Abstract
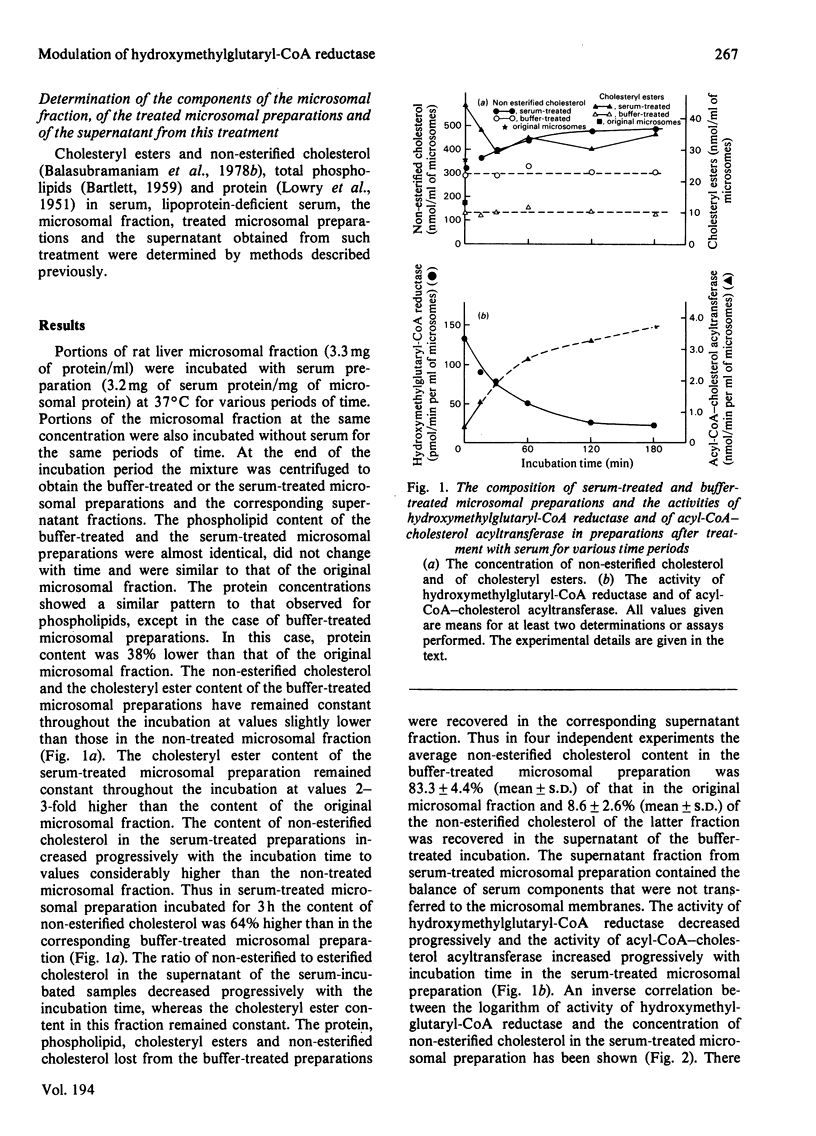
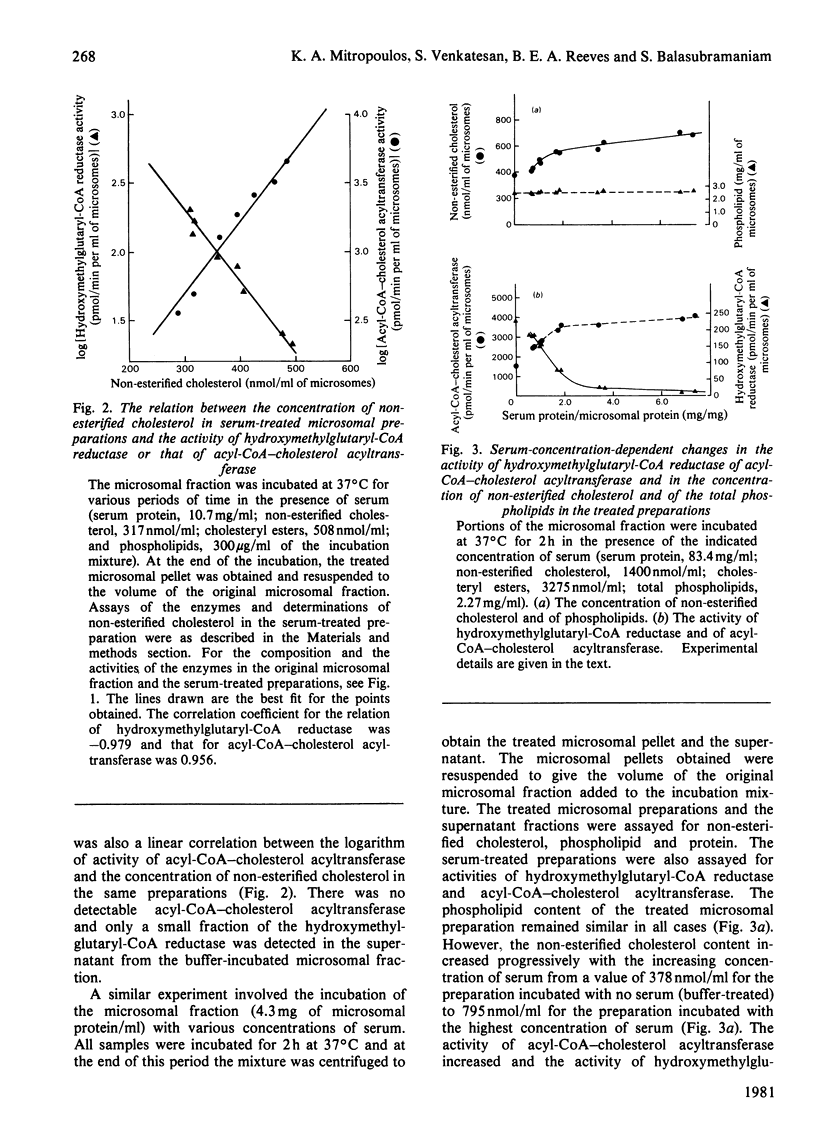
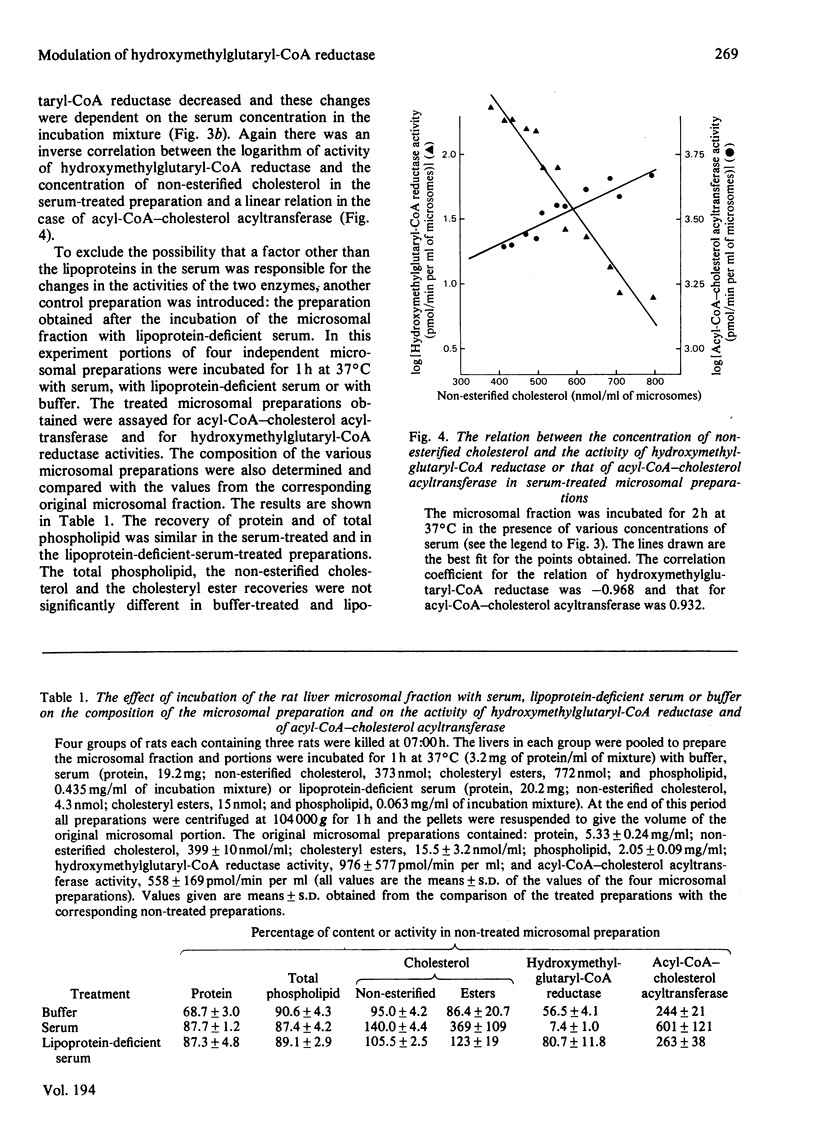
The incubation of rat liver microsomal fraction with a serum preparation followed by the re-isolation of the microsomal membranes has resulted in an increase in the concentration of non-esterified cholesterol, a considerable decrease in the activity of 3-hydroxy-3-methylglutaryl-CoA reductase and in an increase in the activity of acyl-CoA–cholesterol acyltransferase in the treated microsomal preparation. These effects were related to the concentration of serum in the incubation mixture and to the duration of the incubation. The transfer of non-esterified cholesterol was specific in that the content of protein and the total phospholipids were similar in the original microsomal fraction and the serum-treated microsomal preparation. The incubation of the microsomal fraction with lipoprotein-deficient serum or with no serum resulted in both cases in small changes in the non-esterified cholesterol, the esterified cholesterol and the total phospholipid content in the treated preparations compared with these concentrations in the original microsomal fraction, whereas the activity of acyl-CoA–cholesterol acyltransferase and of 3-hydroxy-3-methylglutaryl-CoA reductase was similar in the lipoprotein-deficient-serum-treated and the buffer-treated microsomal preparations. The activity of 3-hydroxy-3-methylglutaryl-CoA reductase was lower and the activity of acyl-CoA–cholesterol acyltransferase was higher in the lipoprotein-deficient-serum-treated and the buffer-treated microsomal preparations as compared with these activities in the original microsomal fraction. However, the serum-treated microsomal preparation had considerably lower activity of 3-hydroxy-3-methylglutaryl-CoA reductase and considerably higher activity of acyl-CoA–cholesterol acyltransferase than these activities in buffer-treated and in lipoprotein-deficient-serum-treated microsomal preparations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar-Costesec A., Wibo M., Thinès-Sempoux D., Beaufay H., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. IV. Biochemical, physical, and morphological modifications of microsomal components induced by digitonin, EDTA, and pyrophosphate. J Cell Biol. 1974 Sep;62(3):717–745. doi: 10.1083/jcb.62.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balasubramaniam S., Mitropoulos K. A., Venkatesan S. Rat-liver acyl-CoA: cholesterol acyltransferase. Eur J Biochem. 1978 Oct;90(2):377–383. doi: 10.1111/j.1432-1033.1978.tb12614.x. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S., Venkatesan S., Mitropoulos K. A., Peters T. J. The submicrosomal localization of acyl-coenzyme A-cholesterol acyltransferase and its substrate, and of cholesteryl esters in rat liver. Biochem J. 1978 Sep 15;174(3):863–872. doi: 10.1042/bj1740863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell F. P. Lipid exchange and transfer between biological lipid-protein structures. Prog Lipid Res. 1978;17(2):207–243. doi: 10.1016/0079-6832(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Arner E. C., Wiley J. S., Shattil S. J. Modification of red cell membrane structure by cholesterol-rich lipid dispersions. A model for the primary spur cell defect. J Clin Invest. 1975 Jan;55(1):115–126. doi: 10.1172/JCI107901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Brown M. S. Effect of alterations of the specific activity of the intracellular acetyl CoA pool on apparent rates of hepatic cholesterogenesis. J Lipid Res. 1974 Sep;15(5):508–516. [PubMed] [Google Scholar]
- Gould R. G. Some aspects of the control of hepatic cholesterol biosynthesis. Expos Annu Biochim Med. 1977;33:13–38. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Dayton S. Stimulation of cholesterol esterification in hepatic microsomes by lipoproteins from normal and hypercholesterolemic rabbit serum. Biochim Biophys Acta. 1979 May 25;573(2):354–360. doi: 10.1016/0005-2760(79)90068-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Dayton S. Studies of the mechanism of augmented synthesis of cholesteryl ester in atherosclerotic rabbit aortic microsomes. Atherosclerosis. 1977 Dec;28(4):447–452. doi: 10.1016/0021-9150(77)90071-5. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Packie R. M. Comparison of the effects of some C27-, C21-, and C19-steroids upon hepatic sterol synthesis and hydroxymethylglutaryl-CoA reductase activity. Arch Biochem Biophys. 1970 Sep;140(1):122–130. doi: 10.1016/0003-9861(70)90016-0. [DOI] [PubMed] [Google Scholar]
- Linn T. C. The effect of cholesterol feeding and fasting upon beta-hydroxy-beta-methylglutaryl coenzyme A reductase. J Biol Chem. 1967 Mar 10;242(5):990–993. [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S., Myant N. B. The effect of interruption of the enterophecatic circulation of bile acids and of cholesterol feeding on cholesterol 7 alpha-hydroxylase in relation to the diurnal rhythm in its activity. Biochim Biophys Acta. 1973 Dec 20;326(3):428–438. doi: 10.1016/0005-2760(73)90143-4. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S. The role of glucocorticoids in the regulation of the diurnal rhythm of hepatic beta-hydroxy-beta-methylglutaryl-coenzyme A reductase and cholesterol 7 alpha-hydroxylase. Biochem J. 1976 Oct 15;160(1):49–55. doi: 10.1042/bj1600049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S., Venkatesan S., Reeves B. E. On the mechanism for the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, of cholesterol 7alpha-hydroxylase and of acyl-coenzyme A:cholesterol acyltransferase by free cholesterol. Biochim Biophys Acta. 1978 Jul 25;530(1):99–111. doi: 10.1016/0005-2760(78)90130-3. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Balasubramaniam S. Compartmentation and supply of cholesterol: two important factors in the co-ordinate regulation of hydroxymethyglutary-CoA reductase and cholesterol 7alpha-hydroxylase. Biochem Soc Trans. 1978;6(5):878–883. doi: 10.1042/bst0060878. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Balasubramaniam S. On the mechanism of regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and of acyl coenzyme A:cholesterol acyltransferase by dietary fat. Biochim Biophys Acta. 1980 Aug 11;619(2):247–257. doi: 10.1016/0005-2760(80)90073-9. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Balasubramaniam S., Peters T. J. The submicrosomal localization of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase, cholesterol 7alpha-hydroxylase and cholesterol in rat liver. Eur J Biochem. 1978 Jan 16;82(2):419–429. doi: 10.1111/j.1432-1033.1978.tb12036.x. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S. The influence of cholesterol on the activity, on the isothermic kinetics and on the temperature-induced kinetics of 3-hydroxy-3-methylglutaryl coenzyme a reductase. Biochim Biophys Acta. 1977 Oct 24;489(1):126–142. doi: 10.1016/0005-2760(77)90239-9. [DOI] [PubMed] [Google Scholar]
- RADDING C. M., STEINBERG D. Studies on the synthesis and secretion of serum lipoproteins by rat liver slices. J Clin Invest. 1960 Oct;39:1560–1569. doi: 10.1172/JCI104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., GUEST M. J. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960 Apr;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Rodwell V. W. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol synthesis. J Biol Chem. 1971 May 25;246(10):3210–3216. [PubMed] [Google Scholar]
- Shattil S. J., Anaya-Galindo R., Bennett J., Colman R. W., Cooper R. A. Platelet hypersensitivity induced by cholesterol incorporation. J Clin Invest. 1975 Mar;55(3):636–643. doi: 10.1172/JCI107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M. An efficient method for modulation of cholesterol level in cell membranes. FEBS Lett. 1978 Jan 15;85(2):317–320. doi: 10.1016/0014-5793(78)80482-7. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Rivnay B. Degree of exposure of membrane proteins determined by fluorescence quenching. Biochemistry. 1977 Mar 8;16(5):982–986. doi: 10.1021/bi00624a027. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Fischkoff S., Chance B., Cooper R. A. Fluorescent probe analysis of the lipid architecture of natural and experimental cholesterol-rich membranes. Biochemistry. 1974 Apr 9;13(8):1589–1595. doi: 10.1021/bi00705a006. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Mitropoulos K. A., Balasubramaniam S., Peters T. J. Biochemical evidence for the heterogeneity of membranes from rat liver endoplasmic reticulum. Studies on the localization of acyl-CoA: cholesterol acyltransferase. Eur J Cell Biol. 1980 Jun;21(2):167–174. [PubMed] [Google Scholar]


