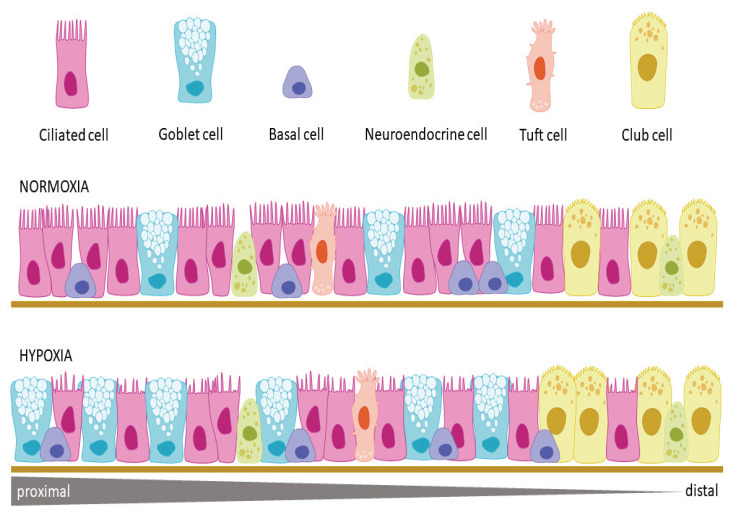
The airway epithelium lines the conductive portion of the respiratory tract from the nasal cavity to the smallest bronchioles. Although several cell types are present in both, the airway epithelium differs in large and small airways. According to classic morphological descriptions, the large airways like nasal cavity, larynx, trachea, and bronchi are lined with a pseudostratified columnar epithelium containing mostly ciliated cells, goblet cells, and basal cells, while the small terminal and respiratory bronchioles are lined with a simple columnar to cuboidal epithelium where mucus-secreting goblet cells are gradually replaced by multifunctional club cells and ciliated and basal cells decrease in number and density [66]. However, further studies and mainly novel single-cell RNA sequencing (scRNA-seq) have uncovered enormous cellular heterogeneity within the airway epithelium. For example, ion-transporting pulmonary ionocytes, several types of tuft cells, variable pulmonary neuroendocrine cells, hillock cells, and pulmonary microfold cells were described [67–69]. Basic cell types and their prospective quantitative changes under hypoxic conditions are visualized in Fig. 2.
Fig. 2.
The normal airway epithelium lining the tracheobronchial tree contains more cell types. The main cell types in the upper part of the tree are represented by ciliated cells, goblet cells and basal cells, in lower branches these types are gradually replaced by club cells. Among main cells the minor cell types are irregularly interspersed. Hypoxia influences both cellular proliferation and cilia formation. The proliferation was observed mainly in the population of secreting cells - goblet cells and club cells. Cilia of ciliated cells under hypoxic conditions become shorter and sparse.
Hypoxia in airways can, due to various causes, affect the airway system globally as well as locally. Local hypoxia can arise as the result of obstruction (e.g. the obstruction of nasal sinuses) or the aggregation of mucus (e.g. cystic fibrosis). Mucus hyperproduction and disruption of epithelial barrier function by the production of VEGF and down-regulation of junctional proteins caused by local obstruction of nasal sinuses led to overexpression of HIF-1 in epithelial cells. Moreover, hypoxia-induced inflammation by highmobility group box 1 (HMGB1) protein translocation into the cytoplasm resulted in the release of IL-8 through a ROS-dependent mechanism in upper airway epithelium [70]. In patients with cystic fibrosis, the thick mucus not only led to partial obstructive luminal hypoxia, but also created particularly hypoxic niches in the airway epithelium. Epithelial hypoxia in this case was potentiated by increased epithelial oxygen consumption associated with increased epithelial Na+ channel (ENaC) mediated Na+ absorption [71].
The general hypoxia can influence the airway epithelium from both luminal and basal sides. In our previous studies, we tested the effect of inhalation of a 10 % hypoxic atmosphere on the epithelium of large and small airways in rabbits at the level of transmission electron microscopy [72,73]. After four-day exposure, the most affected cells were tracheal goblet cells, which were stimulated to mucus release. After rapid mucus discharge, the overstimulated goblet cells mostly did not take part in further secretory cycles but they degenerated and gradually sloughed off. We have demonstrated that a high level of stimulation of secretory cells in the airway epithelium accompanied by degeneration of about 50 % of goblet cells induced a massive differentiation of new secretory elements [74,75]. As the differentiating goblet cells retained the ability to divide [76], the result of this process was hyperplasia of secretory elements followed by changes in their distribution in the epithelium [74,75]. Indeed, differentiating goblet cells represented almost one-fifth of secretory elements and the formation of intraepithelial mucous glands was recorded [72]. Ultrastructural changes of the epithelium in terminal bronchioles were not so prominent, but they corresponded with the findings described in the tracheal epithelium. Cytoplasmic alteration was found both in ciliated and club cells, but degenerative changes were observed only in some club cells. Observed significant increase of club cell relative number in hypoxic rabbits could reflect their compensatory proliferation [73].
Secretory cells
Although the airway epithelial cells’ gross morphology in the light microscope can be described as intact in hypoxic conditions, many metabolic pathways are up- or downregulated [13], which can explain the discrete changes in the cellular ultrastructure found in our studies [72,73]. The secretory cell stimulation and hyperplasia seem to be a common response of the airway epithelium to the hypoxic conditions. The goblet cell hyperplasia mediated by HIF-1α was described in human bronchial epithelial (HBE) cell cultures of patients with chronic obstructive pulmonary disease (COPD) [77]. Hypoxia in mouse bronchial epithelium activated FoxM1 (proliferative factor for club cells) and bronchial cell growth factors RELMα and RELMβ via HIF2α. This activation led to a proliferation of bronchial epithelial cells (Ki67 staining has shown proliferation activity in 10–15 % of cells). Detailed analysis revealed that 78 % of them were club cells, 8 % ciliated cells, and the remaining portion was probably represented by basal cells [78]. Hypoxia-induced HIF-1 stabilization also increased the mucin 5AC (MUC5AC) expression in HBE and this mechanism led to the elevation of its secretion [79]. In a recent study, chronic hypoxia of HBE was associated with an increase in mucus concentration and MUC5AC transcription. The high mucus concentration could be also explained by ion-transport dysregulation via an epithelial Na+ channel (ENaC) beta and gamma subunits hyperexpression, which is HIF dependent [13]. Accumulation of mucus in airways can cause harm in more ways: by aggregation of pollution and immunomodulatory effect [80], by induction of inflammation and epigenetic regulation of macrophages [81].
Neuroendocrine cells
Shivaraju et al. were concerned with the neuroendocrine (NE) cell population in airways. The authors of this study filtered possible other influences that could mimic or modify the effect of pure hypoxia. Hypoxia led to significant proliferation of NE cells. Although some new NE cells could be derived from solitary NE cells, most newly differentiated NE cells arose from basal stem cells. Moreover, some basal stem cells displayed NE-specific vesicles [82]. On the other hand, NE cell proliferation as a reaction to hypoxia was not observed in the later study [13] and our personal observation did not reveal any increase in NE cell density, either [72,73].
Epithelial barrier
Epithelium represents a complicated barrier between luminal content and tissues that can be influenced by hypoxia. HIFs contribute to the expression of barrier-related genes and act in the regulation of barrier-adaptive responses within the mucosa [83]. Although more was described for the hypoxic effect on epithelial barrier function in the gastrointestinal tract [84], the general principles would probably be similar in epithelia, including the airway epithelium [83]. HIF-1 in the intestinal mucosa impacts two ways of barrier maintenance: 1) continual proliferation and epithelium renewal (via expression of regulation genes as WNT/β-catenin and Notch), 2) tight sealing of the barrier (via expression regulation of genes involved in mucus composition, permeability and integrity of tight junctions) [8]. Airway epithelium barrier impairment due to hypoxia affecting various mechanisms here described was observed [85–87]. On the other hand, if the barrier function was impaired by other conditions, then hypoxia-activated HIF-1 improved the epithelial barrier function [58].
Cilia
Oxygen is needed for motile cilia differentiation in airway ciliated cells. If cultivated in submersion, hypoxia blocks the cilia growth through impact on Notch signaling pathway activation and influences the expression of multicilin and Forkhead box J1 (FoxJ1) genes, both strongly involved in cilia formation [88] - multicilin in the early regulation stage of ciliogenesis, FoxJ1 gene in the later one [89]. The importance of the Notch signaling pathway for ciliogenesis can be illustrated also in tumors derived from multiciliated cells. Notch activation led to reduced multiciliation in choroid plexus tumors because of Notch inhibition of Geminin Coiled-Coil Domain Containing (GMNC) and multiciliate differentiation and DNA synthesis associated cell cycle protein (MCIDAS) [90]. GMNC and MCIDAS were previously described as having a crucial role in the ciliogenesis of multiciliated cells [89,91]. As described above, hypoxia can impact the organization and growth of cytoskeletal elements, including microtubules. Centrosomes, too, as microtubular complexes, can be influenced by hypoxia. Hypoxia plays an important role in centrosome amplification through the polo-like kinase 4 (PLK4) receptor, leading to their abnormal size, shape, number and position [92,93]. As PLK4 plays an important role in the early stages of centrosome duplication [94], there is a possibility that motile cilia could be affected by this mechanism under hypoxic conditions in multiciliated cells. Indeed, a significant decrease in the number of kinocilia/mm2, an increase in the percentage of altered cilia and morphological signs of impairment of the vital self-cleaning ability were recorded in the rabbit tracheal epithelium after 4-day normobaric hypoxia [72]. In human airway epithelial cells the decrease of kinocilia number due to hypoxia was described in ALI (air-liquid interface) cell cultures derived from two COPD patients [87].
Conclusion
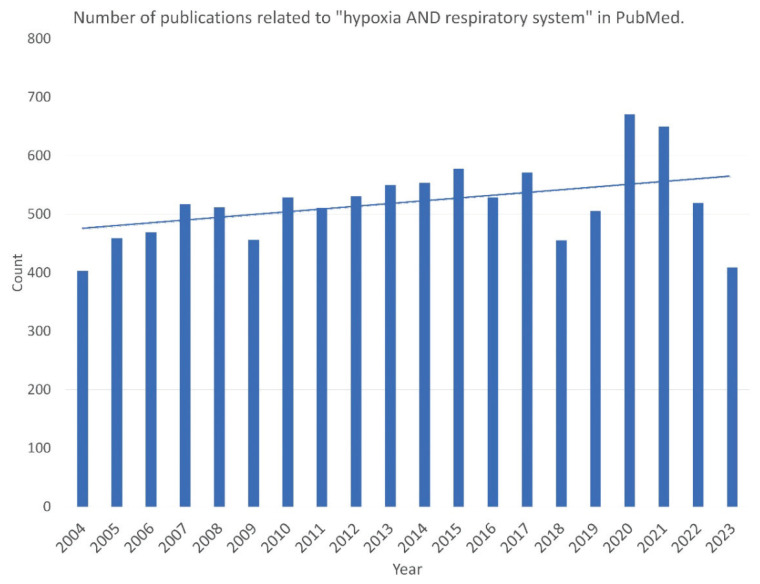
Hypoxia arouses significant interest in the scientific community, as seen in the increasing number of publications concerning hypoxia published in the last decades. Particularly the COVID-19 pandemic focused the interest of the scientific community not only on hypoxia in general [95] but specifically on the hypoxia of the respiratory system, as visualized in Fig. 3. The unique position of the airway epithelium at the interface between ambient and vascular oxygen supply makes it interesting for the comprehensive understanding of the hypoxia influence on the respiratory system. Therefore, we attempted to review the literature and summarize the current understanding of this topic. The common effects of the hypoxic conditions, either local or general, seem to be 1) secretory cell stimulation, hypersecretion and subsequent proliferation, 2) epithelial barrier functional changes, and 3) ciliogenesis impairment. All the described changes can exacerbate the cycle of impaired mucociliary clearance, infection, and inflammation leading to damage of airway epithelium and subsequent airway wall remodeling. The modulation of hypoxia regulatory mechanisms may be one of the strategies for the prevention of airway remodeling changes and the treatment of obstructive respiratory diseases or diseases with mucus hyperproduction.
Fig. 3.
Number of publications related to “hypoxia AND respiratory system” in the last two decades. Particular increase can be observed in the time of the COVID-19 pandemic.
Acknowledgements
The work was supported by the Charles University Grant Agency, grant No. 239123. We sincerely thank Mgr. Vladimír Šmilauer for his help with the graphic design of the figures.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. Journal of Applied Physiology. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 2.Hsia CCW, Schmitz A, Lambertz M, Perry SF, Maina JN. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol. 2013;3:849–915. doi: 10.1002/cphy.c120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richalet JP. The invention of hypoxia. J Appl Physiol (1985) 2021;130:157315–82. doi: 10.1152/japplphysiol.00936.2020. [DOI] [PubMed] [Google Scholar]
- 4.Sargon MF. Lungs and hypoxia: a review of the literature. Anatomy. 2021;15:76–83. doi: 10.2399/ana.21.841001. [DOI] [Google Scholar]
- 5.Ošt’ádal B, Kolář F. Myocardial Hypoxia and Ischemia. In: OŠŤÁDAL B, KOLÁŘ F, editors. Cardiac Ischemia: From Injury to Protection. Boston, MA: Springer US; 1999. pp. 1–44. https://doi.org/10.1007/978-1-4757-3025-8_1, https://doi.org/10.1007/978-1-4757-3025-8_1. [Google Scholar]
- 6.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 7.Wakeland AK, Soncin F, Moretto-Zita M, Chang CW, Horii M, Pizzo D, et al. Hypoxia directs human extravillous trophoblast differentiation in a hypoxia-inducible factor-dependent manner. Am J Pathol. 2017;187:767–780. doi: 10.1016/j.ajpath.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robrahn L, Jiao L, Cramer T. Barrier integrity and chronic inflammation mediated by HIF-1 impact on intestinal tumorigenesis. Cancer Letters. 2020;490:186–192. doi: 10.1016/j.canlet.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano H, Ide H, Ogasa T, Osanai S, Imada M, Nonaka S, et al. Ambient oxygen regulates epithelial metabolism and nitric oxide production in the human nose. J Applied Physiol. 2002;93:189–194. doi: 10.1152/japplphysiol.00096.2002. [DOI] [PubMed] [Google Scholar]
- 12.Nossol C, Diesing AK, Walk N, Faber-Zuschratter H, Hartig R, Post A, et al. Air-liquid interface cultures enhance the oxygen supply and trigger the structural and functional differentiation of intestinal porcine epithelial cells (IPEC) Histochem Cell Biol. 2011;136:103–115. doi: 10.1007/s00418-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikami Y, Grubb BR, Rogers TD, Dang H, Asakura T, Kota P, et al. Chronic airway epithelial hypoxia exacerbates injury in muco-obstructive lung disease through mucus hyperconcentration. Sci Transl Med. 2023;15:eabo7728. doi: 10.1126/scitranslmed.abo7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledford H, Callaway E. Biologists who decoded how cells sense oxygen win medicine Nobel. Nature. 2019;574:161–2. doi: 10.1038/d41586-019-02963-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen K, Song D, Weinstein J, Garcia OA, Pearson LN, Inclán M, et al. High-altitude andean H194R HIF2A allele is a hypomorphic allele. Mol Biol Evolution. 2023;40:msad162. doi: 10.1093/molbev/msad162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15:55–64. doi: 10.1038/nrc3844. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Khalil RA. Chapter Four - Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. In: KHALIL RA, editor. Progress in Molecular Biology and Translational Science. Academic Press; 2017. pp. 87–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbi ME, Semenza GL. Regulation of cell proliferation by hypoxia-inducible factors. Am J Physiol Cell Physiol. 2015;309:C775–82. doi: 10.1152/ajpcell.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SL, Wu C, Xiong ZF, Fang X. Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review) Mol Med Rep. 2015;12:2411–2416. doi: 10.3892/mmr.2015.3689. [DOI] [PubMed] [Google Scholar]
- 22.Tolonen JP, Heikkilä M, Malinen M, Lee HM, Palvimo JJ, Wei GH, et al. A long hypoxia-inducible factor 3 isoform 2 is a transcription activator that regulates erythropoietin. Cell Mol Life Sci. 2020;77:3627–3642. doi: 10.1007/s00018-019-03387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slawski J, Jaśkiewicz M, Barton A, Kozioł S, Collawn JF, Bartoszewski R. Regulation of the HIF switch in human endothelial and cancer cells. Eur J Cell Biol. 2024;103:151386. doi: 10.1016/j.ejcb.2024.151386. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFα Targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 27.Aronow ME, Wiley HE, Gaudric A, Krivosic V, Gorin MB, Shields CL, et al. Von Hippel-Lindau Disease: update on pathogenesis and systemic aspects. Retina. 2019;39:2243–2253. doi: 10.1097/IAE.0000000000002555. [DOI] [PubMed] [Google Scholar]
- 28.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang K, Yu Y, Zhu L, Xu P, Chen J, Ma J, et al. Hypoxia-reprogrammed tricarboxylic acid cycle promotes the growth of human breast tumorigenic cells. Oncogene. 2019;38:6970–6984. doi: 10.1038/s41388-019-0932-1. [DOI] [PubMed] [Google Scholar]
- 31.Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, Louis DN, et al. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell. 1998;1:959–968. doi: 10.1016/S1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- 32.Kurban G, Duplan E, Ramlal N, Hudon V, Sado Y, Ninomiya Y, et al. Collagen matrix assembly is driven by the interaction of von Hippel-Lindau tumor suppressor protein with hydroxylated collagen IV alpha 2. Oncogene. 2008;27:1004–1012. doi: 10.1038/sj.onc.1210709. [DOI] [PubMed] [Google Scholar]
- 33.Ohh M, Taber CC, Ferens FG, Tarade D. Hypoxia-inducible factor underlies von Hippel-Lindau disease stigmata. Elife. 2022;11:e80774. doi: 10.7554/eLife.80774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thoma CR, Toso A, Gutbrodt KL, Reggi SP, Frew IJ, Schraml P, et al. VHL loss causes spindle misorientation and chromosome instability. Nat Cell Biol. 2009;11:994–1001. doi: 10.1038/ncb1912. [DOI] [PubMed] [Google Scholar]
- 35.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]
- 36.Cao H, Yu D, Yan X, Wang B, Yu Z, Song Y, et al. Hypoxia destroys the microstructure of microtubules and causes dysfunction of endothelial cells via the PI3K/Stathmin1 pathway. Cell Biosci. 2019;9:20. doi: 10.1186/s13578-019-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schermer B, Ghenoiu C, Bartram M, Müller RU, Kotsis F, Höhne M, et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. The Journal of cell biology. 2006;175:547–54. doi: 10.1083/jcb.200605092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasanov E, Chen G, Chowdhury P, Weldon J, Ding Z, Jonasch E, et al. Ubiquitination and regulation of AURKA identifies a hypoxia-independent E3 ligase activity of VHL. Oncogene. 2017;36:3450–63. doi: 10.1038/onc.2016.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Resnick A. HIF stabilization weakens primary cilia. PLOS ONE. 2016;11:e0165907. doi: 10.1371/journal.pone.0165907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verghese E, Zhuang J, Saiti D, Ricardo SD, Deane JA. In vitro investigation of renal epithelial injury suggests that primary cilium length is regulated by hypoxia-inducible mechanisms. Cell Biol Int. 2011;35:909–913. doi: 10.1042/CBI20090154. [DOI] [PubMed] [Google Scholar]
- 41.Esteban MA, Harten SK, Tran MG, Maxwell PH. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J Am Soc Nephrol. 2006;17:1801–1806. doi: 10.1681/ASN.2006020181. [DOI] [PubMed] [Google Scholar]
- 42.Pfirrmann T, Gerhardt C. Life-Saver or Undertaker: The relationship between primary cilia and cell death in vertebrate embryonic development. J Dev Biol. 2022;10:52. doi: 10.3390/jdb10040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST. Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol. 2019;15:199–219. doi: 10.1038/s41581-019-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mill P, Christensen ST, Pedersen LB. Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat Rev Genet. 2023;24:421–441. doi: 10.1038/s41576-023-00587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majmundar AJ, Wong WJ, Simon MC. Hypoxia inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semenza GL. A compendium of proteins that interact with HIF-1α. Exp Cell Res. 2017;356:128–135. doi: 10.1016/j.yexcr.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blouin CC, Pagé EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 48.Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohwer N, Jumpertz S, Erdem M, Egners A, Warzecha KT, Fragoulis A, et al. Non-canonical HIF-1 stabilization contributes to intestinal tumorigenesis. Oncogene. 2019;38:5670–5685. doi: 10.1038/s41388-019-0816-4. [DOI] [PubMed] [Google Scholar]
- 51.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, et al. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proceedings of the National Academy of Sciences. 2010;107:11459–64. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Labie D. L’adaptation aux très hautes altitudes - Sur quels gènes une pression sélective s’est-elle exercée ? Med Sci (Paris) 2010;26:1038–9. doi: 10.1051/medsci/201026121038. [DOI] [PubMed] [Google Scholar]
- 53.Eichstaedt CA, Pagani L, Antao T, Inchley CE, Cardona A, Mörseburg A, et al. Evidence of Early-Stage Selection on EPAS1 and GPR126 Genes in Andean High Altitude Populations. Sci Rep. 2017;7:13042. doi: 10.1038/s41598-017-13382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eichstaedt C, Pagani L, Antao T, Inchley C, Cardona A, Mörseburg A, et al. New evidence of genetic adaptation to high altitude in Andean populations. Europ Respir J. 2018;52:PA1274. https://erj.ersjournals.com/content/52/suppl_62/PA1274, https://doi.org/10.1183/13993003.congress-2018.PA1274. [Google Scholar]
- 55.Song D, Navalsky BE, Guan W, Ingersoll C, Wang T, Loro E, et al. Tibetan PHD2, an allele with loss-of-function properties. Proc Natl Acad Sci U S A. 2020;117:12230–8. doi: 10.1073/pnas.1920546117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brutsaert TD, Kiyamu M, Elias Revollendo G, Isherwood JL, Lee FS, Rivera-Ch M, et al. Association of EGLN1 gene with high aerobic capacity of Peruvian Quechua at high altitude. Proc Natl Acad Sci U S A. 2019;116:24006–11. doi: 10.1073/pnas.1906171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gale DP, Harten SK, Reid CDL, Tuddenham EGD, Maxwell PH. Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood. 2008;112:919–921. doi: 10.1182/blood-2008-04-153718. [DOI] [PubMed] [Google Scholar]
- 58.Olson N, Hristova M, Heintz NH, Lounsbury KM, van der Vliet A. Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. Am J Physiol Lung Cellular and Molecular Physiology. 2011;301:L993–1002. doi: 10.1152/ajplung.00250.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proceedings of the National Academy of Sciences. 2013;110:18351–2. doi: 10.1073/pnas.1318345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palazon A, Goldrath A, Nizet V, Johnson RS. HIF Transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SH, Lee SH, Kim CH, Yang KS, Lee EJ, Min KH, et al. Increased expression of vascular endothelial growth factor and hypoxia inducible factor-1α in lung tissue of patients with chronic bronchitis. Clin Biochem. 2014;47:552–559. doi: 10.1016/j.clinbiochem.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Hammond FR, Lewis A, Elks PM. If it’s not one thing, HIF’s another: immunoregulation by hypoxia inducible factors in disease. FEBS. 2020;287:3907–3916. doi: 10.1111/febs.15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGettrick AF, O’Neill LAJ. The Role of HIF in Immunity and Inflammation. Cell Metabolism. 2020;32:524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Polke M, Seiler F, Lepper PM, Kamyschnikow A, Langer F, Monz D, et al. Hypoxia and the hypoxia-regulated transcription factor HIF-1α suppress the host defence of airway epithelial cells. Innate Immun. 2017;23:373–380. doi: 10.1177/1753425917698032. [DOI] [PubMed] [Google Scholar]
- 65.Page LK, Staples KJ, Spalluto CM, Watson A, Wilkinson TMA. Influence of hypoxia on the epithelial-pathogen interactions in the lung: implications for respiratory disease. Front Immunol. 2021;12:653969. doi: 10.3389/fimmu.2021.653969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breeze R, Turk M. Cellular structure, function and organization in the lower respiratory tract. Environ Health Perspect. 1984;55:3–24. doi: 10.1289/ehp.84553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021;14:978–990. doi: 10.1038/s41385-020-00370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dudchenko O, Ordovas-Montanes J, Bingle CD. Respiratory epithelial cell types, states and fates in the era of single-cell RNA-sequencing. Biochem J. 2023;480:921–939. doi: 10.1042/BCJ20220572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russell RJ, Boulet LP, Brightling CE, Pavord ID, Porsbjerg C, Dorscheid D, et al. The airway epithelium: an orchestrator of inflammation, a key structural barrier and a therapeutic target in severe asthma. Eur Respir J. 2024;63:2301397. doi: 10.1183/13993003.01397-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho HJ, Kim CH. Oxygen matters: hypoxia as a pathogenic mechanism in rhinosinusitis. BMB Reports. 2018;51:59–64. doi: 10.5483/BMBRep.2018.51.2.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montgomery ST, Mall MA, Kicic A, Stick SM. Hypoxia and sterile inflammation in cystic fibrosis airways: mechanisms and potential therapies. Eur Respir J. 2017;49:1600903. doi: 10.1183/13993003.00903-2016. [DOI] [PubMed] [Google Scholar]
- 72.Konrádová V, Uhlík J, Vajner L, Herget J, Adášková J. Exposure to hypoxia injures tracheal epithelium (ultrastructural study) Veterinární medicína. 2002;47:270–276. doi: 10.17221/5834-VETMED. [DOI] [Google Scholar]
- 73.Uhlik J, Konradova V, Vajner L, Adaskova J. Normobaric hypoxia induces mild damage to epithelium of terminal bronchioles in rabbits (ultrastructural study) Veterinární medicína. 2005;50:432–438. doi: 10.17221/5645-VETMED. [DOI] [Google Scholar]
- 74.Konrádová V, Kanta J, Sulová J. Effect of bronchoalveolar lavage on the ultrastructure of the tracheal epithelium in rabbits. Respiration. 1990;57:14–20. doi: 10.1159/000195813. [DOI] [PubMed] [Google Scholar]
- 75.Konrádová V, Uhlík J, Vajner L, Zocová J. Reaction of the goblet cells to cholinergic stimulation. Acta Vet Brno. 1996;65:175–180. doi: 10.2754/avb199665030175. [DOI] [Google Scholar]
- 76.Becci PJ, McDowell EM, Trump BF. The respiratory epithelium. II. Hamster trachea, bronchus, and bronchioles. J Natl Cancer Inst. 1978;61:551–561. [PubMed] [Google Scholar]
- 77.Polosukhin VV, Cates JM, Lawson WE, Milstone AP, Matafonov AG, Massion PP, et al. Hypoxia-inducible factor-1 signalling promotes goblet cell hyperplasia in airway epithelium. J Pathol. 2011;224:203–211. doi: 10.1002/path.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torres-Capelli M, Marsboom G, Li QOY, Tello D, Rodriguez FM, Alonso T, et al. Role Of Hif2α oxygen sensing pathway in bronchial epithelial club cell proliferation. Sci Rep. 2016;6:25357. doi: 10.1038/srep25357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou X, Tu J, Li Q, Kolosov VP, Perelman JM. Hypoxia induces mucin expression and secretion in human bronchial epithelial cells. Translational Research. 2012;160:419–427. doi: 10.1016/j.trsl.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367:537–550. doi: 10.1007/s00441-016-2562-z. [DOI] [PubMed] [Google Scholar]
- 81.Hey J, Paulsen M, Toth R, Weichenhan D, Butz S, Schatterny J, et al. Epigenetic reprogramming of airway macrophages promotes polarization and inflammation in muco-obstructive lung disease. Nat Commun. 2021;12:6520. doi: 10.1038/s41467-021-26777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shivaraju M, Chitta UK, Grange RMH, Jain IH, Capen D, Liao L, et al. Airway stem cells sense hypoxia and differentiate into protective solitary neuroendocrine cells. Science. 2021;371:52–57. doi: 10.1126/science.aba0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Glover LE, Colgan SP. Epithelial barrier regulation by hypoxia-inducible factor. Ann Am Thorac Soc. 2017;14:S233–6. doi: 10.1513/AnnalsATS.201608-610MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and Mucosal Inflammation. Annu Rev Pathol. 2016;11:77–100. doi: 10.1146/annurev-pathol-012615-044231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song HA, Kim YS, Cho HJ, Kim SI, Kang MJ, Kim JH, et al. Hypoxia modulates epithelial permeability via regulation of vascular endothelial growth factor in airway epithelia. Am J Respir Cell Mol Biol. 2017;57:527–535. doi: 10.1165/rcmb.2016-0080OC. [DOI] [PubMed] [Google Scholar]
- 86.Zhou W, Yu T, Hua Y, Hou Y, Ding Y, Nie H. Effects of hypoxia on respiratory diseases: perspective view of epithelial ion transport. Am J Physiol-Lung Cellular and Molecular Physiology. 2022;323:L240–250. doi: 10.1152/ajplung.00065.2022. [DOI] [PubMed] [Google Scholar]
- 87.Dale TP, Santer MD, Haris M, Zuo W, Forsyth NR. Hypoxic conditions promote a proliferative, poorly differentiated phenotype in COPD lung tissue progenitor cells in vitro. Exp Lung Res. 2023;49:12–26. doi: 10.1080/01902148.2022.2158404. [DOI] [PubMed] [Google Scholar]
- 88.Gerovac BJ, Valencia M, Baumlin N, Salathe M, Conner GE, Fregien NL. Submersion and hypoxia inhibit ciliated cell differentiation in a notch-dependent manner. Am J Respir Cell Mol Biol. 2014;51:516–525. doi: 10.1165/rcmb.2013-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brooks ER, Wallingford JB. Multiciliated Cells. Current Biol. 2014;24:R973–982. doi: 10.1016/j.cub.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Q, Han Z, Singh N, Terré B, Fame R, Arif U, et al. Disruption of GMNC-MCIDAS multiciliogenesis program is critical in choroid plexus carcinoma development. Cell Death & Differentiation. 2022;29:1–15. doi: 10.1038/s41418-022-00950-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vladar EK, Mitchell BJ. It’s a family act: the geminin triplets take center stage in motile ciliogenesis. The EMBO Journal. 2016;35:904–906. doi: 10.15252/embj.201694206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahane D, Dhingra T, Chalavady G, Datta A, Ghosh B, Rana N, et al. Hypoxia and its effect on the cellular system. Cell Biochemistry and Function. 2024;42:e3940. doi: 10.1002/cbf.3940. [DOI] [PubMed] [Google Scholar]
- 93.Ozcan SC, Kalkan BM, Cicek E, Canbaz AA, Acilan C. Prolonged overexpression of PLK4 leads to formation of centriole rosette clusters that are connected via canonical centrosome linker proteins. Sci Rep. 2024;14:4370. doi: 10.1038/s41598-024-53985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LoMastro GM, Drown CG, Maryniak AL, Jewett CE, Strong MA, Holland AJ. PLK4 drives centriole amplification and apical surface area expansion in multiciliated cells. eLife. 11:e80643. doi: 10.7554/eLife.80643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salyha N, Oliynyk I. Hypoxia modeling techniques: A review. Heliyon. 2023;9:e13238. doi: 10.1016/j.heliyon.2023.e132382. [DOI] [PMC free article] [PubMed] [Google Scholar]