Abstract
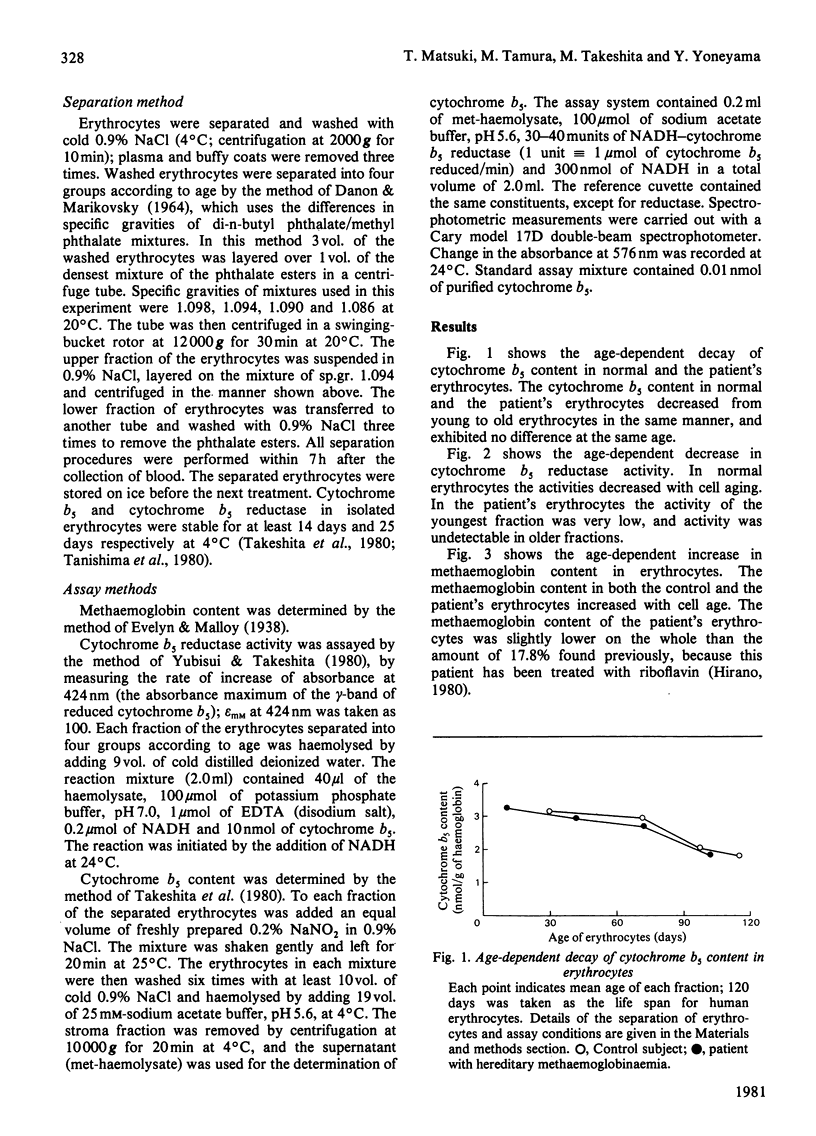
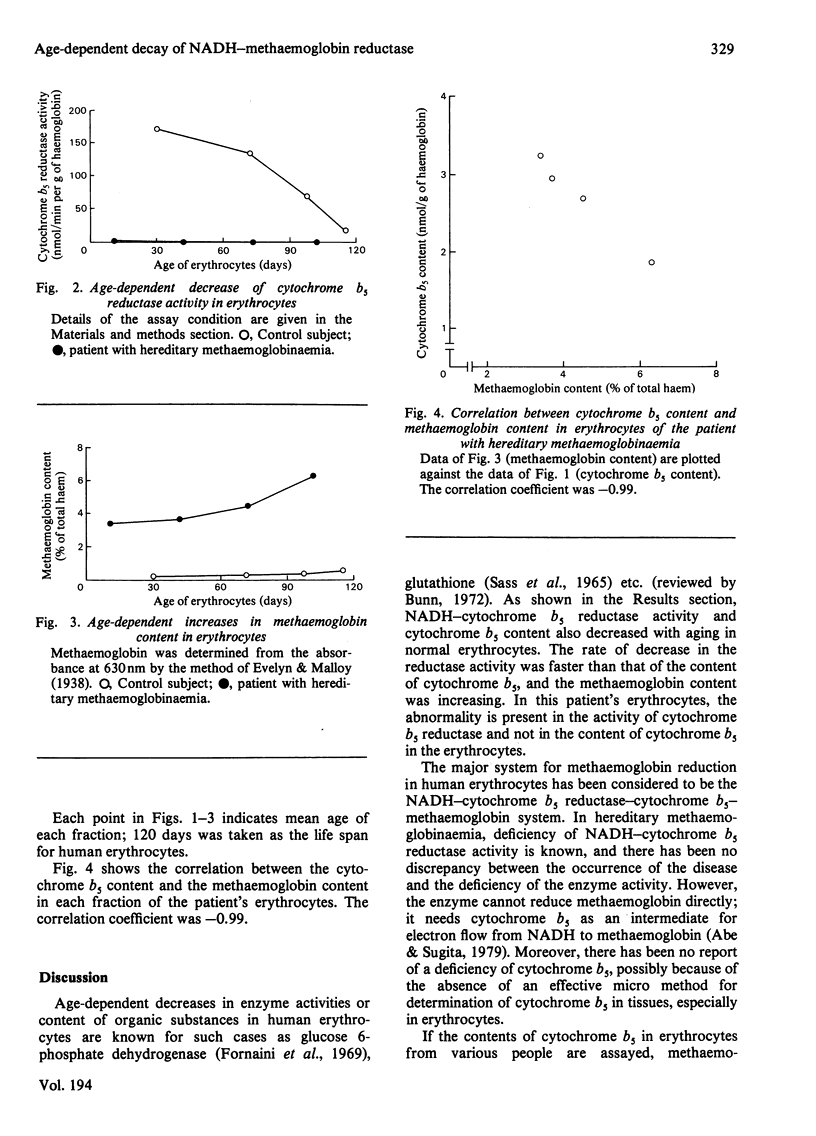
Age-dependent decrease in cytochrome b5 was observed in erythrocytes from both a normal person and a patient with hereditary methaemoglobinaemia without neurological symptoms. With aging, concentrations of cytochrome b5 in erythrocytes from the patient were almost the same as those in the control. Age-dependent decrease in cytochrome b5 reductase activity in the control erythrocytes was also shown; however, the reductase activity was very low in erythrocytes from the patient over the whole age range. Our studies show that methaemoglobin content of erythrocytes seems to be dependent on the content of cytochrome b5 in the cells, both in the control subject and in the patient.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Sugita Y. Properties of cytochrome b5 and methemoglobin reduction in human erythrocytes. Eur J Biochem. 1979 Nov;101(2):423–428. doi: 10.1111/j.1432-1033.1979.tb19735.x. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. Erythocyte destruction and hemoglobin catabolism. Semin Hematol. 1972 Jan;9(1):3–17. [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- Enoch H. G., Strittmatter P. Cytochrome b5 reduction by NADPH-cytochrome P-450 reductase. J Biol Chem. 1979 Sep 25;254(18):8976–8981. [PubMed] [Google Scholar]
- Feig S. A., Nathan D. G., Gerald P. S., Zarkowski H. S. Congenital methemoglobinemia: the result of age-dependent decay of methemoglobin reductase. Blood. 1972 Mar;39(3):407–414. [PubMed] [Google Scholar]
- Fornaini G., Leoncini G., Segni P., Calabria G. A., Dachà M. Relationship between age and properties of human and rabbit erythrocyte glucose-6-phosphate dehydrogenase. Eur J Biochem. 1969 Jan;7(2):214–222. doi: 10.1111/j.1432-1033.1969.tb19594.x. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E., Passon P. G. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat New Biol. 1971 Feb 24;229(8):252–254. doi: 10.1038/newbio229252a0. [DOI] [PubMed] [Google Scholar]
- Keitt A. S., Smith T. W., Jandl J. H. Red-cell "pseudomosaicism" in congenital methemoglobinemia. N Engl J Med. 1966 Aug 25;275(8):399–405. doi: 10.1056/NEJM196608252750801. [DOI] [PubMed] [Google Scholar]
- Leroux A., Junien C., Kaplan J., Bamberger J. Generalised deficiency of cytochrome b5 reductase in congenital methaemoglobinaemia with mental retardation. Nature. 1975 Dec 18;258(5536):619–620. doi: 10.1038/258619a0. [DOI] [PubMed] [Google Scholar]
- Matsuki T., Yubisui T., Tomoda A., Yoneyama Y., Takeshita M., Hirano M., Kobayashi K., Tani Y. Acceleration of methaemoglobin reduction by riboflavin in human erythrocytes. Br J Haematol. 1978 Aug;39(4):523–528. doi: 10.1111/j.1365-2141.1978.tb03621.x. [DOI] [PubMed] [Google Scholar]
- Passon P. G., Reed D. W., Hultquist D. E. Soluble cytochrome b 5 from human erythrocytes. Biochim Biophys Acta. 1972 Jul 12;275(1):51–61. doi: 10.1016/0005-2728(72)90023-0. [DOI] [PubMed] [Google Scholar]
- SASS M. D., CARUSO C. J., O'CONNELL D. J. DECREASED GLUTATHIONE IN AGING RED CELLS. Clin Chim Acta. 1965 Apr;11:334–340. doi: 10.1016/0009-8981(65)90223-8. [DOI] [PubMed] [Google Scholar]
- Sannes L. J., Hultquist D. E. Effects of hemolysate concentration, ionic strength and cytochrome b5 concentration on the rate of methemoglobin reduction in hemolysates of human erythrocytes. Biochim Biophys Acta. 1978 Dec 18;544(3):547–554. doi: 10.1016/0304-4165(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Nomura S., Yoneyama Y. Purification of reduced pyridine nucleotide dehydrogenase from human erythrocytes and methemoglobin reduction by the enzyme. J Biol Chem. 1971 Oct 10;246(19):6072–6078. [PubMed] [Google Scholar]
- Tanishima K., Matsuki T., Fukuda N., Takeshita M., Yoneyama Y. NADH-cytochrome b5 reductase in platelets and leukocytes with special reference to normal levels and to levels in carriers of hereditary methemoglobinemia with or without neurological symptoms. Acta Haematol. 1980;63(1):7–12. doi: 10.1159/000207361. [DOI] [PubMed] [Google Scholar]
- Vetrella M., Astedt B., Barthelmai W., Neuvians D. Activity of NADH- and NADPH-dependent methemoglobin reductases in erythrocytes from fetal to adult age. A parallel assessment. Klin Wochenschr. 1971 Sep 1;49(17):972–977. doi: 10.1007/BF01489462. [DOI] [PubMed] [Google Scholar]
- Yubisui T., Matsuki T., Takeshita M., Yoneyama Y. Characterization of the purified NADPH-flavin reductase of human erythrocytes. J Biochem. 1979 Mar;85(3):719–728. [PubMed] [Google Scholar]
- Yubisui T., Takeshita M. Characterization of the purified NADH-cytochrome b5 reductase of human erythrocytes as a FAD-containing enzyme. J Biol Chem. 1980 Mar 25;255(6):2454–2456. [PubMed] [Google Scholar]


