Abstract
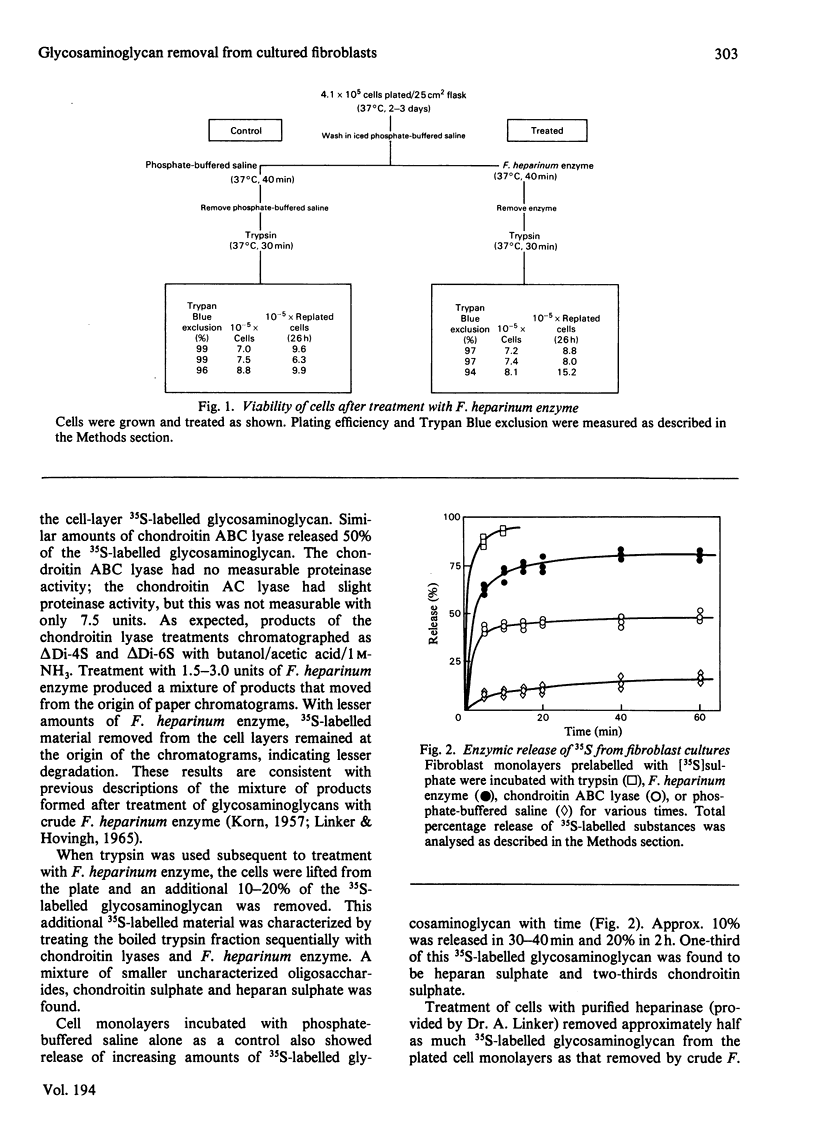
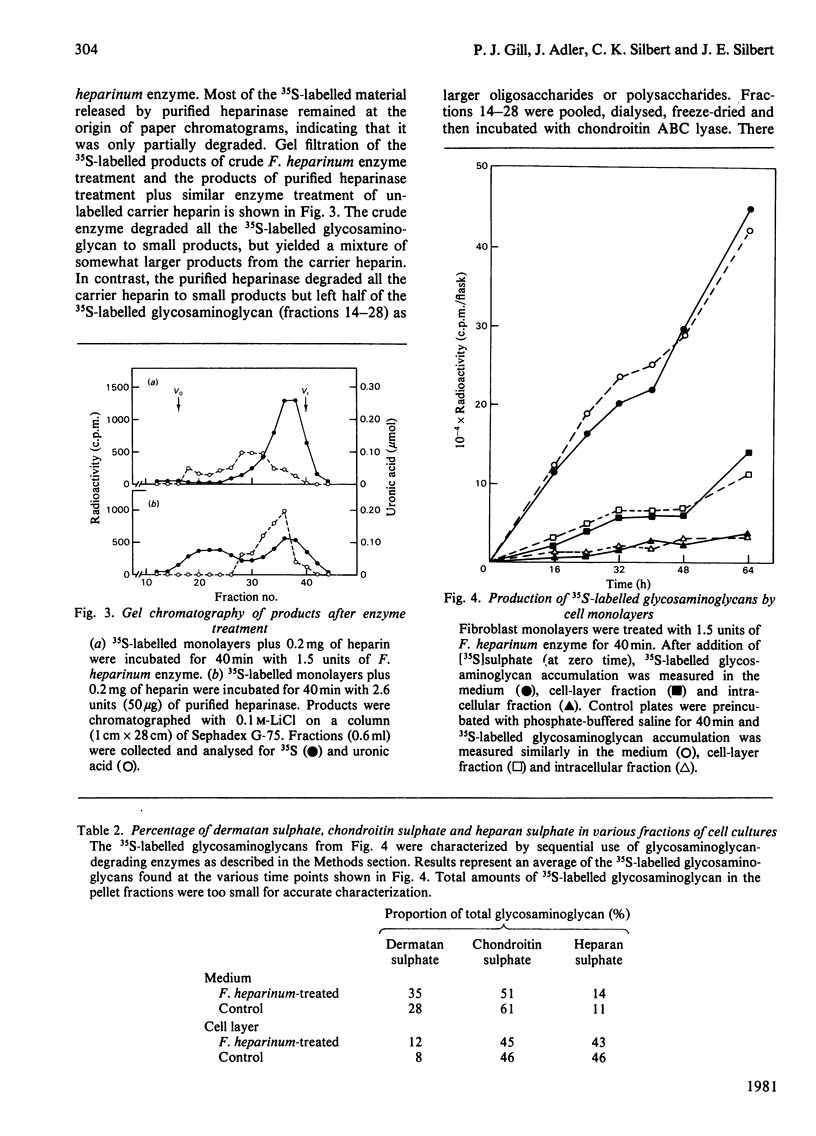
Early-passage human skin fibroblasts were grown as monolayers for 2-3 days in minimum essential medium containing [35S]sulphate, [3H]glucosamine, [3H]fucose, [3H]proline or [3H]leucine to label proteoglycans, glycoproteins or collagen and other proteins. A crude enzyme preparation obtained from a supernatant from sonicated freeze-dried Flavobacter heparinum was added to the cell monolayers. This treatment removed most of the 35S-labelled glycosaminoglycans, with no appreciable removal of the 3H-labelled proteins or 3H-labelled glycoproteins. The cells remained attached and viable as a monolayer. The formation of 35S-labelled glycosaminoglycans was examined after pretreating cultures with crude F. heparinum enzyme, followed by addition of fresh growth medium containing [35S]sulphate. The F. heparinum enzyme did not significantly alter the amount or type of 35S-labelled glycosaminoglycans produced. Thus F. heparinum enzyme can be used to provide cultured-cell monolayers depleted of surface glycosaminoglycans. These cells remain attached, viable and subsequently synthesize normal amounts and type of glycosaminoglycans.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Root M. Enzymatic degradation of heparin-related mucopolysaccharides from the surface of endothelial cell cultures. Biochim Biophys Acta. 1975 Mar 14;385(1):1–10. doi: 10.1016/0304-4165(75)90067-7. [DOI] [PubMed] [Google Scholar]
- Fratantoni J. C., Hall C. W., Neufeld E. F. The defect in Hurler's and Hunter's syndromes: faulty degradation of mucopolysaccharide. Proc Natl Acad Sci U S A. 1968 Jun;60(2):699–706. doi: 10.1073/pnas.60.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D. The degradation of heparin by bacterial enzymes. III. A comparison of the degradation of heparin, hyaluronic acid, and chondroitin sulfate. J Biol Chem. 1957 Jun;226(2):841–844. [PubMed] [Google Scholar]
- Kleinman H. K., Silbert J. E., Silbert C. K. Heparan sulfate of skin fibroblasts grown in culture. Connect Tissue Res. 1975;4(1):17–23. doi: 10.3109/03008207509152193. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The enzymatic degradation of heparin and heparitin sulfate. I. The fractionation of a crude heparinase from flavobacteria. J Biol Chem. 1965 Oct;240(10):3724–3728. [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Hurler's syndrome: biosynthesis of acid mucopolysaccharides in tissue culture. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1310–1316. doi: 10.1073/pnas.56.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Nevo Z., Dorfman A. Stimulation of chondromucoprotein synthesis in chondrocytes by extracellular chondromucoprotein. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2069–2072. doi: 10.1073/pnas.69.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M. K., Jr Measurement of growth and viability of cells in culture. Methods Enzymol. 1979;58:141–152. doi: 10.1016/s0076-6879(79)58132-4. [DOI] [PubMed] [Google Scholar]
- Perkins M. E., Ji T. H., Hynes R. O. Cross-linking of fibronectin to sulfated proteoglycans at the cell surface. Cell. 1979 Apr;16(4):941–952. doi: 10.1016/0092-8674(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Culp L. A. Glycosaminoglycans in the substrate adhesion sites of normal and virus-transformed murine cells. Biochemistry. 1979 Jan 9;18(1):141–148. doi: 10.1021/bi00568a022. [DOI] [PubMed] [Google Scholar]
- Saito H., Uzman B. G. Production and secretion of chondroitin sulfates and dermatan sulfate by established mammalian cell lines. Biochem Biophys Res Commun. 1971 May 21;43(4):723–728. doi: 10.1016/0006-291x(71)90675-9. [DOI] [PubMed] [Google Scholar]
- Sjöberg I., Carlstedt I., Cöster L., Malmström A., Fransson L. A. Structure and metabolism of sulphated glycosaminoglycans in cultures of human fibroblasts. Structural characteristics of co-polymeric galactosaminoglycans in sequential extracts of fibroblasts during pulse-chase experiments. Biochem J. 1979 Feb 15;178(2):257–270. doi: 10.1042/bj1780257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Gross J. The extracellular matrix of the regenerating newt limb: synthesis and removal of hyaluronate prior to differentiation. Dev Biol. 1971 May;25(1):57–77. doi: 10.1016/0012-1606(71)90019-4. [DOI] [PubMed] [Google Scholar]
- Truppe W., Kresse H. Uptake of proteoglycans and sulfated glycosaminoglycans by cultured skin fibroblasts. Eur J Biochem. 1978 Apr 17;85(2):351–356. doi: 10.1111/j.1432-1033.1978.tb12246.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wasteson A., Glimelius B., Busch C., Westermark H., Heldin C. H., Norling B. Effect of a platelet endoglycosidase on cell surface associated heparan sulphate of human culturei endothelial and glial cells. Thromb Res. 1977 Sep;11(3):309–321. doi: 10.1016/0049-3848(77)90184-0. [DOI] [PubMed] [Google Scholar]
- Wiebkin O. W., Muir H. Synthesis of proteoglycans by suspension and monolayer cultures of adult chondrocytes and de novo cartilage nodules-the effect of hyaluronic acid. J Cell Sci. 1977;27:199–211. doi: 10.1242/jcs.27.1.199. [DOI] [PubMed] [Google Scholar]
- Yue B. Y., Baum J. L. The synthesis of glycosaminoglycans by cultures of rabbit corneal endothelial and stromal cells. Biochem J. 1976 Sep 15;158(3):567–573. doi: 10.1042/bj1580567. [DOI] [PMC free article] [PubMed] [Google Scholar]


