Abstract
Background
Blood loss during elective liver resection is one of the main factors affecting the surgical outcome. Different parenchymal transection techniques have been suggested to decrease blood loss.
Objectives
To assess the benefits and risks of the different techniques of parenchymal transection during liver resections.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (March 2008).
Selection criteria
We considered for inclusion all randomised clinical trials comparing different methods of parenchymal dissection irrespective of the method of vascular occlusion or any other measures used for lowering blood loss.
Data collection and analysis
Two authors identified the trials and extracted the data on the population characteristics, bias risk, mortality, morbidity, blood loss, transection speed, and hospital stay independently of each other. We calculated the odds ratio (OR), mean difference (MD), or standardised mean difference (SMD) with 95% confidence intervals based on 'interntion‐to‐treat analysis' or 'available case analysis' using RevMan 5.
Main results
We included seven trials randomising 556 patients. The comparisons include CUSA (cavitron ultrasound surgical aspirator) versus clamp‐crush (two trials); radiofrequency dissecting sealer (RFDS) versus clamp‐crush (two trials); sharp dissection versus clamp‐crush technique (one trial); and hydrojet versus CUSA (one trial). One trial compared CUSA, RFDS, hydrojet, and clamp‐crush technique. The infective complications and transection blood loss were greater in the RFDS than clamp‐crush. There was no difference in the blood transfusion requirements, intensive therapy unit (ITU) stay, or hospital stay in this comparison. There was no significant differences in the mortality, morbidity, markers of liver parenchymal injury or liver dysfunction, ITU, or hospital stay in the other comparisons. The blood transfusion requirements were lower in the clamp‐crush technique than CUSA and hydrojet. There was no difference in the transfusion requirements of clamp‐crush technique and sharp dissection. Clamp‐crush technique is quicker than CUSA, hydrojet, and RFDS. The transection speed of sharp dissection and clamp‐crush technique was not compared. There was no clinically or statistically significant difference in the operating time between sharp dissection and clamp‐crush techniques. Clamp‐crush technique is two to six times cheaper than the other methods depending upon the number of surgeries performed each year.
Authors' conclusions
Clamp‐crush technique is advocated as the method of choice in liver parenchymal transection because it avoids special equipment, whereas the newer methods do not seem to offer any benefit in decreasing the morbidity or transfusion requirement.
Keywords: Humans; Blood Loss, Surgical; Blood Loss, Surgical/prevention & control; Blood Transfusion; Blood Transfusion/statistics & numerical data; Hemostasis, Surgical; Hemostasis, Surgical/methods; Hepatectomy; Hepatectomy/methods; Hepatectomy/mortality; Liver; Liver/surgery; Randomized Controlled Trials as Topic
Plain language summary
Clamp‐crush technique seems to be the method of choice in liver parenchymal transection
Liver resection (removal of a part of the liver) is performed mainly for cancerous and non‐cancerous tumours in the liver. About 1000 liver resections are performed each year in the United Kingdom. Blood loss during liver resection is one of the main factors affecting the development of surgical complications. Different parenchymal transection techniques (techniques used to divide the liver) have been suggested to decrease blood loss. In this systematic review of seven randomised clinical trials including 556 patients, various methods of parenchymal transection techniques were compared. The infective complications and transection blood loss were greater in the radio frequency dissecting sealer (RFDS ) than clamp‐crush technique. There were no significant differences in the mortality or in the morbidity between the other techniques of parenchymal transection. There was also no difference in the markers of liver parenchymal injury or liver dysfunction between the different methods used. Intensive therapy unit stay and hospital stay were similar. The blood transfusion requirements were lower in the clamp‐crush technique than CUSA (cavitron ultrasonic surgical aspirator) and hydrojet. There was no difference in the transfusion requirements of clamp‐crush technique and sharp dissection. Clamp‐crush technique is quicker than CUSA, hydrojet, and RFDS. The transection speed of sharp dissection and clamp‐crush technique was not compared. There was no clinically or statistically significant difference in the operating time between sharp dissection and clamp‐crush techniques. Clamp‐crush technique is two to six times cheaper than the other methods depending upon the number of surgeries performed each year. Clamp‐crush technique is advocated as the method of choice in liver parenchymal transection because it avoids the need for special equipment and the newer methods do not seem to offer any benefit in decreasing the morbidity or transfusion requirement.
Background
Elective liver parenchymal resection is performed, among others, for benign and malignant liver tumours (Belghiti 1993). The other main reason for liver resection is living donor liver resection (Bombuy 2004). The malignant tumours may arise primarily within the liver (hepatocellular carcinoma and cholangiocarcinoma) or may be metastases from malignancies of other organs (Belghiti 1993; Fong 1996). More than 1000 elective liver resections are performed annually in the United Kingdom alone (HES 2005).
The liver resections could be anatomical resections (resection of Couinaud segments) or can be non‐anatomical resections (wedge resections or resections that extend across Couinaud's segmental planes) (Liu 2004). The anatomical liver resections (as per International Hepato‐Pancreato‐Biliary Association Brisbane 2000 terminology of liver anatomy and resections) include right hemi‐hepatectomy (Couinaud segments 5‐8 ±1), left hemi‐hepatectomy (segments 2 through 4 ±1), right trisectionectomy (segments 4 through 8 ±1), left trisectionectomy (segments 2 through 5, 8 ±1), right anterior sectionectomy (segments 5, 8), right posterior sectionectomy (segments 6, 7), left medial sectionectomy (segment 4), left lateral sectionectomy (segments 2, 3), segmentectomy (any segment), and bisegmentectomy (any 2 segments in continuity) (Strasberg 2000). Although every liver resection is considered a major surgery, only resection of three or more segments is a considered a major liver resection (Belghiti 1993).
Blood loss during liver resection is one of the factors affecting the peri‐operative outcomes of patients (Shimada 1998; Yoshimura 2004; Ibrahim 2006). Various techniques have been attempted to reduce the blood loss during liver resection. These include lowering the central venous pressure (Wang 2006), hypoventilation (Hasegawa 2002), or vascular occlusion (Belghiti 1996; Belghiti 1999). Various techniques of liver parenchymal transection have been suggested to decrease blood loss. These include the finger fracture technique (Rui 2003), sharp dissection (Smyrniotis 2002; Smyrniotis 2005), Kelly's technique (clamp‐crush technique) (Arita 2005; Koo 2005; Lesurtel 2005), ultrasonic dissector (cavitron ultrasonic surgical aspirator or CUSA) (Rau 1996; Rau 2001; Takayama 2001; Koo 2005; Lesurtel 2005), hydrojet (Rau 1996; Rau 2001; Lesurtel 2005), or a radiofrequency (RF) dissecting sealer (Weber 2002; Arita 2005; Lesurtel 2005). Among these, the finger fracture technique, the clamp‐crush technique, and sharp dissection do not require any special instruments. The finger fracture technique and the clamp‐crush technique are generally considered the standard forms of liver parenchymal transection (Lin 1987).
Lesurtel et al found that the clamp‐crushing technique results in lower operative blood loss and decreased parenchymal transection time than CUSA, hydrojet, and RF dissecting sealer (Lesurtel 2005). Arita et al found no significant difference in the blood loss or parenchymal transection time between the clamp‐crushing technique and the dissecting sealer (Arita 2005).
Both these studies did not find any difference in plasma enzyme markers of liver damage, ie, aspartate transaminase (AST) (Arita 2005; Lesurtel 2005) and alanine transaminase (ALT) (Lesurtel 2005) activities. Both these studies did not find any difference in the morbidity between the different techniques (Arita 2005; Lesurtel 2005). Koo 2005 found higher number of air emboli in the right heart after liver parenchymal transection using CUSA than that found after liver parenchymal transection using clamp crush technique.
We were not able to identify any systematic reviews or meta‐analyses related to parenchymal transection techniques in liver resection.
Objectives
To assess the benefits and harms of the different techniques of parenchymal transection during elective liver resections.
Methods
Criteria for considering studies for this review
Types of studies
We considered for the review only randomised clinical trials (irrespective of language, blinding, or publication status).
We excluded quasi‐randomised studies, where the method of allocating participants to a treatment are not strictly random (eg, date of birth, hospital record number, alternation), cohort studies, and case‐control studies).
Types of participants
Patients who are about to undergo elective liver resection for benign or malignant liver tumour or living donor liver resection.
Types of interventions
We included trials comparing one method of parenchymal transection with another method of parenchymal transection irrespective of whether the underlying liver was normal or has chronic liver disease; vascular occlusion was used; the method of management of the raw surface; or whether liver resection was associated with or without bile duct excision (ie, with or without bilio‐enteric anastomoses).
Co‐interventions (including radioablation) were allowed provided that they are used equally in the intervention groups.
Types of outcome measures
Primary outcomes
Mortality (peri‐operative mortality and mortality at maximal follow‐up).
Peri‐operative morbidity (such as re‐operations for bleeding, bile leakage, etc).
Secondary outcomes
Blood loss (during resection and total operative blood loss) and transfusion requirements (number of units, number of patients requiring blood transfusion).
Biochemical markers of liver damage (aspartate aminotransferase (AST), alanine aminotransferase (ALT)), and markers of liver function (bilirubin, prothrombin time) (liver function tests).
Parenchymal transection time; speed; total operating time.
Hospital stay (intensive therapy unit stay or total hospital stay).
Costs as reported by authors.
Search methods for identification of studies
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2008), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003). We have given the search strategies in Appendix 1 with the time span for the searches.
We also searched the references of the identified trials to identify further relevant trials.
We also contacted manufacturers of liver parenchymal transection devices (Radionics (ValleyLab): manufacturers of CUSA; Salient Surgical Technologies (TissueLink) and Angiodynamics: manufacturers of RFDS; Mitsubishi MC Machinery Systems and ERBE: manufacturers of hydrojet and inquired of any unpublished trials. Angiodynamics and Erbe sent us replies in November, but there were no new trials.
Data collection and analysis
Trial selection and extraction of data
We did not apply any language or publication status restrictions. KGS and VP, independently of each other, identified the trials for inclusion. We have also listed the excluded trials with the reasons for the exclusion.
KGS and VP extracted the following data independently:
Year and language of publication.
Country of study.
Year of study.
Inclusion and exclusion criteria.
Sample size.
Population characteristics such as age and gender ratio.
Major or minor liver resections.
Normal or cirrhotic livers.
Method of vascular occlusion.
Management of the raw surface.
Outcomes mentioned above.
Methodological quality (described below).
KGS and VP also assessed the methodological quality of the trials independently, without masking of the study names. Any unclear or missing information was sought by contacting the authors of the individual trials. There was no doubt whether the trials shared the same patients ‐ completely or partially.
The authors resolved any differences in opinion through discussion.
Assessment of methodological quality
The authors followed the instructions given in The Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2008) and The Cochrane Hepato‐Biliary Group Module (Gluud 2008).
Due to the risk of overestimation of intervention effects in randomised trials with inadequate methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), we looked at the influence of methodological quality of the trials on the trial results by evaluating the reported randomisation and follow‐up procedures in each trial. If information was not available in the published trial, we contacted the authors in order to assess the trials correctly. We assessed the following components:
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice was considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers was used for the allocation of patients. These studies are known as quasi‐randomised and were excluded from the review.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, or sealed envelopes. In addition, if there was no blinding in the trials, the allocation concealment was considered adequate only if blocked randomisation was not used or if the blocks were of variable size or if the blocks were distributed across multiple centres such that it is not possible to predict the block size in a single centre.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described. In addition, if there was no blinding in the trials, the allocation concealment was considered unclear if it was not clear whether blocked randomisation was used or if the method of blocked randomisation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised (such studies were excluded). In addition, if there was no blinding in the trials, the allocation concealment was considered inadequate if it was possible to predict future assignments of participants based on previous assignments such as when fixed size blocks were used in a single centre trial. However, such trials were considered for inclusion in the review
Blinding
It is not possible to blind the health‐care provider (surgeon) to the groups. However, it is possible to blind the patients and the outcome assessors. So, blinding was considered adequate if patients and outcome assessors were blinded.
Adequate, if the patients and outcome assessors were blinded, and the method of blinding was described.
Unclear, if the patients and outcome assessors were blinded, and the method of blinding was not described.
Inadequate, if the patients and outcome assessors were not blinded.
Incomplete data outcomes
Adequate, if there were no post‐randomisation drop‐outs or withdrawals or if the post‐randomisation drop‐outs were balanced in both groups or reasons for missing data were unlikely to be related to true outcome (for example, patients did not undergo surgery after randomisation).
Unclear, if it is not clear whether there were any drop‐outs or withdrawals or if the reasons for these drop‐outs were not clear.
Inadequate, if the reasons for missing data are likely to be related to true outcomes, 'as‐treated' analysis was performed, potentially inappropriate application of simple imputation, potential for patients with missing outcomes to induce clinically relevant bias in effect estimate or effect size.
Selective outcome reporting
Adequate, if all the important outcomes were reported or if the trial's protocol was available and all of the trial's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported.
Unclear, if there is insufficient information to assess whether the risk of selective outcome reporting is present.
Inadequate, if not all the pre‐specified outcomes were reported or if the primary outcomes were changed or if some of the important outcomes were incompletely reported.
Other biases
Baseline imbalance
Adequate, if there was no baseline imbalance in important characteristics.
Unclear, if the baseline characteristics were not reported.
Inadequate, if there was a baseline imbalance due to chance or due to imbalanced exclusion after randomisation.
Early stopping
Adequate (sample size calculation was reported and the trial was not stopped or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was low).
Unclear (sample size calculations were not reported and it is not clear whether the trial was stopped early or not).
Inadequate (the trial was stopped early due to an informal stopping rule or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high).
Academic bias
Adequate (the author of the trial has not conducted previous trials addressing the same interventions).
Unclear (It is not clear if the author has conducted previous trials addressing the same interventions).
Inadequate (the author of the trial has conducted previous trials addressing the same interventions).
Sponsor bias
Adequate, if the trial was unfunded or was not funded by an equipment manufacturer.
Unclear, if the source of funding was not clear.
Inadequate, if the trial was funded by an equipment manufacturer.
Statistical methods
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2008). We used the software package RevMan 5 (RevMan 2008) provided by the Cochrane Collaboration. For dichotomous outcomes, we calculated the odds ratio with 95% confidence interval. For continuous outcomes, we calculated mean difference (MD) or standardised mean difference (SMD) with 95% confidence interval (CI). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In case of discrepancy between the two models, we reported both results; otherwise we have reported only the results from the fixed‐effect model. Heterogeneity was explored by chi‐squared test with significance set at P value 0.10, and the quantity of heterogeneity was measured by I2 (Higgins 2002). An I2 > 30% was considered statistically significant heterogeneity. We performed the analysis on an 'intention‐to‐treat' basis (Newell 1992) whenever possible. Otherwise, we adopted the 'available case analysis'. In case we found 'zero‐event' trials for statistically significant outcomes, we planned to perform a sensitivity analysis with and without empirical continuity correction factors as suggested by Sweeting et al (Sweeting 2004). However, we did not find any such outcomes.
Subgroup analysis
We planned to perform the subgroup analyses for: ‐ Normal livers and chronic liver disease. ‐ Liver resections versus living donor retrievals. ‐ Minor and major liver resections. ‐ Different techniques of vascular occlusion. ‐ Different techniques of management of raw surface. ‐ Trials with low and high risk of bias.
However, we did not perform any of the subgroup analysis because of the few trials included under each outcome.
Sensitivity analysis
One of the trials used vascular occlusion in the clamp‐crush technique only (Lesurtel 2005). We performed a sensitivity analysis excluding this trial from all comparisons involving clamp‐crush technique. This was a post‐hoc decision following comments from peer reviewers and editors.
Bias exploration
We planned to use a funnel plot to explore bias (Egger 1997; Macaskill 2001). Asymmetry in funnel plot of trial size against treatment effect was to be used to assess bias. We also intended to perform linear regression approach described by Egger et al to determine the funnel plot asymmetry (Egger 1997). However, we did not perform any of the above because of the few trials included under each outcome.
Results
Description of studies
We identified a total of 887 references through the electronic searches of The Cochrane Hepato‐Biliary Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 107), MEDLINE (n = 393), EMBASE (n = 242), and Science Citation Index Expanded (n = 145). We excluded 288 duplicates and 591 clearly irrelevant references through reading abstracts. Eight references were retrieved for further assessment. No references were identified through scanning reference lists of the identified randomised trials. We excluded one reference (Rau 1996) because of the reason listed under the table 'Characteristics of excluded studies'. The remaining seven references were references of seven completed randomised trials, which fulfilled the inclusion criteria (Rau 2001; Takayama 2001; Arita 2005; Koo 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007). Details of the trials are shown in the table 'Characteristics of included studies'.
Participants
A total of 556 participants undergoing elective liver resection were randomised in the seven trials. The number of participants in each trial ranged from 50 to 132. We were not able to extract relevant data on the sex ratio of the participants from one trial (Takayama 2001). The proportion of females was 32.4% in the remaining trials. The mean or median age in the trials varied between 52.7 years and 68 years. Information on the number of major resections was not available in one trial (Koo 2005). The proportion of major liver resections was 45.3% in the remaining trials. None of the trials included living donor liver retrievals.
Comparisons
The different comparisons are stated in the 'Characteristics of included studies'. The comparisons include CUSA versus clamp‐crush technique (two trials ‐ Takayama 2001; Koo 2005); radio frequency dissecting sealer (RFDS) versus clamp‐crush (two trials ‐ Arita 2005; Lupo 2007); sharp dissection versus clamp‐crush technique (one trial ‐ Smyrniotis 2005); and hydrojet versus CUSA (one trial ‐ Rau 2001). One trial (Lesurtel 2005) compared CUSA, RFDS, hydrojet, and clamp‐crush technique.
Outcome measures
The outcome measures reported by the different trials were peri‐operative mortality (Takayama 2001; Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007), surgery related complications (Rau 1996; Takayama 2001; Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007), air embolism (Koo 2005), blood loss (Rau 2001; Takayama 2001; Arita 2005; Koo 2005; Lesurtel 2005; Smyrniotis 2005); number of patients transfused (Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007); amount of blood transfused (Rau 1996; Rau 2001; Arita 2005; Koo 2005), operating time (Koo 2005; Smyrniotis 2005), transection time (Rau 2001; Takayama 2001; Arita 2005; Koo 2005), transection speed (Rau 1996; Rau 2001; Takayama 2001; Arita 2005; Lesurtel 2005), markers of liver parenchymal injury (Arita 2005; Lesurtel 2005), markers of liver dysfunction (Lesurtel 2005), intensive therapy unit (ITU) stay (Lesurtel 2005; Smyrniotis 2005), and hospital stay (Arita 2005; Lesurtel 2005; Smyrniotis 2005; Lupo 2007).
The other outcome measures reported by the trials were air embolisms to the heart (Koo 2005), costs (Lesurtel 2005), and tumour exposure at margin (Takayama 2001).
Risk of bias in included studies
The risk of bias is summarised in Figure 1 and Figure 2. Out of the seven trials, five (71.4%) had adequate generation of the allocation sequence (Rau 2001; Takayama 2001; Arita 2005; Lesurtel 2005; Lupo 2007); three trials (42.9%) had adequate allocation concealment (Takayama 2001;Arita 2005;Lesurtel 2005). None of the trials reported on blinding of patients or outcome assessors. All the trials had addressed incomplete outcome data adequately. Five trials (71.4%) reported on the important outcomes and were free of selective outcome reporting (Takayama 2001;Arita 2005;Lesurtel 2005;Smyrniotis 2005;Lupo 2007). Three of these five trials (42.9%) were free from all the other biases (Takayama 2001;Arita 2005;Lupo 2007). All the trials were considered to be of high risk of bias because of the lack of blinding in all the trials.
1.
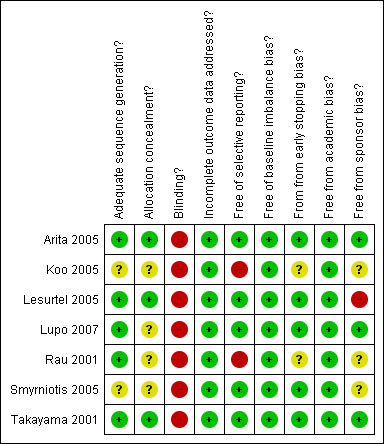
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
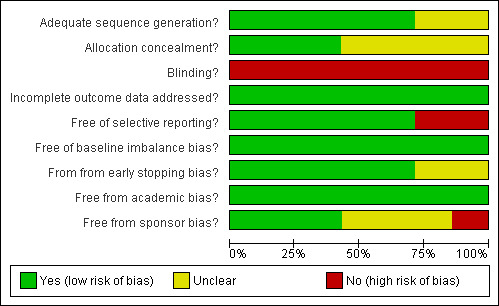
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
This review is based on seven trials including 556 patients. None of the trials reported long term mortality. So, all the mortality reported was peri‐operative mortality.
CUSA versus clamp‐crush technique
In the three trials that provided comparison between CUSA and clamp‐crush (Takayama 2001; Koo 2005; Lesurtel 2005), 232 patients were randomised to either CUSA (116 patients) or clamp‐crush (116 patients) techniques.
Since one trial (Lesurtel 2005) compared CUSA without vascular occlusion and clamp‐crush technique with vascular occlusion, we considered this trial to be different from the other two (Takayama 2001; Koo 2005) and analysed it separately.
Trials where vascular occlusion was equal between groups
Mortality and morbidity
There was no statistically significant difference between the two groups in the mortality or in morbidity. The number of patients with air embolism detected in the heart by echocardiography was statistically significantly higher in the CUSA group (odds ratio OR 24.77, 95% CI 1.34 to 457.61). However, none of the patients had clinically significant air embolism.
Blood loss and transfusion requirements
There was no difference in the operative blood loss, in the median transection blood loss (330 versus 325 ml/sq cm), or in the amount of blood transfused.
Transection speed
There was no statistically significant difference in the operating time or the transection time.
Trial of CUSA without vascular occlusion and clamp‐crush technique with vascular occlusion
Mortality and morbidity
There was no statistically significant difference between the two groups in the mortality and morbidity.
Blood loss and transfusion requirements
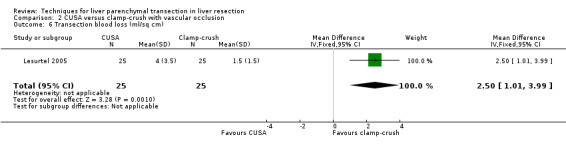
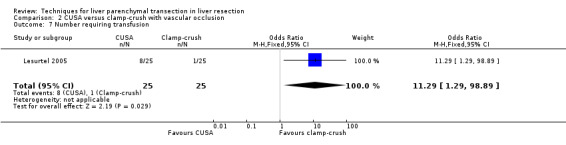
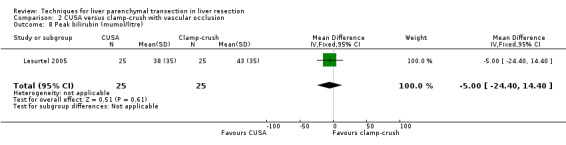
There was a statistically significant difference in the amount of blood loss per sq cm (mean difference MD 2.50, 95% CI 1.01 to 3.99). A statistically significant higher number of people undergoing liver transection by CUSA technique required blood transfusion than those undergoing liver resection by clamp‐crush technique (OR 11.29, 95% CI 1.29 to 98.89).
Liver function tests
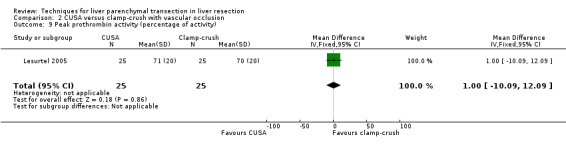
There was no statistically significant difference in the AST, or ALT, or bilirubin level or prothrombin activity.
Transection speed
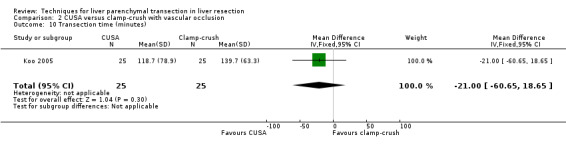
The transection speed was statistically significantly quicker (MD 1.60 sq cm/min, 95% CI 0.89 to 2.31) in the clamp‐crush method than the CUSA.
Stay
There was no statistically significant difference in the median intensive therapy unit (ITU) or hospital stay between the two groups.
Costs
Costs were calculated in one trial (Lesurtel 2005) based on the transection speed, blood loss, and cost of the maintenance of the instrument. The CUSA was 3 to 6 times costlier than clamp‐crush technique depending upon the number of cases performed per year.
RFDS versus clamp‐crush technique
In the three trials that provided a comparison between RFDS and clamp‐crush (Arita 2005; Lesurtel 2005; Lupo 2007), 180 patients were randomised to either the RFDS (89 patients) or clamp‐crush (91 patients) techniques. The results of the meta‐analysis and the data from the trials that could not be included in a meta‐analysis are tabulated in Table 1.
1. Radiofrequency dissecting sealer versus clamp‐crush.
| Outcome | Number of studies | Number of patients | Meta‐analysis | Other trials |
| Peri‐operative mortality | 3 | 180 | Not estimable | ‐ |
| Liver failure | 2 | 100 | 0.74 [0.14, 3.95] | ‐ |
| Bleeding requiring percutaneous drainage | 1 | 50 | Not estimable | ‐ |
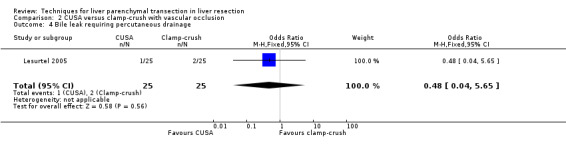
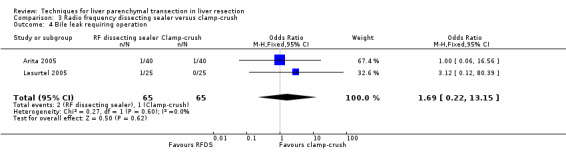
| Bile leak requiring operation | 2 | 130 | 1.69 [0.22, 13.15] | |
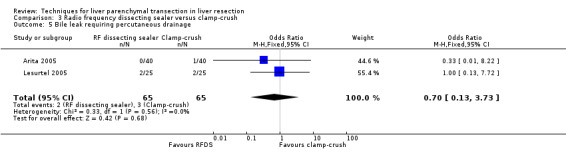
| Bile leak requiring percutaneous drainage | 2 | 130 | 0.70 [0.13, 3.73] | ‐ |
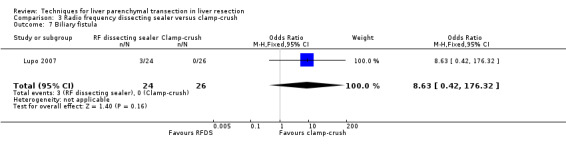
| Biliary fistula | 1 | 50 | 8.63 [0.42, 176.32] | |
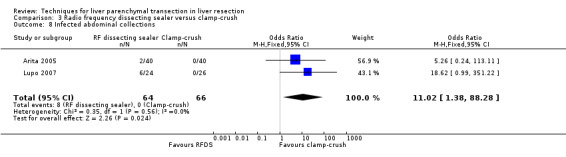
| Infected abdominal collections | 2 | 130 | 11.02 [1.38, 88.28] | ‐ |
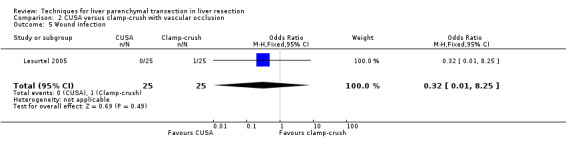
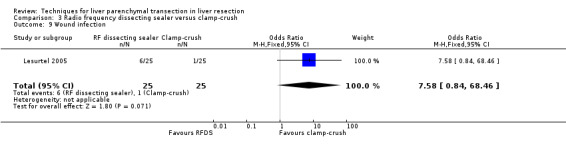
| Wound infection | 1 | 50 | 7.58 [0.84, 68.46] | ‐ |
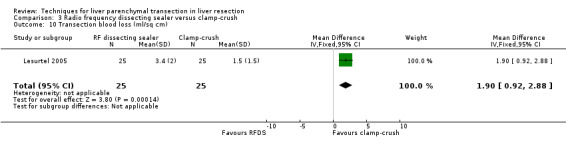
| Transection blood loss (ml/sq cm) | 1 | 50 | 1.90 [0.92, 2.88] | ‐ |
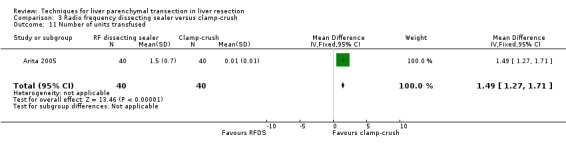
| Blood transfused (units) | ‐ | ‐ | ‐ | 1.5 units vs 0 units (Arita 2005) |
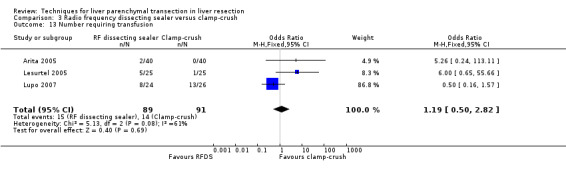
| Number requiring transfusion | 3 | 180 | 1.19 [0.50, 2.82] | ‐ |
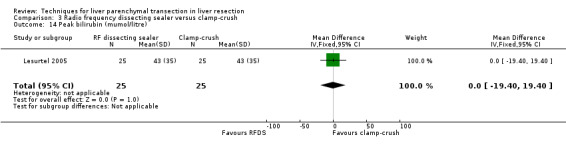
| Peak bilirubin (mumol/litre) | 1 | 50 | 0.00 [‐19.40, 19.40] | ‐ |
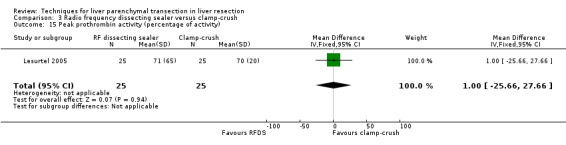
| Peak prothrombin activity (percentage of activity) | 1 | 50 | 1.00 [‐25.66, 27.66] | ‐ |
| Aspartate transaminase (AST) and alanine transaminase (ALT) | 1 | 50 | ‐ | No difference (Lesurtel 2005) |
| Transection speed (sq cm/minute) | 1 | 50 | ‐1.40 [‐2.23, ‐0.57] | ‐ |
| Operating time (minutes) | 1 | 50 | ‐ | 292 vs 278 (Lupo 2007) |
| Intensive therapy unit stay (days) | 1 | 50 | ‐ | 1 vs 1 median (Lesurtel 2005) |
| Hospital stay (days) | 2 | 100 | ‐ | 9 vs 9 median (Lesurtel 2005) 16 vs 18 median (Arita 2005) |
| Costs (Euros per patient) | 1 | not applicable | ‐ | 1618 vs 497 Lesurtel 2005 |
cm = centimetre ml = millilitre mumol = micromole sq = square
Mortality and morbidity
There was no mortality in either group. The infected intra‐abdominal collections were significantly higher in the RFDS group than clamp‐crush group (OR 11.02, 95% CI 1.38 to 88.28). Wound infection approached statistical significance favouring the clamp‐crush technique (OR 7.58, 95% CI 0.8 to 68.46).
Blood loss and transfusion requirements
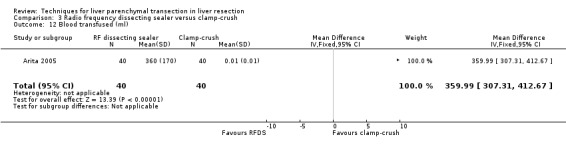
There was a higher transection blood loss in the RFDS group than clamp‐crush group (MD 1.90, 95% CI 0.92 to 2.88). There was no difference in the number of people requiring blood transfusion between the two groups (OR 1.19, 95% CI 0.50 to 2.82). The amount of blood transfused could not be estimated as none in the clamp‐crush group underwent blood transfusion in the only trial (Arita 2005) that reported on this outcome. By imputing a value of 0.01 and 0.01 for mean and standard deviation instead of 0 and 0, we could calculate a mean difference. This was statistically significantly lower in the clamp‐crush method (number of units: MD 1.49 units; 95% CI 1.27 to 1.71; amount of blood transfused: MD 359.99 ml; 95% CI 307.31 to 412.67).
Liver function tests
There was no statistically significant difference in the AST, or ALT, or bilirubin level, or prothrombin activity.
Transection speed
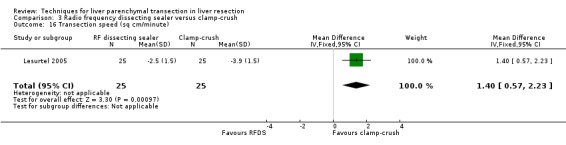
The transection speed was statistically significantly quicker (MD 1.40 sq cm/min, 95% CI 0.57 to 2.23) in the clamp‐crush method than the RFDS in the only trial, which reported on this outcome (Lesurtel 2005). There was no difference in the median operating time between the two groups in the only trial, which reported on the operating time (Lupo 2007).
Stay
There was no statistically significant difference in the median intensive therapy unit (ITU) or hospital stay between the two groups.
Costs
Costs were calculated in one trial (Lesurtel 2005) based on the transection speed, blood loss, and cost of the maintenance of the instrument. The RFDS was approximately 3 times costlier than clamp‐crush technique.
Sentivity analysis
On exclusion of the trial, which used vascular occlusion in the clamp‐crush group alone (Lesurtel 2005), infected intraabdominal collections favouring clamp‐crush technique and the amount of blood transfused (after imputing the mean and standard deviation as mentioned previously) were the only statistically significant differences between the groups. This was because transection speed and costs were reported only in the trial, which was excluded in the sensitivity analysis (Lesurtel 2005).
Hydrojet versus clamp‐crush technique
In the only trial that provided comparison between hydrojet and clamp‐crush (Lesurtel 2005), 50 patients were randomised to either hydrojet (25 patients) or clamp‐crush (25 patients) techniques. In this trial, vascular occlusion was used only in the clamp‐crush group.
Mortality and morbidity
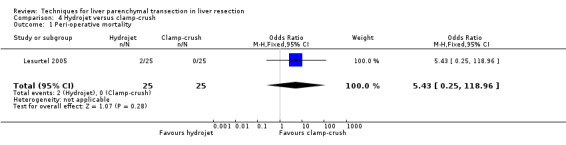
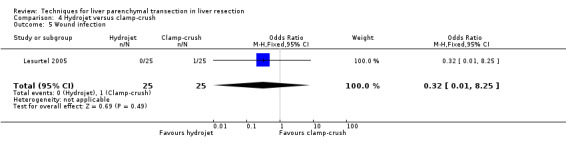
There was no statistically significant difference between the two groups in the mortality (OR 5.43, 95% CI 0.25 to 118.96) or in morbidity.
Blood loss and transfusion requirements
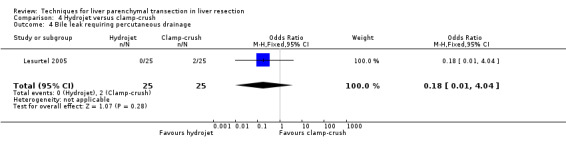
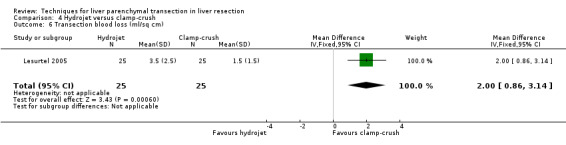
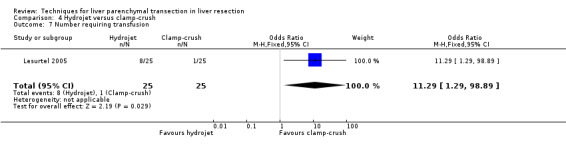
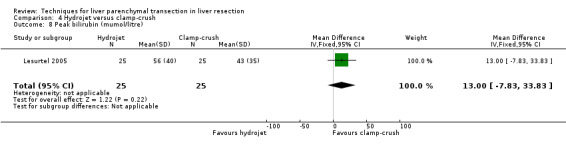
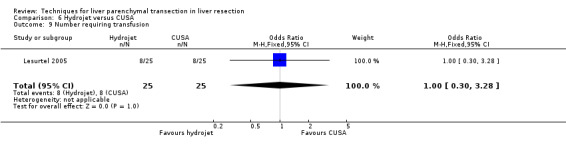
There was greater blood loss (MD 2.00 ml/cm2, 95% CI 0.86 to 3.14) and higher number of people requiring blood transfusion in the hydrojet group than the clamp‐crush group (OR 11.29, 95% 1.29 to 98.89).
Liver function tests
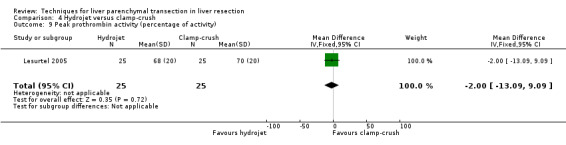
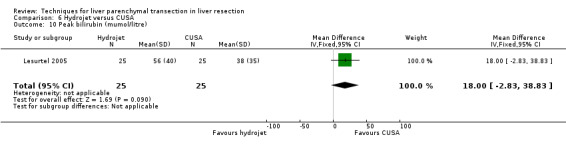
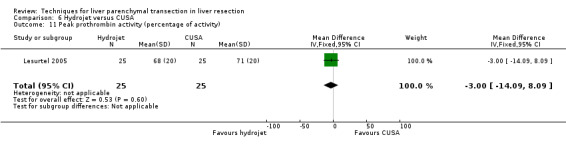
There was no statistically significant difference in the AST or ALT or bilirubin level or prothrombin activity.
Transection speed
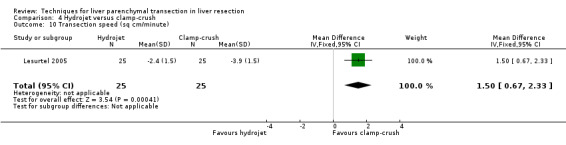
The transection speed was statistically significantly quicker (MD 1.50 sq cm/min, 95% CI 0.67 to 2.33) in the clamp‐crush method than the hydrojet.
Stay
There was no statistically significant difference in the median intensive therapy unit (ITU) (1 day in both groups) or hospital stay (9 days in both groups) between the two groups.
Costs
Costs were calculated based on the transection speed, blood loss, and cost of the maintenance of the instrument. The hydrojet was approximately 2 to 4 times costlier than clamp‐crush technique depending upon the number of cases operated per year.
Sharp dissection versus clamp‐crush technique
In the only trial that provided comparison between sharp dissection (SD) and clamp‐crush (CC) techniques (Smyrniotis 2005), 82 patients were randomised to either sharp dissection (41 patients) or clamp‐crush (41 patients) techniques.
Mortality and morbidity
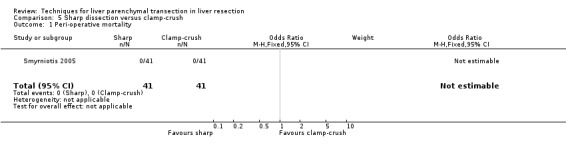
There was no mortality in either group. There was no statistically significant difference between the two groups in operative morbidity.
Blood loss and transfusion requirements
There was no statistically significant difference in the median operative blood loss (500 ml SD versus 460 ml CC) or the number of people requiring blood transfusion (OR 0.80, 95% CI 0.32 to 2.01).
Transection speed
There was no statistically significant difference in the median operating time (205 min SD versus 211 min CC).
Stay
There was no statistically significant difference in the median intensive therapy unit (ITU) (1 day in both groups) or hospital stay (10 days SD versus 11 days CC) between the two groups.
Hydrojet versus CUSA
In the two trials that provided comparison between hydrojet and CUSA (Rau 2001; Lesurtel 2005), 111 patients were randomised to either hydrojet (56 patients) or CUSA (55 patients) techniques. The results of the meta‐analysis and the data from the trials that could not be included for the meta‐analysis are tabulated in Table 2.
2. Hydrojet versus cavitron ultrasonic surgical aspirator.
| Outcome | Number of studies | Number of patients | Meta‐analysis | Other studies |
| Peri‐operative mortality | 1 | 50 | 1.00 [0.13, 7.72] | ‐ |
| Liver failure | 1 | 50 | 1.00 [0.06, 16.93] | ‐ |
| Bleeding requiring percutaneous drainage | 1 | 50 | 3.12 [0.12, 80.39] | ‐ |
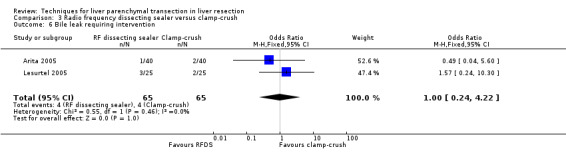
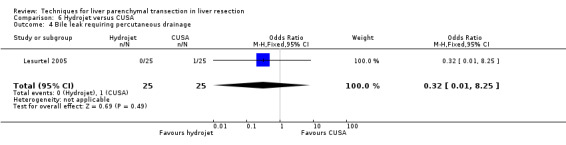
| Bile leak requiring intervention | 1 | 50 | 0.32 [0.01, 8.25] | ‐ |
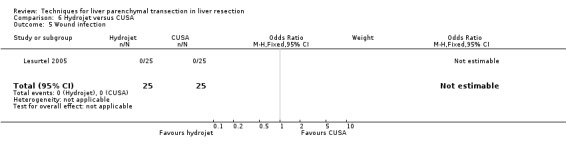
| Wound infection | 1 | 50 | Not estimable | ‐ |
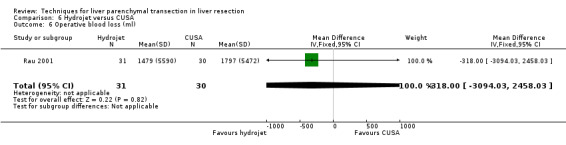
| Operative blood loss (ml) | 1 | 61 | ‐318.00 [‐3094.03, 2458.03] | ‐ |
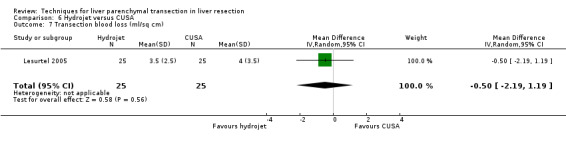
| Transection blood loss (ml/sq) | 1 | 50 | ‐0.50 [‐2.19, 1.19] | ‐ |
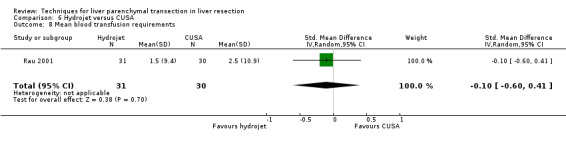
| Mean blood transfusion requirements | 1 | 61 | ‐0.10 [‐0.60, 0.41] | ‐ |
| Number requiring transfusion | 1 | 50 | 1.00 [0.30, 3.28] | ‐ |
| Peak bilirubin (mumol/litre) | 1 | 50 | 18.00 [‐2.83, 38.83] | ‐ |
| Peak prothrombin activity (percentage of activity) | 1 | 50 | ‐3.00 [‐14.09, 8.09] | ‐ |
| Aspartate transaminase (AST) and alanine transaminase (ALT) | 1 | 50 | ‐ | No difference (Lesurtel 2005) |
| Operating time (minutes) | 1 | 50 | ‐27.30 [‐58.63, 4.03] | ‐ |
| Transection time (minutes) | 1 | 61 | ‐18.00 [‐61.03, 25.03] | ‐ |
| Transection speed (sq cm/minute) | 2 | 111 | 0.01 [‐0.36, 0.38] | ‐ |
| Intensive therapy unit stay (days) | 1 | 50 | ‐ | 1 vs 1 median (Lesurtel 2005) |
| Hospital stay (days) | 1 | 50 | ‐ | 9 vs 9 median (Lesurtel 2005) |
| Costs (Euros per patient) | 1 | not applicable | ‐ | 1125 to 2235 vs 1587 to 2912 (depending upon the volume) (Lesurtel 2005) |
cm = centimetre ml = millilitre mumol = micromole sq = square
Mortality and morbidity
There was no statistically significant difference between the two groups in the mortality (OR 1.00, 95% CI 0.13 to 7.72) or in morbidity.
Blood loss and transfusion requirements
There was no statistically significant difference in the transection blood loss, operative blood loss, the number of people requiring transfusion or the mean transfusion requirements.
Liver function tests
There was no statistically significant difference in the AST, or ALT, or bilirubin level or prothrombin activity.
Transection speed
There was no statistically significant difference in the transection time or transection speed between the two groups.
Stay
There was no statistically significant difference in the median intensive therapy unit (ITU) or hospital stay between the two groups.
Costs
Costs were calculated in one trial based on the transection speed, blood loss, and cost of the maintenance of the instrument. The hydrojet was approximately a third cheaper than CUSA (Lesurtel 2005).
RFDS versus CUSA
In the only trial that provided comparison between RFDS and CUSA (Lesurtel 2005), 50 patients were randomised to either RFDS (25 patients) or CUSA (25 patients) techniques.
Mortality and morbidity
There was no statistically significant difference between the two groups in the mortality (OR 5.43, 95% CI 0.25 to 118.96) or in morbidity.
Blood loss and transfusion requirements
There was no statistically significant transection blood loss or the number of people requiring blood transfusion between the two groups.
Liver function tests
There was no statistically significant difference in the AST, or ALT, or bilirubin level, or prothrombin activity.
Transection speed
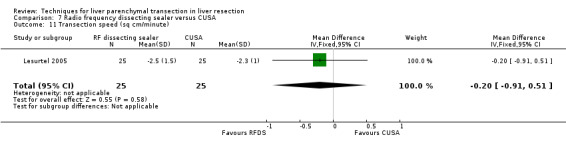
There was no statistically significant difference in the transection speed between the two groups.
Stay
There was no statistically significant difference in the median intensive therapy unit (ITU) (1 day in both groups) or hospital stay (9 days in both groups) between the two groups.
Costs
Costs were calculated based on the transection speed, blood loss, and cost of the maintenance of the instrument. Depending upon the number of cases operated, RFDS costs were approximately 50% to 100% of that of CUSA.
RFDS versus hydrojet
In the only trial that provided comparison between RFDS and hydrojet (Lesurtel 2005), 50 patients were randomised to either RFDS (25 patients) or hydrojet (25 patients) techniques.
Mortality and morbidity
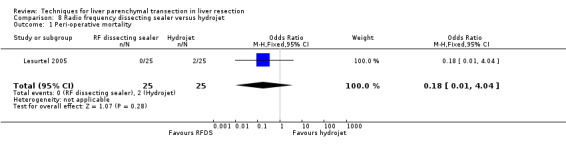
There was no statistically significant difference between the two groups in the mortality (OR 0.18, 95% CI 0.01 to 4.04) or in morbidity.
Blood loss and transfusion requirements
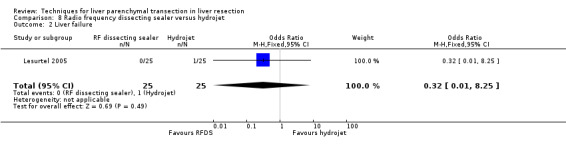
There was no statistically significant transection blood loss or the number of people requiring blood transfusion between the two groups.
Liver function tests
There was no statistically significant difference in the AST, or ALT, or bilirubin level or prothrombin activity.
Transection speed
There was no statistically significant difference in the transection speed between the two groups.
Stay
There was no statistically significant difference in the median intensive therapy unit (ITU) (1 day in both groups) or hospital stay (9 days in both groups) between the two groups.
Costs
Costs were calculated based on the transection speed, blood loss, and cost of the maintenance of the instrument. RFDS costs about 25% more than the hydrojet.
Funnel plots
Exploration of bias was not done because of the few trials included under each outcome.
Subgroup analysis
No subgroup analysis was performed because of the few trials included under each outcome.
Discussion
In this systematic review, there were no significant differences in the mortality or in the morbidity (including bile leak) of liver resection irrespective of the method used for parenchymal transection. However, the trials were not adequately powered to identify significant differences in the mortality or morbidity. Markers of liver parenchymal injury or liver dysfunction were also similar and there was no difference in the ITU or hospital stay between the different groups.
In the trial by Koo et al (Koo 2005), there was a significantly higher number of air embolisms detected in the heart in the CUSA group than the clamp‐crush group. All the embolisms that filled half or more of the diameter of the right heart (including some which filled the entire right heart) were in the CUSA group (Koo 2005). However, none of the patients in either group developed clinical symptoms. The importance of this finding in the absence of clinical symptoms is not clear. But it is likely to reflect the risk of a massive air embolism with CUSA.
Clamp‐crush technique appears to have the lowest blood loss and lowest transfusion requirements compared to the different techniques. The trial (Lesurtel 2005), which compared clamp‐crush technique with three other techniques (CUSA, RFDS, and hydrojet), continuous portal trial clamping was used in the clamp‐crush technique, while no inflow occlusion was used for the other techniques (CUSA, RFDS, hydrojet). Another trial (Koo 2005) comparing clamp‐crush technique with CUSA did not employ vascular occlusion. This trial did not find any difference in the blood transfusion requirements between the two groups. The third trial comparing the clamp‐crush technique with CUSA employed intermittent vascular occlusion in both groups. This trial did not report on blood transfusion requirements but reported that the median transection blood loss was similar in the two groups. Thus, it is likely that vascular occlusion played an important role in decreasing the blood transfusion requirements in the clamp‐crush technique in the trial where vascular occlusion was used in the clamp‐crush technique only. However, the transection speed of clamp‐crush technique is higher than the other techniques enabling safe vascular occlusion.Techniques should be assessed as a 'package', ie, a parenchymal transection technique in combination with a particular method of vascular occlusion to find out the best combination.
The transection speed is given more importance than the operating time as this takes the transection area into account. Clamp‐crush technique is quicker than CUSA, hydrojet, and RFDS. The transection speed of sharp dissection and clamp‐crush technique was not compared. There was no clinically or statistically significant difference in the operating time between sharp dissection and clamp‐crush techniques. Since the primary aim of vascular occlusion is reducing the blood loss, the use of vascular occlusion is clearly a confounding factor. Whether vascular occlusion is necessary and whether there is an 'ideal' method of vascular occlusion is a matter of controversy (Gurusamy 2007). In this review, we found that intermittent vascular occlusion is safe and decreases blood loss and blood transfusion requirements, but it did not affect the morbidity. Thus, the ideal vascular occlusion method has not been established. The other confounding factors like low central venous pressure, hypoventilation, and use of intravenous drugs like tranexamic acid were not stated in most trials.
The clamp‐crush technique and sharp dissection technique do not involve any additional instruments. All the other techniques involve additional equipments. There is no increased mortality or morbidity associated with clamp‐crush technique. While the trials were not powered to measure the mortality and morbidity, the sample sizes were enough to detect differences in enzyme markers of liver injury. The clamp‐crush technique was not associated with a higher raise of these enzymes than other techniques. None of the trials demonstrated a reduction in transfusion requirements by using special instruments. So, there is no evidence of superiority of any technique over clamp‐crush technique. Clamp‐crush technique is also quicker than most other interventions (as represented by the transection speed). A cost comparison between clamp‐crush technique and other techniques revealed that the clamp‐crush technique is two to six times cheaper than the other methods depending upon the number of surgeries performed each year (Lesurtel 2005).
The main drawback of this review is the small number of trials in each comparison making it impossible to perform subgroup analyses. The number of patients included for different comparisons ranged from 50 (hydrojet versus clamp‐crush; RFDS versus CUSA; RFDS versus hydrojet) to 282 (CUSA versus clamp‐crush). This sample size is not sufficiently powered to detect clinically significant differences in the primary outcomes. All the trials were of high risk of bias mainly because of the lack of blinding. While patient blinding can be easily achieved, even this was not reported in the trials, and it is not safe to assume that the patients were blinded to the groups. The outcome assessor blinding is more difficult to achieve. The bias due to lack of blinding can be minimised by using objective outcomes whenever feasible and by involving a second team of surgeons (Wood 2008). The trials were also not adequately powered to measure differences in the mortality and morbidity in liver resection. So, adequately powered low bias‐risk trials are necessary to compare the different techniques of liver resection.
Until low bias‐risk randomised clinical trials employing factorial designs to identify the effect of confounding factors such as the method of vascular occlusion, low CVP, and hypoventilation are performed, clamp‐crush technique is advocated as the method of choice in liver parenchymal transection because of the low costs and avoidance of special equipment whilst minimising morbidity.
Authors' conclusions
Implications for practice.
Clamp‐crush technique is advocated as the method of choice in liver parenchymal transection because it avoids the need for special equipment whereas the newer methods do not seem to offer any benefit in decreasing the morbidity or transfusion requirement.
Implications for research.
Further randomised clinical trials are needed to compare the different liver parenchymal transection techniques. They have to be with a sufficient sample size, low risk of bias, and employ patient blinding and outcome assessment by blinded assessors. These trials should be reported according to the CONSORT guidelines (http://www.consort‐statement.org).
History
Protocol first published: Issue 1, 2008 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 14 April 2008 | Amended | Converted to new review format. |
Acknowledgements
TC Mahendran, Chennai, my first surgical teacher. Martyn Parker, Peterborough District Hospital, Peterborough who inspired me to write Cochrane Reviews. The Cochrane Hepato‐Biliary Group for their support. Peer Reviewers: P Ghaneh, UK; S Gaujoux, France; D Piccolboni, Italy. Contact Editor: N Alexakis, Greece.
Appendices
Appendix 1. Search strategies
| Database | Period of Search | Search Strategy |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | March 2008 | (((liver OR hepatic) AND (segmentectomy OR resection OR transection)) OR hepatectomy) AND ( "blood loss" OR "blood losses" OR hemorrhage OR hemorrhages OR haemorrhage OR haemorrhages OR hemostasis OR hemostases OR haemostasis OR haemostases) |
| Cochrane Central Register of Controlled Trials in The Cochrane Library (CENTRAL) | Issue 1, 2008 | #1 liver OR hepatic #2 MeSH descriptor Liver explode all trees #3 (#1 OR #2) #4 segmentectomy OR resection OR transection #5 (#3 AND #4) #6 MeSH descriptor Hepatectomy explode all trees #7 (#5 OR #6) #8 MeSH descriptor Hemorrhage explode all trees #9 MeSH descriptor Hemostasis, Surgical explode all trees #10 MeSH descriptor Hemostasis explode all trees #11 "blood loss" OR "blood losses" OR hemorrhage OR hemorrhages OR haemorrhage OR haemorrhages OR hemostasis OR hemostases OR haemostasis OR haemostases #12 (#8 OR #9 OR #10 OR #11) #13 (#7 AND #12) |
| MEDLINE (PubMed) | 1950 to March 2008 | ((("Liver"[MeSH] OR liver OR hepatic) AND (segmentectomy OR resection OR transection)) OR "Hepatectomy"[MeSH]) AND ("Hemorrhage"[MeSH] OR "Hemostasis, Surgical"[MeSH] OR "Hemostasis"[MeSH] OR "blood loss" OR "blood losses" OR hemorrhage OR hemorrhages OR haemorrhage OR haemorrhages OR hemostasis OR hemostases OR haemostasis OR haemostases) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) AND humans [mh]) |
| EMBASE (Dialog Datastar) | 1980 to March 2008 | 1 Bleeding#.W..DE. OR blood ADJ loss OR blood ADJ losses OR hemorrhage OR hemorrhages OR haemorrhage OR haemorrhages OR hemostasis OR hemostases OR haemostasis OR haemostases 2 LIVER OR HEPATIC OR HEPATO 3 SEGMENTECTOMY OR RESECTION 4 2 AND 3 5 HEPATECTOMY OR LIVER‐RESECTION.DE. 6 4 OR 5 7 1 AND 6 8 RANDOM$ OR FACTORIAL$ OR CROSSOVER$ OR CROSS ADJ OVER$ OR PLACEBO$ OR DOUBL$ ADJ BLIND$ OR SINGL$ ADJ BLIND$ OR ASSIGN$ OR ALLOCAT$ OR VOLUNTEER$ OR CROSSOVER‐PROCEDURE#.MJ. OR DOUBLE‐BLIND‐PROCEDURE#.DE. OR SINGLE‐BLIND‐PROCEDURE#.DE. OR RANDOMIZED‐CONTROLLED‐TRIAL#.DE. 9 7 AND 8 |
| Science Citation Index Expanded (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | 1970 to March 2008 | #1 TS=(liver or hepatic) #2 TS=(segmentectomy OR resection OR transection) #3 #2 AND #1 #4 TS=(hepatectomy) #5 #4 OR #3 #6 TS=("blood loss" OR "blood losses" OR hemorrhage OR hemorrhages OR haemorrhage OR haemorrhages OR hemostasis OR hemostases OR haemostasis OR haemostases) #7 TS=(random* OR blind* OR placebo* OR meta‐analysis) #8 #7 AND #6 AND #5 |
Data and analyses
Comparison 1. CUSA versus clamp‐crush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Air embolism (clinical) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Air embolism (Echocardiogram) | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 24.77 [1.34, 457.61] |
| 4 Bile leak requiring intervention | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Abdominal collections requiring drainage | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.18, 22.96] |
| 6 Infected abdominal collections | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.18, 22.96] |
| 7 Wound infection | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.33] |
| 8 Operative blood loss (ml) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐83.70 [‐381.83, 214.43] |
| 9 Blood transfused (ml) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐83.1 [‐216.31, 50.11] |
| 10 Operating time (minutes) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐27.30 [‐58.63, 4.03] |
| 11 Transection time (minutes) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐19.00 [‐60.65, 18.65] |
| 12 Tumour exposure at resection margin | 1 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.32 [0.86, 12.85] |
1.1. Analysis.
Comparison 1 CUSA versus clamp‐crush, Outcome 1 Peri‐operative mortality.
1.2. Analysis.
Comparison 1 CUSA versus clamp‐crush, Outcome 2 Air embolism (clinical).
1.3. Analysis.
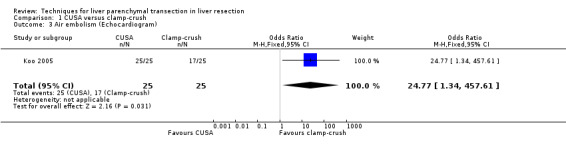
Comparison 1 CUSA versus clamp‐crush, Outcome 3 Air embolism (Echocardiogram).
1.4. Analysis.
Comparison 1 CUSA versus clamp‐crush, Outcome 4 Bile leak requiring intervention.
1.5. Analysis.
Comparison 1 CUSA versus clamp‐crush, Outcome 5 Abdominal collections requiring drainage.
1.6. Analysis.
Comparison 1 CUSA versus clamp‐crush, Outcome 6 Infected abdominal collections.
1.7. Analysis.
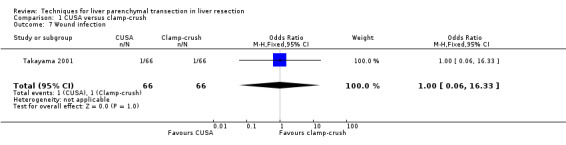
Comparison 1 CUSA versus clamp‐crush, Outcome 7 Wound infection.
1.8. Analysis.
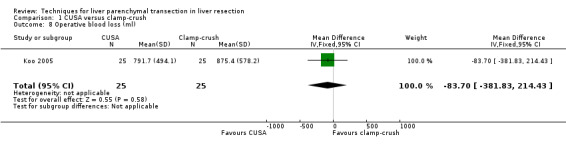
Comparison 1 CUSA versus clamp‐crush, Outcome 8 Operative blood loss (ml).
1.9. Analysis.
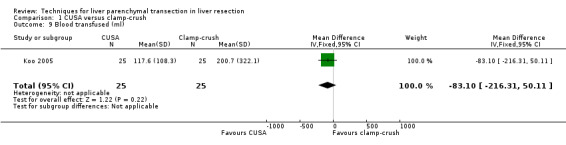
Comparison 1 CUSA versus clamp‐crush, Outcome 9 Blood transfused (ml).
1.10. Analysis.
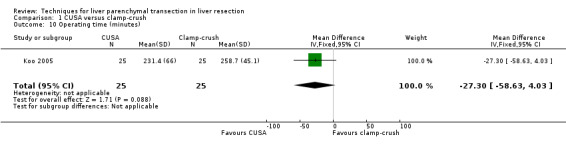
Comparison 1 CUSA versus clamp‐crush, Outcome 10 Operating time (minutes).
1.11. Analysis.
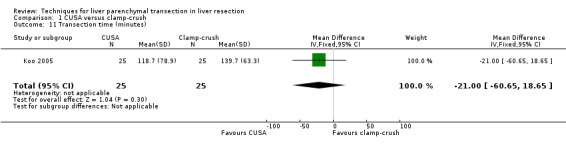
Comparison 1 CUSA versus clamp‐crush, Outcome 11 Transection time (minutes).
1.12. Analysis.
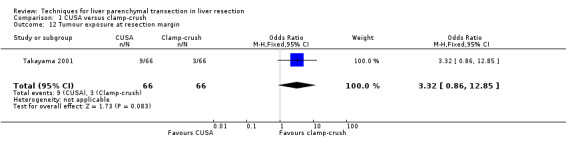
Comparison 1 CUSA versus clamp‐crush, Outcome 12 Tumour exposure at resection margin.
Comparison 2. CUSA versus clamp‐crush with vascular occlusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.43 [0.25, 118.96] |
| 2 Liver failure | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.93] |
| 3 Bleeding requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Bile leak requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.65] |
| 5 Wound infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 6 Transection blood loss (ml/sq cm) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.01, 3.99] |
| 7 Number requiring transfusion | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.29 [1.29, 98.89] |
| 8 Peak bilirubin (mumol/litre) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐24.40, 14.40] |
| 9 Peak prothrombin activity (percentage of activity) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐10.09, 12.09] |
| 10 Transection time (minutes) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐19.00 [‐60.65, 18.65] |
| 11 Transection speed (sq cm/minute) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [0.89, 2.31] |
2.1. Analysis.
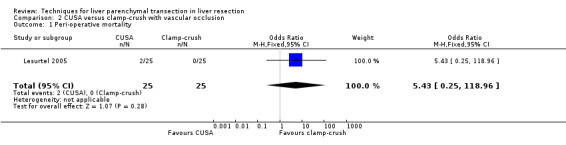
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 1 Peri‐operative mortality.
2.2. Analysis.
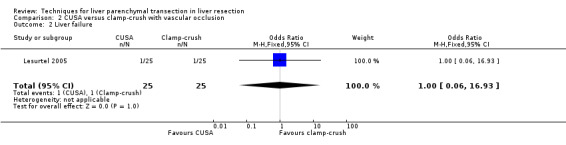
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 2 Liver failure.
2.3. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 3 Bleeding requiring percutaneous drainage.
2.4. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 4 Bile leak requiring percutaneous drainage.
2.5. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 5 Wound infection.
2.6. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 6 Transection blood loss (ml/sq cm).
2.7. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 7 Number requiring transfusion.
2.8. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 8 Peak bilirubin (mumol/litre).
2.9. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 9 Peak prothrombin activity (percentage of activity).
2.10. Analysis.
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 10 Transection time (minutes).
2.11. Analysis.
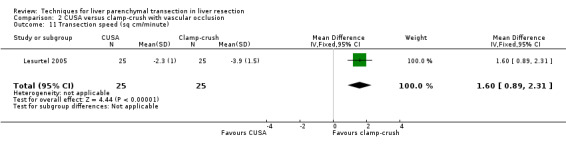
Comparison 2 CUSA versus clamp‐crush with vascular occlusion, Outcome 11 Transection speed (sq cm/minute).
Comparison 3. Radio frequency dissecting sealer versus clamp‐crush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Liver failure | 2 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.14, 3.95] |
| 3 Bleeding requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Bile leak requiring operation | 2 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.22, 13.15] |
| 5 Bile leak requiring percutaneous drainage | 2 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.13, 3.73] |
| 6 Bile leak requiring intervention | 2 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.24, 4.22] |
| 7 Biliary fistula | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.63 [0.42, 176.32] |
| 8 Infected abdominal collections | 2 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.02 [1.38, 88.28] |
| 9 Wound infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.58 [0.84, 68.46] |
| 10 Transection blood loss (ml/sq cm) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.9 [0.92, 2.88] |
| 11 Number of units transfused | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.49 [1.27, 1.71] |
| 12 Blood transfused (ml) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 359.99 [307.31, 412.67] |
| 13 Number requiring transfusion | 3 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.50, 2.82] |
| 14 Peak bilirubin (mumol/litre) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐19.40, 19.40] |
| 15 Peak prothrombin activity (percentage of activity) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐25.66, 27.66] |
| 16 Transection speed (sq cm/minute) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.57, 2.23] |
3.1. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 1 Peri‐operative mortality.
3.2. Analysis.
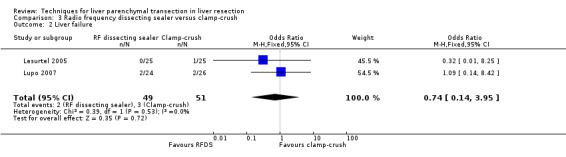
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 2 Liver failure.
3.3. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 3 Bleeding requiring percutaneous drainage.
3.4. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 4 Bile leak requiring operation.
3.5. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 5 Bile leak requiring percutaneous drainage.
3.6. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 6 Bile leak requiring intervention.
3.7. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 7 Biliary fistula.
3.8. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 8 Infected abdominal collections.
3.9. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 9 Wound infection.
3.10. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 10 Transection blood loss (ml/sq cm).
3.11. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 11 Number of units transfused.
3.12. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 12 Blood transfused (ml).
3.13. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 13 Number requiring transfusion.
3.14. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 14 Peak bilirubin (mumol/litre).
3.15. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 15 Peak prothrombin activity (percentage of activity).
3.16. Analysis.
Comparison 3 Radio frequency dissecting sealer versus clamp‐crush, Outcome 16 Transection speed (sq cm/minute).
Comparison 4. Hydrojet versus clamp‐crush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.43 [0.25, 118.96] |
| 2 Liver failure | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.93] |
| 3 Bleeding requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.12, 80.39] |
| 4 Bile leak requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.04] |
| 5 Wound infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 6 Transection blood loss (ml/sq cm) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.86, 3.14] |
| 7 Number requiring transfusion | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.29 [1.29, 98.89] |
| 8 Peak bilirubin (mumol/litre) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐7.83, 33.83] |
| 9 Peak prothrombin activity (percentage of activity) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐13.09, 9.09] |
| 10 Transection speed (sq cm/minute) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [0.67, 2.33] |
4.1. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 1 Peri‐operative mortality.
4.2. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 2 Liver failure.
4.3. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 3 Bleeding requiring percutaneous drainage.
4.4. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 4 Bile leak requiring percutaneous drainage.
4.5. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 5 Wound infection.
4.6. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 6 Transection blood loss (ml/sq cm).
4.7. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 7 Number requiring transfusion.
4.8. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 8 Peak bilirubin (mumol/litre).
4.9. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 9 Peak prothrombin activity (percentage of activity).
4.10. Analysis.
Comparison 4 Hydrojet versus clamp‐crush, Outcome 10 Transection speed (sq cm/minute).
Comparison 5. Sharp dissection versus clamp‐crush.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Bleeding requiring re‐operation | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.25 [0.24, 112.88] |
| 3 Bile leak requiring intervention | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.18, 23.55] |
| 4 Abdominal collections requiring drainage | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.55] |
| 5 Wound infection | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.55] |
| 6 Number requiring transfusion | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.32, 2.01] |
5.1. Analysis.
Comparison 5 Sharp dissection versus clamp‐crush, Outcome 1 Peri‐operative mortality.
5.2. Analysis.
Comparison 5 Sharp dissection versus clamp‐crush, Outcome 2 Bleeding requiring re‐operation.
5.3. Analysis.
Comparison 5 Sharp dissection versus clamp‐crush, Outcome 3 Bile leak requiring intervention.
5.4. Analysis.
Comparison 5 Sharp dissection versus clamp‐crush, Outcome 4 Abdominal collections requiring drainage.
5.5. Analysis.
Comparison 5 Sharp dissection versus clamp‐crush, Outcome 5 Wound infection.
5.6. Analysis.
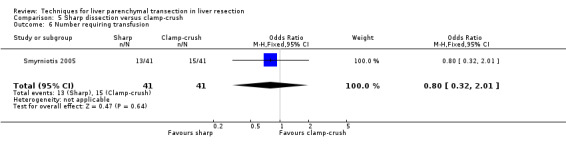
Comparison 5 Sharp dissection versus clamp‐crush, Outcome 6 Number requiring transfusion.
Comparison 6. Hydrojet versus CUSA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.72] |
| 2 Liver failure | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.93] |
| 3 Bleeding requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.12, 80.39] |
| 4 Bile leak requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 5 Wound infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Operative blood loss (ml) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐318.0 [‐3094.03, 2458.03] |
| 7 Transection blood loss (ml/sq cm) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐2.19, 1.19] |
| 8 Mean blood transfusion requirements | 1 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.60, 0.41] |
| 9 Number requiring transfusion | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.28] |
| 10 Peak bilirubin (mumol/litre) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐2.83, 38.83] |
| 11 Peak prothrombin activity (percentage of activity) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐14.09, 8.09] |
| 12 Transection time (minutes) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐18.0 [‐61.03, 25.03] |
| 13 Transection speed (sq cm/minute) | 2 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.38, 0.36] |
6.1. Analysis.
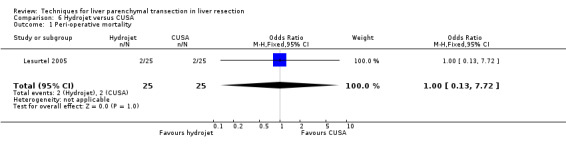
Comparison 6 Hydrojet versus CUSA, Outcome 1 Peri‐operative mortality.
6.2. Analysis.
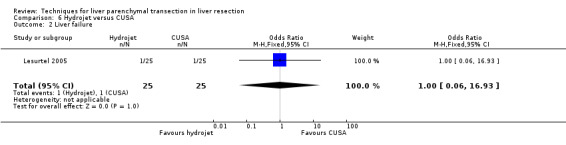
Comparison 6 Hydrojet versus CUSA, Outcome 2 Liver failure.
6.3. Analysis.
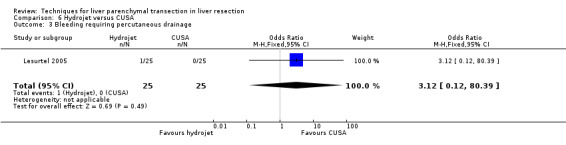
Comparison 6 Hydrojet versus CUSA, Outcome 3 Bleeding requiring percutaneous drainage.
6.4. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 4 Bile leak requiring percutaneous drainage.
6.5. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 5 Wound infection.
6.6. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 6 Operative blood loss (ml).
6.7. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 7 Transection blood loss (ml/sq cm).
6.8. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 8 Mean blood transfusion requirements.
6.9. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 9 Number requiring transfusion.
6.10. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 10 Peak bilirubin (mumol/litre).
6.11. Analysis.
Comparison 6 Hydrojet versus CUSA, Outcome 11 Peak prothrombin activity (percentage of activity).
6.12. Analysis.
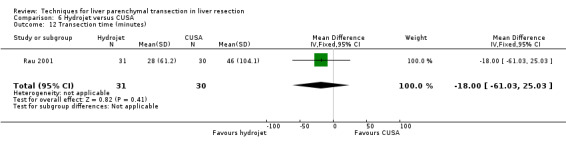
Comparison 6 Hydrojet versus CUSA, Outcome 12 Transection time (minutes).
6.13. Analysis.
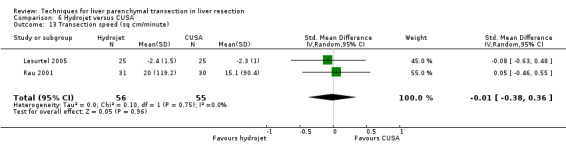
Comparison 6 Hydrojet versus CUSA, Outcome 13 Transection speed (sq cm/minute).
Comparison 7. Radio frequency dissecting sealer versus CUSA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.04] |
| 2 Liver failure | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 3 Bleeding requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Bile leak requiring operation | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.12, 80.39] |
| 5 Bile leak requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.18, 24.61] |
| 6 Wound infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 17.0 [0.90, 320.37] |
| 7 Transection blood loss (ml/sqcm) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.18, 0.98] |
| 8 Number requiring transfusion | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.93] |
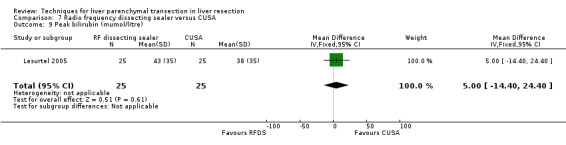
| 9 Peak bilirubin (mumol/litre) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐14.40, 24.40] |
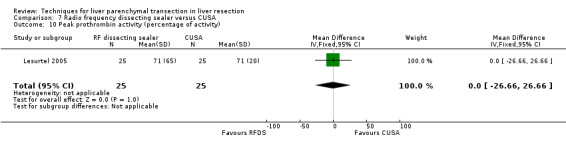
| 10 Peak prothrombin activity (percentage of activity) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐26.66, 26.66] |
| 11 Transection speed (sq cm/minute) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.91, 0.51] |
7.1. Analysis.
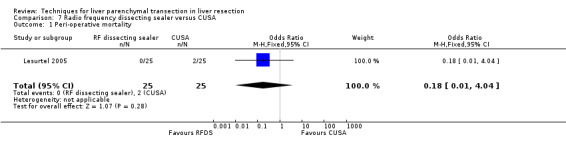
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 1 Peri‐operative mortality.
7.2. Analysis.
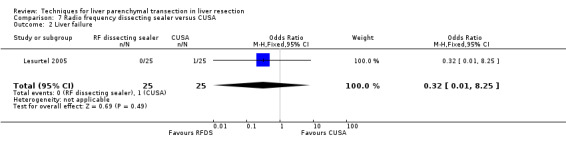
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 2 Liver failure.
7.3. Analysis.
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 3 Bleeding requiring percutaneous drainage.
7.4. Analysis.
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 4 Bile leak requiring operation.
7.5. Analysis.
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 5 Bile leak requiring percutaneous drainage.
7.6. Analysis.
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 6 Wound infection.
7.7. Analysis.
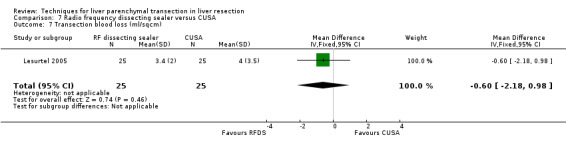
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 7 Transection blood loss (ml/sqcm).
7.8. Analysis.
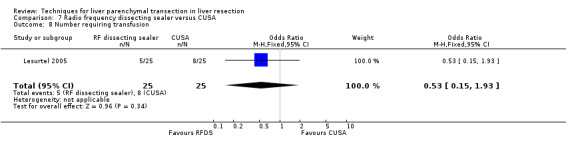
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 8 Number requiring transfusion.
7.9. Analysis.
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 9 Peak bilirubin (mumol/litre).
7.10. Analysis.
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 10 Peak prothrombin activity (percentage of activity).
7.11. Analysis.
Comparison 7 Radio frequency dissecting sealer versus CUSA, Outcome 11 Transection speed (sq cm/minute).
Comparison 8. Radio frequency dissecting sealer versus hydrojet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Peri‐operative mortality | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.04] |
| 2 Liver failure | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
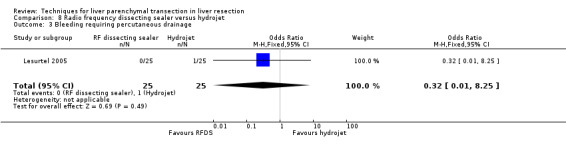
| 3 Bleeding requiring percutaneous drainage | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.25] |
| 4 Bile leak requiring intervention | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.93 [0.39, 162.07] |
| 5 Wound infection | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 17.0 [0.90, 320.37] |
| 6 Transection blood loss (ml/sqcm) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.68, 1.48] |
| 7 Number requiring transfusion | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.93] |
| 8 Peak prothrombin activity (percentage of activity) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐23.66, 29.66] |
| 9 Peak bilirubin (mumol/litre) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐33.83, 7.83] |
| 10 Transection speed (sq cm/minute) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.93, 0.73] |
8.1. Analysis.
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 1 Peri‐operative mortality.
8.2. Analysis.
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 2 Liver failure.
8.3. Analysis.
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 3 Bleeding requiring percutaneous drainage.
8.4. Analysis.
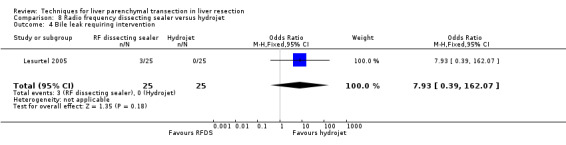
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 4 Bile leak requiring intervention.
8.5. Analysis.
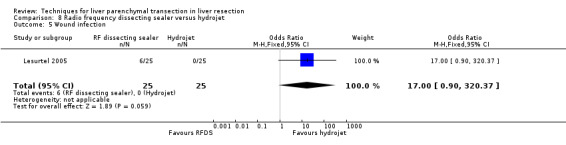
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 5 Wound infection.
8.6. Analysis.
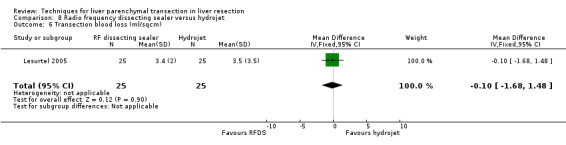
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 6 Transection blood loss (ml/sqcm).
8.7. Analysis.
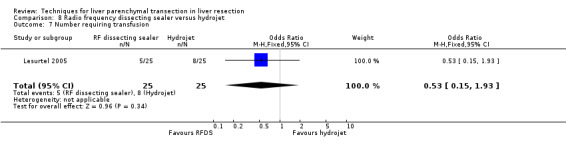
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 7 Number requiring transfusion.
8.8. Analysis.
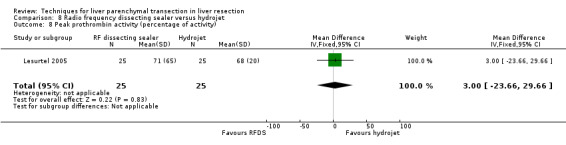
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 8 Peak prothrombin activity (percentage of activity).
8.9. Analysis.
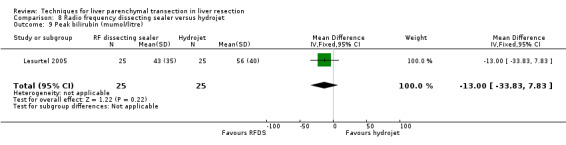
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 9 Peak bilirubin (mumol/litre).
8.10. Analysis.
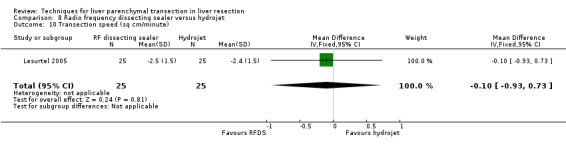
Comparison 8 Radio frequency dissecting sealer versus hydrojet, Outcome 10 Transection speed (sq cm/minute).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arita 2005.
| Methods | Randomised clinical trial. Generation of the allocation sequence: computerised minimisation process (adequate). Allocation concealment: held by third party (adequate). Blinding: inadequate. Incomplete outcome data addressed:adequate. Free from selective reporting: adequate. Free from baseline imbalance bias: adequate. Free from early stopping bias: adequate. Free from sponsor bias: adequate. |
|
| Participants | Country: Japan.
Number randomised: 80.
Median age: 66 (RFDS); 68 (clamp‐crush).
Females: 20 (25%).
Major liver resection: 20 (25%).
Chronic liver disease: 43 (53.8%).
Cirrhosis: 21 (26.3%). Inclusion criteria
Exclusion criteria Inflow occlusion at the hepatic hilum proved impossible at laparotomy. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: RFDS (n = 40) Group 2: Clamp‐crush (n = 40). Co‐interventions
|
|
| Outcomes | The main outcome measures were peri‐operative mortality, peri‐operative morbidity, blood loss and transfusion requirements, liver function tests, transection speed, and hospital stay. | |
| Notes | We requested further information from the authors regarding some outcomes in December 2006. We were unable to obtain the information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate ("The assignments were done by an internet‐accessed registration system administered by the independent randomization service") |
| Allocation concealment? | Low risk | A ‐ Adequate ("The assignments were done by an internet‐accessed registration system administered by the independent randomization service") |
| Blinding? All outcomes | High risk | C ‐ Inadequate |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate Review authors' comment: No post‐randomisation drop‐outs. |
| Free of selective reporting? | Low risk | A ‐ Adequate Review authors' comment: All the important outcomes were reported. |
| Free of baseline imbalance bias? | Low risk | A ‐ Adequate |
| From from early stopping bias? | Low risk | A ‐ Adequate Review author comment: The sample size calculations were reported and the calculated number of patients were recruited. |
| Free from academic bias? | Low risk | A ‐ Adequate Review author comment: No previous publication or conference report of a similar trial by the trial author was identified. |
| Free from sponsor bias? | Low risk | A ‐ Adequate ("This work was supported by a grant from the Kanae Foundation for Life‐Socio‐medical service") |
Koo 2005.
| Methods | Randomised clinical trial. Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: inadequate. Incomplete outcome data addressed:adequate. Free from selective reporting: inadequate. Free from baseline imbalance bias: adequate. Free from early stopping bias: unclear. Free from blocked randomisation bias: unclear. Free from sponsor bias: unclear. |
|
| Participants | Country: Korea.
Number randomised: 50.
Mean age: 52.7 years.
Females: 14 (28%).
Major liver resection: not stated.
Chronic liver disease: not stated.
Cirrhosis: not stated. Inclusion criteria Elective liver resection. Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: CUSA (n = 25) Group 2: Clamp‐crush (n = 25). Co‐interventions
|
|
| Outcomes | The main outcome measures were peri‐operative morbidity, blood loss and transfusion requirements, transection time, and operating time. | |
| Notes | We requested further information from the authors regarding some outcomes in December 2006. We were unable to obtain the information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | B ‐ Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear ("Randomization was performed by opening a sealed envelope before induction of anesthesia"). However, it was not clear whether randomisation was performed in blocks. |
| Blinding? All outcomes | High risk | C ‐ Inadequate |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate Review authors' comment: No post‐randomisation drop‐outs. |
| Free of selective reporting? | High risk | C ‐ Inadequate Review authors' comment: Important outcomes were not reported. |
| Free of baseline imbalance bias? | Low risk | A ‐ Adequate ("Differences in the demographic data and duration of surgery between groups were not significant") |
| From from early stopping bias? | Unclear risk | B ‐ Unclear |
| Free from academic bias? | Low risk | A ‐ Adequate Review author comment: No previous publication or conference report of a similar trial by the trial author was identified. |
| Free from sponsor bias? | Unclear risk | B ‐ Unclear |
Lesurtel 2005.
| Methods | Randomised clinical trial. Generation of the allocation sequence: urn randomisation (adequate). Allocation concealment: sealed envelope (adequate). Blinding: inadequate. Incomplete outcome data addressed:adequate. Free from selective reporting: adequate. Free from baseline imbalance bias: adequate. Free from early stopping bias: adequate.Free from sponsor bias: inadequate. |
|
| Participants | Country: Switzerland.
Number randomised: 100.
Mean age: 56 years.
Females: 47(47%).
Major liver resection: 61(61%).
Chronic liver disease: not stated.
Cirrhosis: none. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to four groups. Group 1: CUSA (n = 25) Group 2: Hydrojet (n = 25) Group 3: RFDS (n = 25) Group 4: Clamp‐crush (n = 25). Co‐interventions
|
|
| Outcomes | The main outcome measures were peri‐operative mortality, peri‐operative morbidity, blood loss and transfusion requirements, liver function tests, transection speed, stay, and costs. | |
| Notes | Authors provided information on allocation sequence generation; and questions related to morbidity and liver enzymes in December 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate ("The generation of the randomisation sequence was performed with sealed envelopes using urn randomisation") |
| Allocation concealment? | Low risk | A ‐ Adequate ("The generation of the randomisation sequence was performed with sealed envelopes using urn randomisation") |
| Blinding? All outcomes | High risk | C ‐ Inadequate |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate Review authors' comment: No post‐randomisation drop‐outs. |
| Free of selective reporting? | Low risk | A ‐ Adequate Review authors' comment: All the important outcomes were reported. |
| Free of baseline imbalance bias? | Low risk | A ‐ Adequate |
| From from early stopping bias? | Low risk | A ‐ Adequate Review author comment: The sample size calculations were reported and the calculated number of patients were recruited. |
| Free from academic bias? | Low risk | A ‐ Adequate Review author comment: No previous publication or conference report of a similar trial by the trial author was identified. |
| Free from sponsor bias? | High risk | C ‐ Inadequate ("The authors thank Tyco Healthcare (Mansfield, MA), Erbe (Tubingen, Germany), and TissueLink (Dover, NH) for their sponsorship") |
Lupo 2007.
| Methods | Randomised clinical trial. Generation of the allocation sequence: random number table (adequate). Allocation concealment: unclear. Blinding: inadequate. Incomplete outcome data addressed:adequate. Free from selective reporting: adequate. Free from baseline imbalance bias: adequate. Free from early stopping bias: adequate. Free from sponsor bias: adequate. |
|
| Participants | Country: Italy.
Number randomised: 51 (1 did not undergo resection because of advanced malignancy noted only during malignancy).
Median age: 62
Females: 14 (28%).
Major liver resection: 21 (42%).
Chronic liver disease: not stated.
Cirrhosis: 7 (14%). Inclusion criteria Curative liver resection for primary or secondary liver cancer. Exclusion criteria Patients not considered eligible for radical treatment after laparotomy. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: RFDS (n = 24) Group 2: Clamp‐crush (n = 26). Co‐interventions
|
|
| Outcomes | The main outcome measures were peri‐operative mortality, peri‐operative morbidity, transfusion requirements, operating time, and hospital stay. | |
| Notes | The trial authors provided information on allocation concealment and location of abscesses in March 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate ("The allocation was performed by random numbers tables with sealed envelopes"). |
| Allocation concealment? | Unclear risk | B ‐ Unclear ("The allocation was performed by random numbers tables with sealed envelopes"). However, it was not clear if randomisation was performed using blocks. |
| Blinding? All outcomes | High risk | C ‐ Inadequate |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate Review authors' comment: One patient who allocated to RFDS group had advanced cancer on laparotomy and did not receive the intervention. However, we think that this post‐randomisation drop‐out was not related to the outcomes. |
| Free of selective reporting? | Low risk | A ‐ Adequate Review authors' comment: All the important outcomes were reported. |
| Free of baseline imbalance bias? | Low risk | A ‐ Adequate |
| From from early stopping bias? | Low risk | A ‐ Adequate Review author comment: The sample size calculations were reported and the calculated number of patients were recruited. |
| Free from academic bias? | Low risk | A ‐ Adequate Review author comment: No previous publication or conference report of a similar trial by the trial author was identified. |
| Free from sponsor bias? | Low risk | A ‐ Adequate ("The authors thank the Hospital Service Spa (Aprilia) for helping with the technical development of the devices used") |
Rau 2001.
| Methods | Randomised clinical trial. Generation of the allocation sequence: lots (adequate). Allocation concealment: unclear. Blinding: inadequate. Incomplete outcome data addressed:adequate. Free from selective reporting: inadequate. Free from baseline imbalance bias: adequate. Free from early stopping bias: unclear. Free from sponsor bias: unclear. |
|
| Participants | Country: Germany.
Number randomised: 61.
Mean age: 62.3 years.
Females: 25 (41.0%).
Major liver resection: 24 (39.3%).
Chronic liver disease: Not stated.
Cirrhosis: Not stated. Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: Waterjet (n = 31) Group 2: CUSA (n = 30). Co‐interventions
|
|
| Outcomes | The main outcome measures were blood loss and transfusion requirements, and transection speed. | |
| Notes | Authors provided information on allocation sequence generation and allocation concealment in March 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate ("The selection of letters was performed by the leading nurse of the operation theatre") |
| Allocation concealment? | Unclear risk | B ‐ Unclear ("when the decision for liver resection was made, the letter was opened with the information on the dissection device"). However, it was not clear whether randomisation was performed in blocks. |
| Blinding? All outcomes | High risk | C ‐ Inadequate |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate Review authors' comment: No post‐randomisation drop‐outs. |
| Free of selective reporting? | High risk | C ‐ Inadequate Review authors' comment: Important outcomes were not reported. |
| Free of baseline imbalance bias? | Low risk | A ‐ Adequate |
| From from early stopping bias? | Unclear risk | B ‐ Unclear |
| Free from academic bias? | Low risk | A ‐ Adequate Review author comment: No previous publication or conference report of a similar trial by the trial author was identified. |
| Free from sponsor bias? | Unclear risk | B ‐ Unclear |
Smyrniotis 2005.
| Methods | Randomised clinical trial. Generation of the allocation sequence: unclear. Allocation concealment: unclear. Blinding: inadequate. Incomplete outcome data addressed:adequate. Free from selective reporting: adequate. Free from baseline imbalance bias: adequate. Free from early stopping bias: adequate. Free from sponsor bias: unclear. |
|
| Participants | Country: Greece.
Number randomised: 82.
Median age: 63 years.
Females: 17 (28%).
Major liver resection: 60 (73%).
Chronic liver disease: not stated.
Cirrhosis: 12 (14.6%). Inclusion criteria Liver resection for benign or malignant conditions. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: Sharp transection (n = 41) Group 2: Clamp‐crush (n = 41). Co‐interventions
|
|
| Outcomes | The main outcome measures were peri‐operative mortality, peri‐operative morbidity, blood loss and transfusion requirements, operating time, and stay. | |
| Notes | The authors provided information on allocation concealment and morbidity in December 2006. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | B ‐ Unclear |
| Allocation concealment? | Unclear risk | B ‐ Unclear ("Patients were randomized in the operating room using sealed envelopes"). However, it was not clear whether randomisation was performed in blocks. |
| Blinding? All outcomes | High risk | C ‐ Inadequate |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate Review authors' comment: No post‐randomisation drop‐outs. |
| Free of selective reporting? | Low risk | A ‐ Adequate Review authors' comment: All the important outcomes were reported. |
| Free of baseline imbalance bias? | Low risk | A ‐ Adequate |
| From from early stopping bias? | Low risk | A ‐ Adequate Review author comment: The sample size calculations were reported and the calculated number of patients were recruited. |
| Free from academic bias? | Low risk | A ‐ Adequate Review author comment: No previous publication or conference report of a similar trial by the trial author was identified. |
| Free from sponsor bias? | Unclear risk | B ‐ Unclear |
Takayama 2001.
| Methods | Randomised clinical trial. Generation of the allocation sequence: computerised minimisation process (adequate). Allocation concealment: held by third party (adequate). Blinding: inadequate. Incomplete outcome data addressed:adequate. Free from selective reporting: adequate. Free from baseline imbalance bias: adequate. Free from early stopping bias: adequate. Free from sponsor bias: adequate. |
|
| Participants | Country: Japan.
Number randomised: 132.
Median age: 61 years (group 1); 63 years (group 2).
Females: Not stated.
Major liver resection: 43 (32.6%).
Chronic liver disease: not stated.
Cirrhosis: not stated. Inclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: CUSA (n = 66) Group 2: Clamp‐crush (n = 66). Co‐interventions
|
|
| Outcomes | The main outcome measures were peri‐operative mortality, peri‐operative morbidity, blood loss and transfusion requirements, and transection speed. | |
| Notes | The authors provided information on randomisation procedure and morbidity in January 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A ‐ Adequate ("The randomisation was generated by the minimization method using Microsoft Excel (for Windows) and its Visual Basic"). |
| Allocation concealment? | Low risk | A ‐ Adequate ("The randomisation was generated by the minimization method using Microsoft Excel (for Windows) and its Visual Basic"). |
| Blinding? All outcomes | High risk | C ‐ Inadequate |
| Incomplete outcome data addressed? All outcomes | Low risk | A ‐ Adequate Review authors' comment: No post‐randomisation drop‐outs. |
| Free of selective reporting? | Low risk | A ‐ Adequate Review authors' comment: All the important outcomes were reported. |
| Free of baseline imbalance bias? | Low risk | A ‐ Adequate |
| From from early stopping bias? | Low risk | A ‐ Adequate Review author comment: The sample size calculations were reported and the calculated number of patients were recruited. |
| Free from academic bias? | Low risk | A ‐ Adequate Review author comment: No previous publication or conference report of a similar trial by the trial author was identified. |
| Free from sponsor bias? | Low risk | A ‐ Adequate ("This work was supported in part by a grant‐in‐aid for cancer research from the Ministiy of Health and Welfare, Tokyo, Japan") |
ALT = alanine transaminase AST = aspartate transaminase CUSA = cavitron ultrasonic surgical aspirator CVP = central venous pressure HVE = hepatic vascular exclusion IPC = ischaemic pre‐conditioning ITU = intensive therapy unit IV = intravenous PTC = portal triad clamping RFDS = radiofrequency dissection sealer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Rau 1996 | Randomisation was stopped because of technical difficulties. |
Differences between protocol and review
The outcomes have now been classified into primary and secondary outcomes. The method of assessment of bias‐risk has been updated in line with the methodology stated in the Cochrane Handbook (Higgins 2008). A sensitivity analysis excluding a trial, which used vascular occlusion in one group was performed following comments from peer reviewers and editors.
Contributions of authors
KG identified trials for inclusion, extract data, analyse data, and wrote the draft review. VP is the second author who independently identified trials for inclusion and extracted data. DS and BRD critically commented on the review. All authors approved of the review.
Sources of support
Internal sources
none, Not specified.
External sources
none, Not specified.
Declarations of interest
None known.
New
References
References to studies included in this review
Arita 2005 {published data only}
- Arita J, Hasegawa K, Kokudo N, Sano K, Sugawara Y, Makuuchi M. Randomized clinical trial of the effect of a saline‐linked radiofrequency coagulator on blood loss during hepatic resection. The British Journal of Surgery 2005;92(8):954‐9. [DOI] [PubMed] [Google Scholar]
Koo 2005 {published data only}
- Koo BN, Kil HK, Choi JS, Kim JY, Chun DH, Hong YW. Hepatic resection by the Cavitron Ultrasonic Surgical Aspirator increases the incidence and severity of venous air embolism. Anesthesia Analgesia 2005;101(4):966‐70, table of contents. [DOI] [PubMed] [Google Scholar]
Lesurtel 2005 {published and unpublished data}
- Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed? a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Annals of Surgery 2005;242(6):814‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lupo 2007 {published and unpublished data}
- Lupo L, Gallerani A, Panzera P, Tandoi F, Palma G, Memeo V. Randomized clinical trial of radiofrequency‐assisted versus clamp‐crushing liver resection. The British Journal of Surgery 2007;94(3):287‐91. [DOI] [PubMed] [Google Scholar]
Rau 2001 {published and unpublished data}
- Rau HG, Wichmann MW, Schinkel S, Buttler E, Pickelmann S, Schauer R, et al. [Surgical techniques in hepatic resections: Ultrasonic aspirator versus Jet‐Cutter. A prospective randomized clinical trial]. Zentralblatt fur Chirurgie 2001;126(8):586‐90. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2005 {published and unpublished data}
- Smyrniotis V, Arkadopoulos N, Kostopanagiotou G, Farantos C, Vassiliou J, Contis J, et al. Sharp liver transection versus clamp crushing technique in liver resections: a prospective study. Surgery 2005;137(3):306‐11. [DOI] [PubMed] [Google Scholar]
Takayama 2001 {published and unpublished data}
- Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Archives of Surgery 2001;136(8):922‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Rau 1996 {published data only}
- Rau HG, Meyer G, Jauch KW, Cohnert TU, Buttler E, Schildberg FW. [Liver resection with the water jet: conventional and laparoscopic surgery]. Der Chirurg 1996;67(5):546‐51. [PubMed] [Google Scholar]
Additional references
Belghiti 1993
- Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Annals of Surgery 1993;218(6):748‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belghiti 1996
- Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Annals of Surgery 1996;224(2):155‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belghiti 1999
- Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Annals of Surgery 1999;229(3):369‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bombuy 2004
- Bombuy E, Fondevila C, Rodriguez‐Laiz G, Ferrer J, Amador A, Valentini M, et al. Ischemic preconditioning in adult living donor liver transplantation, a pilot study [EASL abstract]. Journal of Hepatology 2004;40(Suppl 1):39. [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fong 1996
- Fong Y, Brennan MF, Brown K, Heffernan N, Blumgart LH. Drainage is unnecessary after elective liver resection. American Journal of Surgery 1996;171(1):158‐62. [DOI] [PubMed] [Google Scholar]
Gluud 2008
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2008, Issue 4. Art. No.: LIVER.
Gurusamy 2007
- Gurusamy KS, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006409.pub2] [DOI] [PubMed] [Google Scholar]
Hasegawa 2002
- Hasegawa K, Takayama T, Orii R, Sano K, Sugawara Y, Imamura H, et al. Effect of hypoventilation on bleeding during hepatic resection: a randomized controlled trial. Archives of Surgery 2002;137(3):311‐5. [DOI] [PubMed] [Google Scholar]
HES 2005
- Hospital Episode Statistics. Main operations. 3 character: 2004‐05. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=205 (accessed 9 September 2008).
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention 5.0.0 [updated February 2008]. The Cochrane Colloboration, 2008. Available from www.cochrane‐handbook.org. [Google Scholar]
Ibrahim 2006
- Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transplantation 2006;12(6):950‐7. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lin 1987
- Lin TY, Lee CS, Chen KM, Chen CC. Role of surgery in the treatment of primary carcinoma of the liver: a 31‐year experience. The British Journal of Surgery 1987;74(9):839‐42. [PUBMED: 2822201] [DOI] [PubMed] [Google Scholar]
Liu 2004
- Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Annals of Surgery 2004;239(2):194‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Rui 2003
- Rui JA, Wang SB, Chen SG, Zhou L. Right trisectionectomy for primary liver cancer. World Journal of Gastroenterology 2003;9(4):706‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Shimada 1998
- Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. British Journal of Surgery 1998;85(2):195‐8. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2002
- Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, et al. Total versus selective hepatic vascular exclusion in major liver resections. American Journal of Surgery 2002;183(2):173‐8. [DOI] [PubMed] [Google Scholar]
Strasberg 2000
- Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB Surgery 2000;2(3):333‐9. [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World Journal of Gastroenterology 2006;12(6):935‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2002
- Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Annals of Surgery 2002;236(5):560‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshimura 2004
- Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World Journal of Surgery 2004;28(10):982‐6. [DOI] [PubMed] [Google Scholar]


