Abstract
Several neuropeptides present in bone tissues, produced by nerve fibers and bone cells, have been reported to play a role in regulating the fine‐tuning of osteoblast and osteoclast functions to maintain bone homeostasis. This study aims to characterize the influence of the neuropeptide vasoactive intestinal peptide (VIP) on the differentiation process of human mesenchymal stem cells (MSCs) into osteoblasts and on their anabolic function. We describe the mRNA and protein expression profile of VIP and its receptors in MSCs as they differentiate into osteoblasts, suggesting the presence of an autocrine signaling pathway in these cells. Our findings reveal that VIP enhances the expression of early osteoblast markers in MSCs under osteogenic differentiation and favors both bone matrix formation and proper cytoskeletal reorganization. Finally, our data suggest that VIP could be exerting a direct modulatory role on the osteoblast to osteoclast signaling by downregulating the receptor activator of nuclear factor‐κB ligand/osteoprotegerin ratio. These results highlight the potential of VIP as an osteoinductive differentiation factor, emerging as a key molecule in the maintenance of human bone homeostasis.
Keywords: actin cytoskeleton, bone metabolism, human mesenchymal stem cells, osteogenesis, VIP
Vasoactive intestinal peptide (VIP) acts as a promoter of human osteogenesis, enhancing the expression of early osteoblast markers and favoring both bone matrix formation and proper cytoskeletal reorganization. Moreover, VIP could be exerting a direct modulatory role on the osteoblast to osteoclast signaling by downregulating the receptor activator of nuclear factor‐κB ligand/osteoprotegerin (RANKL/OPG) ratio.
1. INTRODUCTION
Physiological bone remodeling and bone homeostasis depend to a large extent on the dynamic coupling between osteoclastic resorption and osteoblastic osteogenesis. The fine‐tuning of this system is essential for the proper maintenance of the skeleton and for fracture healing. 1 Therefore, when such a fine equilibrium is pathologically disrupted in favor of bone erosion, homeostasis is compromised. In some pathological conditions, such as osteoporosis, resorption exceeds formation, compromising bone integrity. In others, resorption occurs autonomously without direct association with subsequent bone formation, leading to focal bone loss, as observed in rheumatoid arthritis (RA). 2 , 3
Osteoblasts are cuboidal shaped cells responsible for bone matrix synthesis and mineralization mostly located on active bone‐forming surfaces. These cells arise from the sequential commitment of undifferentiated mesenchymal stem cells (MSCs) to the osteoblastic linage. 4 Upon osteoblastic commitment, preosteoblasts undergo proliferation, exit the cell cycle and initiate an early differentiation to immature osteoblasts, characterized by the secretion of bone matrix proteins. 5 The maturation of extracellular matrix is followed by calcium deposition that depends on the expression of alkaline phosphatase (ALP) by phenotypically mature osteoblasts, thus mediating the extracellular matrix mineralization. 6 Therefore, the osteoblastogenic process actually involves a tightly organized sequence of biochemical events controlled by numerous signals. Among them, the transcription factor Runx2 is recognized as a key molecular switch in osteoblast differentiation, being essential for both the attenuation of osteoblast growth and the activation of genes encoding major osteoid proteins, such as type I collagen. In turn, this osteoblast master regulator is modulated by different upstream signaling pathways, including the Wnt/β‐catenin system. 7 Moreover, cell adhesion dynamics and structural changes in F‐actin networks are important events during osteoblastogenesis. 8 Differentiating osteoblasts adhere to the extracellular matrix through focal adhesion complexes involving vinculin, which plays a key role in the dynamic of these molecular complexes. 9 When MSCs undergo osteogenic differentiation, the actin cytoskeleton becomes more diffuse, and the distribution of focal adhesions changes. 8 This cytoskeletal rearrangement is accompanied by changes in cell morphology and cytoskeletal tension, which are suggested to ultimately regulate the spatiotemporal genetic program that governs osteoblast commitment and maturation. 10 , 11
Multilayered control of MSCs osteodifferentiation is in accordance with the crucial role of osteoblasts in the maintenance of bone homeostasis. In fact, normal bone metabolism relies on the balance between its osteogenic activity and osteoclast‐mediated osteolysis, being further orchestrated by a variety of autocrine and paracrine factors ranging from systemic hormones to local osteotropic cytokines and growth factors. 12 , 13 One of the main cross‐talking pathways between osteoblast and osteoclast is the receptor activator of nuclear factor‐κB ligand/osteoprotegerin (RANKL/OPG) system. RANKL, mainly produced by osteoblasts, binds to its receptor RANK on osteoclast precursors and stimulates their differentiation, activity, and survival. This osteoclastogenic signaling can in turn be blocked by OPG, a decoy receptor for RANKL also secreted by osteoblasts. 14 , 15 Besides, several lines of evidence indicate that neuropeptides released from skeletal nerve fibers, and in some cases, by bone cells, are likewise involved in the complex network of signals regulating bone homeostasis. 16 , 17 , 18 , 19
The vasoactive intestinal peptide (VIP) is a ubiquitous neuropeptide expressed in both the peripheral and central nervous system as well as in immune and endocrine cells. The presence of VIP‐immunoreactive nerve fibers has been demonstrated in bone and periosteum tissue in several mammalian species, including humans. 20 , 21 More recently, immunohistochemistry has shown that VIP is expressed in the subchondral bone, bone marrow cavities, and bone cells of patients with osteoarthritis. 19 According to its wide distribution, VIP exhibits pleiotropic effects in physiological and pathological conditions, which are mainly exerted through two specific G protein‐coupled receptors, VPAC1 and VPAC2. In fact, VIP has shown potent anti‐inflammatory and immunomodulatory properties in different inflammatory/autoimmune contexts. 22 In particular, findings from murine models of RA and human studies have evidenced a protective action of VIP on bone destruction, which is likely associated with a decreased RANKL/OPG ratio. 23 , 24 , 25 Furthermore, VIP has also demonstrated anti‐osteoclastogenic effects in animal models and in human cells, impairing both differentiation and resorption activity. 25 , 26 , 27 , 28 , 29 However, data on human osteoblasts are very scarce and limited to describing the presence of functional VIP receptors in human periosteum‐derived osteoblastic cells (SaM‐1) and human osteosarcoma‐derived cells (SaOS‐2, HOS, and MG‐63). 30 , 31 , 32 , 33
This study aims to address the research gap regarding the influence of VIP on the differentiation process of human MSCs to osteoblasts and on their anabolic function. We further analyze whether such potential osteogenic effects are accompanied by a modulation of the osteoblast regulatory activity on osteoclastogenesis. Overall, our results could represent a key first step in deciphering the involvement of VIP in the maintenance and restoration of human bone homeostasis, as it might play a pivotal role in coordinating osteoblast and osteoclast biology.
2. MATERIALS AND METHODS
2.1. Osteogenic differentiation cell culture
Bone marrow‐human mesenchymal stem cells (BM‐hMSC) from four healthy donors (two men and two women) belonging to different ethnicities and age ranges (30–50 years) were used. These cells were obtained commercially from StemCell Technologies (Catalog #70071) and are characterized for MSC specific markers and tested for their ability to differentiate in vitro into the different linages. Culture wells were previously treated with a commercial attachment solution to facilitate cell adherence. Then, BM‐hMSC were plated at 8 × 103 cells/cm2 in 24‐well plates and 8‐well chambers in MesenCult™‐ACF Plus medium (StemCell) supplemented with 1% penicillin/streptomycin (Cultek), 1% glutamax (Sigma‐Aldrich), and 0.2% of its specific commercial supplement. Once the cells reached 90% confluency, osteogenic induction was performed. MesenCult medium was exchanged for Osteogenic Differentiation medium (StemCell) supplemented with 1% penicillin/streptomycin, 1% glutamax and 20% of its specific commercial supplement, medium was changed every 3 days. At this point, 10−8 M VIP (Bachem A.G.) was added to some of the wells. As previously described, 34 differentiation cultures in the presence and absence of the neuropeptide were maintained over 14 days at 37°C in a humidified atmosphere containing 5% CO2.
The optimal dose of VIP for our study was selected based on a preliminary experiment using osteoblast differentiation cultures of an human telomerase reverse transcriptase (hTERT) immortalized adipose‐derived mesenchymal stem cell line. The effect of different concentrations of VIP (10−6, 10−7, and 10−8 M) on the gene expression of the osteogenic master regulator Runx2 was studied on Days 1, 4, and 7. Results indicated that 10−8 M was the most effective concentration of VIP for upregulating the mRNA levels of Runx2 (data not shown) and therefore the optimal concentration for studying the osteoinductive effects of VIP.
2.2. Real‐time polymerase chain reaction (PCR) analysis
Total RNA from both BM‐hMSC and cells under osteoblast differentiation conditions on Days 1, 4, 7, 10 and 14 was extracted using TriReagent (Sigma‐Aldrich). RNA quantity and purity were measured on NanoDrop®, and 2 μg were used for complementary deoxyribonucleic acid (cDNA) synthesis using a High‐Capacity cDNA Reverse Transcription Kit (Life Technologies).
Specific TaqMan probes for target genes (CTNNB1, RUNX2, COL1A1, TNFSF11, TNFRS11B, VIPR1, and VIPR2) and one house‐keeping gene (glyceraldehyde 3‐phosphate dehydrogenase (GAPDH)) with manufacturer‐predesigned primers were used along with TaqMan Gene Expression Master Mix (Applied Biosystem) to perform real‐time PCR analyses. For the VIP gene, transcript levels were analyzed by semi‐quantitative PCR with SYBR® Green PCR Master Mix (Promega) using GAPDH as the house‐keeping gene. The sequence of primers used and accession numbers of the genes analyzed with SYBR® technology are summarized in Table 1. Assays were made in triplicate, and results were normalized according to the expression levels of GAPDH.
TABLE 1.
Sequence of primers and accession numbers of the genes analyzed.
| Gene | GenBank accession no. | Sequence position | Primers | Sequence |
|---|---|---|---|---|
| VIP | NM_003381.4 | 98–209 | hVIP |
Forward: 5′‐TCAGCACCTAAGACAGCTCCA‐3′ Reverse: 5′‐AGCACACTGAGAAGAGTCAGG‐3′ |
| GAPDH | NM_002046.7 | 57–130 | hGAPDH |
Forward: 5′‐AGCCACATCGCTCAGACAC‐3′ Reverse: 5′‐GAGTTAAAAGCAGCCCTGGTG‐3′ |
2.3. Western blot analysis of VPAC receptors, β‐catenin and Runx2
Protein extracts were obtained in ice‐cold radioimmunoprecipitation assay buffer (Thermofisher). Protein extracts (25 μg for β‐catenin and Runx2 and 30 μg for VIP receptors) were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene difluoride membranes. After blocking, membranes were incubated overnight at 4°C with mouse monoclonal anti‐human β‐catenin (1:1000, Invitrogen), rabbit monoclonal anti‐human Runx2 (1:500, Invitrogen), rabbit polyclonal anti‐human VPAC1 (1:10000, Thermofisher), and mouse monoclonal anti‐human VPAC2 (1:1000, Abnova). Rabbit anti‐GAPDH (1:5000, Sigma‐Aldrich) was used as a loading control. Then, anti‐rabbit and anti‐mouse horseradish peroxidase (HRP)‐conjugated secondary antibodies (1:5000, ECL) were used, and proteins were detected using SuperSignal WestPico Chemiluminiscent System (Cytiva Amersham). Results were analyzed using the Bio‐Rad Quantity One program and normalized against GAPDH.
2.4. Determination of intracellular cyclic adenosine monophosphate (cAMP) concentrations
Levels of cAMP were determined by the HitHunter cAMP Assay for Small Molecules Kit (DiscoverX). On the seventh day of the osteogenic process, 40 × 103 cells were stimulated with 10−8 M VIP, 10−8 M VPAC1 agonist [Lys15Arg16Leu27VIP(1–7)‐GRF(8‐27)] (Bachem, Bubendorf, Switzerland) or 10−8 M VPAC2 agonist (RO 25‐1553; Bachem). After 30 min at 37°C, the reaction was terminated, and cells were lysed. Concentration of cAMP in the lysates was measured according to the manufacturer's instructions.
2.5. Measurement of vasoactive intestinal peptide levels in culture supernatants
Levels of VIP were assessed using a commercially available competitive enzyme‐linked immunosorbent assay (ELISA) kit (Phoenix Pharmaceuticals) according to the manufacturer's instructions. Supernatant samples were concentrated at 2:1 using the Eppendorf Concentrator Plus. Thanks to the establishment of a standard curve with known concentrations and its extrapolation, supernatant VIP levels were determined, applying the corresponding dilution factor. Each sample was assayed twice.
2.6. Evaluation of alkaline phosphatase concentration in culture supernatants
After 10 days of BM‐hMSC culture in osteogenic differentiation medium, evaluation of ALP levels in culture supernatants was assessed by means of a commercial kit (MAK447‐Sigma Aldrich) according to the manufacturer's instructions. The enzyme present in the sample hydrolyzes the p‐nitrophenyl phosphate of the kit's working reagent, resulting in a yellow‐colored reaction. The absorbance at 405 nm was measured at time 0 and after 4 min of said reaction. All generated data were included in the instruction's formula so that ALP units per liter of sample could be calculated.
2.7. Quantification of calcium deposits through alizarin red stain
On Day 10 of osteogenic differentiation cultures, the mineralizing activity of cells was assessed following a method similar to an already published protocol. 35 , 36 Cells were fixed and then stained with alizarin red (pH 4.2, Sigma) for 20 min in the dark at room temperature. After washing, only the calcium deposits produced by the cells were stained bright red. These results were photographed with a microscope coupled to a Nikon DS‐Ri2 camera. To quantify the amount of alizarin red bound to the mineralized matrix, the staining was solubilized in a 10% cetylpyridinium chloride (CPC) solution (pH 7, Sigma) for 30 min. The optical density (OD) values were measured at 562 nm and the results were interpolated in a standard line prepared in the range 0.03–2 mM with alizarin red solubilized in CPC.
2.8. Detection of F‐actin and vinculin by confocal fluorescence microscopy
Immunocytochemistry studies were performed with the Actin Cytoskeleton/Focal Adhesion Staining Kit (FAK100, Sigma‐Aldrich). BM‐hMSC were seeded into 8‐well chambers (Ibidi) and cultured for 4 days under osteogenic differentiation conditions. On Day 4, cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton/phosphate‐buffered saline (PBS) for 5 min. Once cells were blocked with 5% bovine serum albumin (BSA)/PBS for 30 min at room temperature (RT), the samples were incubated for 1 h at RT with mouse anti‐human vinculin (1:250) and TRITC‐conjugated phalloidin (for F‐actin labeling) (1:500). After washing, cells were incubated in darkness with Alexa Fluor 488 goat anti‐mouse (Thermofisher) (1:500) for 1 h at RT. Lastly, nuclei cells were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI) 1:1000. Fluorescence was examined using a Leica SP‐8 LIGHTNING confocal microscope (Leica DM IRE2; objective 40x; Leica Microsystems). Acquired images were processed by FIJI‐Image J software.
2.9. Statistical analysis
Data were analyzed using GraphPad Prism 8.0 software. Parametric variables were analyzed via t‐test and analysis of variance (ANOVA) together with Dunnett's post hoc test, while non‐parametric variables were analyzed using the Mann–Whitney U test. Two‐sided p‐values less than 0.05 were considered significant (*p < 0.05; **p < 0.01; ***p < 0.001). G*Power software was used to estimate the number of independent experiments required for statistical analysis.
3. RESULTS
3.1. Characterizing the VIP/receptor axis in human osteoblast differentiation in vitro
To determine the expression pattern of VIP and its receptors, VPAC1 and VPAC2, mRNA levels were analyzed by real‐time PCR in donor BM‐hMSCs and on Days 1, 4, 7, 10, and 14 of osteogenic differentiation cultures.
VIP gene expression was detected both in mesenchymal cells prior to osteogenic induction and in osteoblasts during the last stages of their differentiation, showing a reduction of VIP transcript levels on Day 7, which was reversed on Days 10 and 14 reaching values above those of the BM‐hMSCs (Figure 1A). VIP was also quantified by ELISA in the culture supernatants after 10 days of osteogenic induction, obtaining a concentration of 517.8 ± 9.4 pg/mL (Figure SS1).
FIGURE 1.
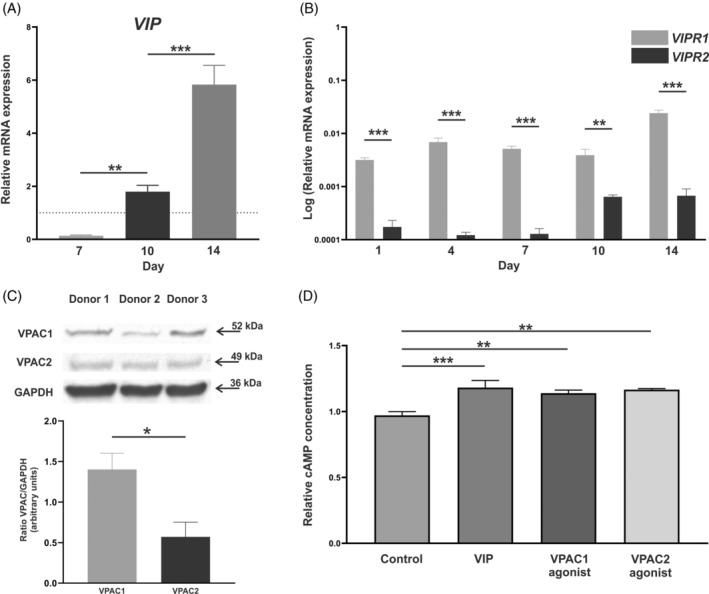
Characterization of the vasoactive intestinal peptide (VIP)/receptor axis during in vitro human osteoblast differentiation. VIP (A), VIPR1 (VPAC1), and VIPR2 (VPAC2) (B) mRNA expression levels in donor bone marrow‐human mesenchymal stem cells (BM‐hMSCs), before differentiation is initiated (dotted line) and after 1, 4, 7, 10, or 14 days of osteogenic induction was determined by real‐time PCR. Results are expressed as relative mRNA expression (relative to GAPDH levels) and, in the case of VIP, referenced to undifferentiated cells (BM‐hMSCs). The means ± standard error of the mean (SEM) of triplicate determinations of five independent experiments are shown. t Tests were performed (**p < 0.01; ***p < 0.001) (C) Protein detection of VIP receptors after 7 days of differentiation was determined by Western blot and GAPDH was used as a control. The intensity of the bands of VPAC1 and VPAC2 was quantified by densitometric analysis and normalized against the intensity of GAPDH. Results represent the means ± SEM of three independent experiments. t‐Test was performed (*p < 0.05). (D) Intracellular levels of cAMP in osteoblast differentiated for 7 days were quantified after stimulation with 10−8 M of VIP or agonist for either VPAC1 or VPAC2. cAMP concentration data are expressed relative to control cells. Means ± SEM of triplicate determinations of three independent experiments are shown, ANOVA was performed and Dunnett's test was applied as post hoc (**p < 0.01; ***p < 0.001).
VPAC1 and VPAC2 gene expression was observed at different time points during the 14 days of human osteoblast differentiation period in vitro. The results revealed a greater expression of VPAC1 compared to VPAC2 (p < 0.001 for Days 1, 4, 7, and 14 and p = 0.003 for Day 10) (Figure 1B). Moreover, this pattern was stable during the osteogenic process, showing no differences in the ratio VPAC1/VPAC2 (data not shown). To confirm the protein expression of VIP receptors in human osteoblasts, VPAC1 and VPAC2 were detected by Western blot and quantified (Figure 1C), confirming the results obtained at the RNA level.
Having characterized the expression of both receptors and knowing that adenylate cyclase activation is the main pathway mediating the effects of VIP, the functionality of VPAC1 and VPAC2 was studied through cAMP quantification. Stimulation with 10−8 M VIP, VPAC1 agonist, or VPAC2 agonist significantly increased intracellular levels of cAMP (p < 0.001, p = 0.001, and p = 0.001, respectively) in osteoblast differentiated for 7 days (Figure 1D).
3.2. VIP upregulates the expression of β‐catenin and Runx2
We next elucidated whether the presence of VIP during in vitro differentiation modulates the expression of two master regulators of this process: β‐catenin (CTNNB1) and Runx2 (RUNX2).
Relative gene expression was analyzed by real‐time PCR in BM‐hMSCs and after 1, 4, and 7 days of osteogenic induction in the presence or absence of VIP 10−8 M. Both genes showed higher expression when the differentiation was performed in the presence of VIP, with significant differences at Day 4 for CTNNB1 (p = 0.0093) (Figure 2A) and at Day 7 for RUNX2 (p < 0.001) (Figure 2B).
FIGURE 2.
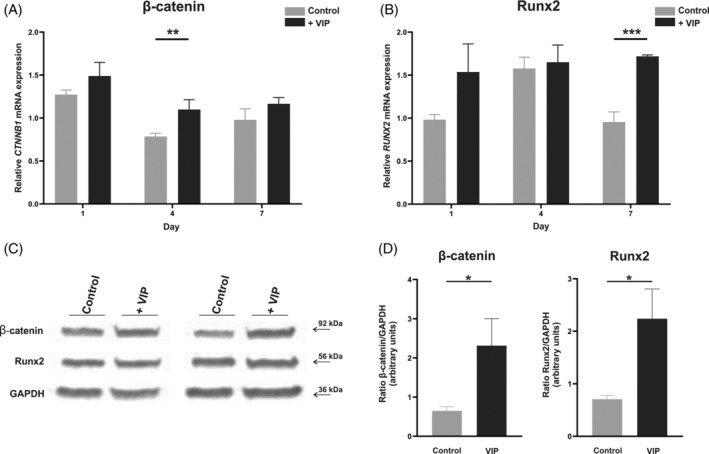
Vasoactive intestinal peptide (VIP) upregulates the expression of β‐catenin and Runx2. CTNNB1 (β‐catenin) (A) and RUNX2 (Runx2) (B) mRNA expression levels after 1, 4, or 7 days of bone marrow‐human mesenchymal stem cells (BM‐hMSCs) osteogenic induction in absence or presence of VIP 10−8 M was determined by real‐time PCR. Results are expressed as relative mRNA expression (relative to GAPDH levels) and referenced to undifferentiated cells (BM‐hMSCs). The means ± SEM of triplicate determinations of four independent experiments are shown. β‐catenin and Runx2 protein quantification at Day 7 of differentiation was determined by Western blot (C). Picture is a representative example of six experiments with similar results. (D) Relative expression levels of protein bands were calculated with the densitometry values of the band corresponding to the protein of interest relative to GAPDH and referenced to basal levels. Mann–Whitney U was performed (*p < 0.05; **p < 0.01; ***p < 0.001).
Protein levels of β‐catenin and Runx2 were also quantified by Western blot after 7 days of culture of Human bone marrow mesenchymal stem cell (hBM‐MSCs) with osteogenic medium in the presence or absence of VIP 10−8 M. The results confirmed the mRNA data, showing a significant increase of β‐catenin (p = 0.030) and Runx2 (p = 0.032) in those cells differentiated in the presence of VIP (Figure 2C,D).
3.3. VIP enhances type I collagen expression and cytoskeletal changes associated with osteoblast differentiation
To explore the influence of VIP on the osteogenic process, we next characterized the genetic expression profile of type I collagen (COL1A1), an early gene marker of osteoblast differentiation, by real‐time PCR at Days 1, 4, 7, and 14 of BM‐hMSCs cultured under osteogenic induction in the presence or absence of VIP 10−8 M. A similar pattern of COL1A1 gene expression was observed throughout osteogenesis in both conditions. However, cells maintained in differentiation conditions in the presence of VIP reached the maximum expression earlier, which also exceeded that of cells cultured under basal conditions (p = 0.009) (Figure 3A).
FIGURE 3.
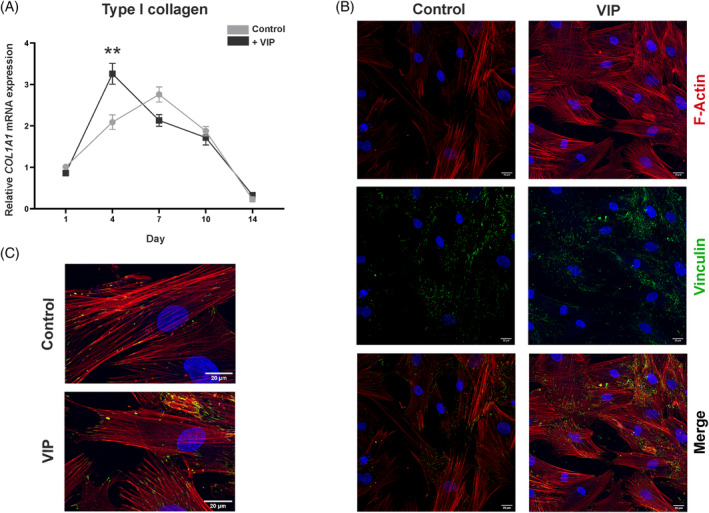
Vasoactive intestinal peptide (VIP) promotes type I collagen gene expression and cytoskeletal reorganization. (A) Type I collagen gene (COL1A1) expression after 1, 4, 7, 10, or 14 days of osteogenic induction in absence or presence of VIP 10−8 M was determined by real‐time PCR. Results are expressed as relative mRNA expression (relative to GAPDH levels) and referenced to undifferentiated cells (bone marrow‐human mesenchymal stem cells [BM‐hMSCs]). The means ± SEM of triplicate determinations of five independent experiments are shown, Mann–Whitney U was performed (**p < 0.01). (B) Photomicrographs of the effect of VIP on the actin cytoskeleton and focal adhesions in differentiating osteoblasts (Leica SP8 Lightning confocal microscope, Scale bar: 20 μm). (C) Magnified image of a representative cell of the cytoskeleton morphology in mesenchymal stem cell under basal osteogenic conditions and with the presence of VIP. Detection of F‐actin was performed using Phalloidin staining (TRITC, Red), specific primary and secondary antibodies were used for the detection of vinculin (Alexa Fluor 488, green), nuclei were counterstained with DAPI.
To elucidate the effect of VIP on cytoskeletal reorganization associated with MSCs osteogenic differentiation, immunofluorescence staining of both F‐actin and vinculin of focal adhesions was performed on Day 4 of the differentiation cultures in presence or absence of VIP. Qualitative observation of the confocal images obtained (Figure 3B) suggested differences in the thickness of actin filaments and in the pattern of focal adhesions. Under basal osteoinduction conditions, spindle‐shaped cells exhibited parallel thick actin bundles traversing their entire length whereas in the presence of VIP, cells showed a network of thinner actin filaments and a more cuboidal morphology accompanied by a change in the distribution of focal adhesions (Figure 3B,C).
3.4. The presence of VIP increases osteoblast bone‐forming activity
After studying the stimulatory effect of VIP on the expression of different osteogenic markers both at the genetic and protein level, its action on the bone‐forming activity of osteoblasts was analyzed. ALP in cell culture supernatants and calcium deposits were quantified after 10 days of osteoblast differentiation cultures in the presence or absence of the neuropeptide at a concentration of 10−8 M. Results revealed a significant increase in both ALP secretion (p = 0.0281) (Figure 4A) and calcium deposition (p = 0.009) when osteogenic induction occurred in the presence of VIP (Figure 4B,C).
FIGURE 4.
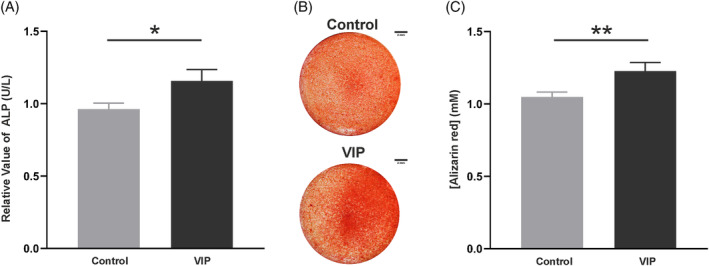
Osteoblast anabolic activity is promoted by vasoactive intestinal peptide (VIP). (A) Alkaline phosphatase (ALP) present in the supernatant was determined after 10 days of bone marrow‐human mesenchymal stem cells culture under osteogenic induction in presence or absence of VIP 10−8 M. The results shown represent the mean ± SEM of the relative concentration of ALP referred to the basal condition obtained in five independent experiments. (B) Photomicrographs of calcium deposits after stained with alizarin red, in the wells of cells subjected to both experimental conditions (scale bar: 2 mm). (C) Quantification of alizarin red staining using spectrophotometry. Data are presented as the means ± SEM of duplicate determinations of six independent experiments referred to control condition. t‐Test was performed (*p < 0.05; **p < 0.01).
3.5. VIP is involved in the crosstalk osteoblast–osteoclast
Finally, to explore the potential osteoprotective mechanism that VIP might mediate, we next analyzed by real‐time PCR the gene expression of RANKL (TNFSF11) and OPG (TNFRSF11B) from in vitro differentiated human osteoblasts after 14 days of osteogenic induction of BM‐hMSCs with or without the addition of VIP 10−8 M. Results showed that although OPG gene expression was not modulated by VIP (Figure 5B), there was a significant decrease in the levels of RANKL transcripts (p < 0.001) (Figure 5A). Therefore, a significant decrease in the RANKL/OPG ratio was obtained (p = 0.018) when the osteogenic process occurred in the presence of VIP (Figure 5C).
FIGURE 5.
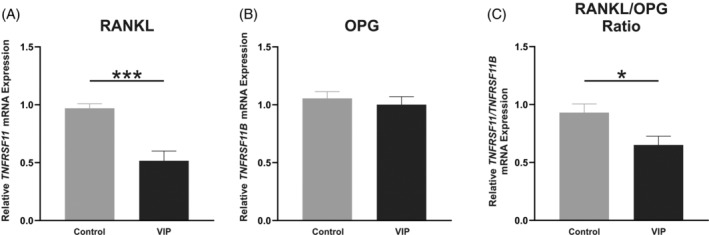
Vasoactive intestinal peptide (VIP) modulates gene expression of mediator molecules in osteoclast–osteoblast communication. Relative mRNA expression of (A) TNFSF11 (RANKL) and (B) TNFRSF11B (OPG) after 14 days of osteoblast differentiation in absence or presence of VIP 10−8 M was determined by real‐time PCR. (C) Receptor activator of nuclear factor‐κB ligand/osteoprotegerin (RANKL/OPG) ratio of gene expression values. Results are expressed as relative mRNA expression (relative to GAPDH levels) and referenced to the control group. The means ± SEM of triplicate determinations of four independent experiments are shown, t test was performed (*p < 0.05; ***p < 0.001).
4. DISCUSSION
An array of systemic factors and mediators produced within the bone microenvironment regulate the fine‐tuning between osteoblast and osteoclast functions, which is necessary for preserving homeostatic bone remodeling. 12 , 13 , 37 , 38 Among them, the neuropeptide VIP has been demonstrated to exhibit anti‐osteoclastogenic activity in both murine and human studies. 23 , 24 , 25 , 26 , 27 , 28 , 29 However, comprehensive knowledge about VIP regulating role in human osteoblast differentiation and anabolic activity remains unknown. In the present study, we have reported, for the first time, the stimulatory influence of VIP on BM‐hMSCs osteogenic differentiation and osteoblast synthetic function. Results revealed that the presence of VIP during in vitro osteoinduction of human BM‐MSCs enhances the expression of osteoblast differentiation‐stage‐specific markers and favors the characteristic reorganization of both actin cytoskeleton and focal adhesion complexes. We further found that such pro‐osteoblastogenic effects are accompanied by an increase in matrix mineralization and an attenuation of osteoblast‐mediated osteoclastogenesis. The findings of this study would confirm in humans the osteoprotective effects of VIP previously demonstrated in animal models, suggesting a promising pivotal role for this neuropeptide in the maintenance of homeostatic human bone metabolism.
According to the neuro‐osteological hypothesis, neuropeptides released by sympathetic fibers innervating bone tissues can exert paracrine effects on cells expressing their receptors in the vicinity of nerve endings. 28 Since Hohman et al. demonstrated in 1986 the existence of VIP‐immunoreactive nerve fibers in rat bone tissue, 20 further findings have confirmed their presence in the rat periosteum, epiphysis, and bone marrow cavity. 39 , 40 More recent studies in human subchondral bone have shown, by immunochemistry, that VIP is expressed not only along the surface of trabecular bone and in bone marrow cavities but also in osteocyte‐like cells. 19 , 41 In line with this, our present data revealed that in vitro differentiated human osteoblasts express the neuropeptide VIP, both at the genetic and protein level. These results appear to be in disagreement with those previously published by Togari et al. showing no expression of VIP transcripts in human periosteum‐derived osteoblastic cells (SaM‐1) and in human osteosarcoma‐derived cells (SaOS‐2, HOS, and MG‐63). 32 However, this discrepancy could be derived from differences in experimental conditions since the cell model studied and the mRNA detection methods that were used are different. Given that we further detected protein levels of VIP in osteoblast supernatants, it is worth speculating that osteoblast lineage cells, including osteoblasts and osteocytes, might represent an additional source of VIP in the bone environment that would allow higher local levels of this neuropeptide.
Regarding VIP receptors, although several studies have shown that mouse calvarial osteoblasts and murine osteosarcoma cell lines are provided with functional VPAC1 and VPAC2 receptors, 31 , 33 , 42 data on human cells are limited to a single report by Togari et al. analyzing their mRNA expression in different human osteosarcoma cell lines and in the human osteoblast SaM‐1 cell line. 32 Therefore, our results show for the first time the expression profile of VIP receptors during the time course of human MSCs osteogenic differentiation. These findings evidence the presence of VPAC1 and VPAC2 receptors in BM‐hMSCs and in mature osteoblasts differentiated in vitro, showing a progressive induction of both transcripts throughout the differentiation process with a constant ratio between their mRNA levels in favor of VPAC1 receptor. While these results are not consistent with the exclusive expression of VPAC1 previously described by Togari et al. in human osteoblast cell lines, 32 it is worthy to note that VPAC1 transcripts have also been shown to be induced during osteogenic differentiation of mouse calvarial osteoblasts, while VPAC2 is constitutively expressed. 33 Nevertheless, discrepancies in the pattern of receptor subtypes observed between these reports are not mutually incompatible, since different cell types, stage of differentiation and species were studied using distinct methods of analysis. Moreover, concerning the signaling pathways elicited by VIP, our data revealed that VPAC receptors are linked to cAMP as an intracellular mediator in human osteoblasts differentiated in vitro, in line with the VIP‐induced adenylate cyclase activation previously described in mouse calvarial osteoblasts, osteosarcoma cell lines, and in human osteoblast‐like cells derived from explants of trabecular bone. 31 , 42 , 43 , 44
Differentiation of MSCs toward the osteoblastic phenotype entails several stages, including the synthesis of the osteoid matrix, changes in cell morphology and cytoskeleton, as well as osteoid mineralization. 4 , 5 On a molecular level, Runx2 is identified as the master transcription factor driving the primary commitment of mesenchymal progenitors to osteoblast lineage, being upstream regulated by the Wnt pathway via the stabilization of cytoplasmic β‐catenin. 7 , 45 , 46 , 47 Our study of β‐catenin and Runx2 levels in BM‐hMSCs cultured under osteoinduction conditions demonstrates an increase in gene and protein expression of both osteogenic factors in the presence of VIP. This is consistent with the recently demonstrated promoting effect of this neuropeptide on both the Wnt/β‐catenin signaling pathway and the expression of the downstream RUNX2 gene, in turn mediating VIP‐enhancing action on the differentiation of rat bone marrow MSCs to osteoblasts. 48
Besides, when we examined the effect of VIP presence on the expression of type I collagen, the main osteoid component and an early marker of osteogenesis, a maximum in its transcript levels was reached at an earlier time point during BM‐hMSCs osteogenic differentiation in vitro, exceeding those measured under basal conditions. This expression profile reflects an increased initial bone matrix formation in the presence of the neuropeptide, in agreement with previous findings in rat osteoblasts differentiated in vitro 48 and as expected based on our results, given that the COL1A1 gene is a direct target of the VIP upregulated Runx2 transcription factor. 49 Although further immunocytochemical quantitative studies are needed to characterize more precisely the modulatory action of VIP on the cytoskeleton organization during MSCs osteogenic differentiation, we described differences in both F‐actin and vinculin distribution based on qualitative visual analysis of confocal immunofluorescence microscopy images. Cells cultured in the presence of VIP exhibited a thinner actin network distributed throughout the cytoplasm, resembling the thin and dense microfilament meshwork filling the interior of terminally differentiated osteoblasts. Changes in their morphology and in the distribution of vinculin present in focal adhesions were also evident. These cells displayed a larger spreading area and a shape resembling polyhedral cells, characteristic of later stages in osteoblast maturation. Given that several studies have reported that changes in cell morphology and actin cytoskeleton are important early events during osteogenesis of MSCs 8 , 11 , 50 , 51 , 52 and that reorganization of focal adhesions is necessary for osteoblast attachment and subsequently for extracellular matrix synthesis, 10 , 11 our results suggest that VIP promotes BM‐hMSCs osteogenesis and anabolic activity of osteoblasts by favoring the rearrangement of the cytoskeleton. Interestingly, modulation of intracellular cAMP concentrations by G protein‐coupled receptor (GPCR) ‐coupled mechanisms has been demonstrated to play a regulatory role in actin cytoskeleton reorganization 53 and bone formation, 54 , 55 which would be in accordance with the reported upregulation of cAMP levels upon stimulation of VPAC receptors in differentiating osteoblasts. Therefore, VIP emerges as a component of the network of mediators regulating human osteoblastic differentiation through the modulation of cytoskeletal structures, possibly using cAMP as an intracellular messenger.
In the present study, we also found a stimulatory effect of the presence of VIP on ALP production and calcium deposits in osteoblastic differentiation cultures of BM‐hMSCs, evidencing an increase in active bone formation and in the mineralizing capacity that supports the ability of the neuropeptide to promote osteogenic functions. This is consistent with a previous study in osteogenically induced rat MSCs, where an increased ALP activity and mineralized nodules formation were observed under VIP treatment. 48 Moreover, in agreement with our data showing that VPAC receptors on human osteoblasts are linked to cAMP formation, other authors have demonstrated that this second messenger acts as a signal transducing mechanism mediating VIP‐induced enhancement of ALP expression in human osteoblast cell lines as well as in primary cultures of mouse calvarial osteoblasts. 44 , 56
Finally, given the important role played by osteoblasts in the regulation of osteoclast biology through the production of RANKL and OPG, and considering that a decrease in the RANKL/OPG ratio has been linked to the VIP osteoprotective effects observed in RA animal models and patients, 23 , 24 , 25 we hypothesized that this neuropeptide may exert a regulatory effect on osteoblast‐mediated osteoclastogenesis by modulating the expression of such cytokines. Our data revealed a significant decrease in RANKL gene expression when osteoblasts were differentiated in the presence of VIP, leading to a consequent reduction of the RANKL/OPG ratio. Furthermore, this is in line with previous data showing the ability of VIP to decrease the 1,25(OH)2‐vitamin D3‐stimulated expression of RANKL in mouse bone marrow cultures. 27 Therefore, the present results suggest that, in addition to our previous demonstration of the direct inhibitory action of VIP on human osteoclasts, 25 this neuropeptide also exerts an indirect anti‐osteoclastogenic effect by regulating osteoblast factors controlling osteoclast biology.
To summarize, although further studies are needed to complete the whole mapping of the VIP/VPAC system in human osteoblast lineage cells, our present in vitro results suggest the existence of an autocrine signaling pathway for VIP in these cells, contributing to the neuro‐osteogenic network involved in the regulation of human bone metabolism. The present findings also support the potential of VIP as an osteoinductive differentiation factor that promotes human MSCs osteogenesis by enhancing the expression of early osteoblast markers and promoting bone matrix formation as well as proper cytoskeletal reorganization. Finally, our data indicate that VIP could be playing a direct modulatory role on the osteoblast to osteoclast signaling by regulating RANKL/OPG ratio, thus emerging as a pivotal molecule in the maintenance of human bone homeostasis. Therefore, while additional studies are needed to evaluate and optimize the suggested osteoinductive effects of VIP, our results represent an initial and relevant step in the exploration of new therapeutic targets for a plethora of musculoskeletal diseases characterized by the disruption of bone remodeling balance.
AUTHOR CONTRIBUTIONS
Conceptualization, MC; methodology, DC‐V, PA‐C, and IG‐L; validation MC; investigation, DC‐V and PA‐C; resources, MC and RPG; data curation, DC‐V, PA‐C, and IG‐L; writing—original draft preparation, MC, DC‐V, and PA‐C; writing—review and editing, DC‐V, PA‐C, IG‐L, IG‐C, SP‐G, AL, RV‐R, AC‐M, KT, CM, YJ, RPG and MC; visualization, DC‐V, PA‐C, and IG‐L; supervision, MC, YJ, and RPG; project administration YJ; funding acquisition, YJ and CM. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This research was funded by grants RD21/0002/004 and PI20/00078 from the Ministerio de Economía y Competitividad (Instituto de Salud Carlos III) and co‐funded by European regional development fund (ERDF) “A way to make Europe.”
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Supporting information
Figure S1. VIP levels at Day 10 of osteogenic differentiation were determined by ELISA in cell culture supernatants. Data are presented as mean ± SEM for each donor.
ACKNOWLEDGMENTS
We would like to thank the Genomics and Fluorescence Microscopy Centres of Complutense University for the use of their facilities. We are also grateful to Mariano R. Rodríguez‐Sosa for his assistance with osteoblast differentiation techniques.
Castro‐Vázquez D, Arribas‐Castaño P, García‐López I, Gutiérrez‐Cañas I, Pérez‐García S, Lamana A, et al. Vasoactive intestinal peptide exerts an osteoinductive effect in human mesenchymal stem cells. BioFactors. 2024;50(6):1148–1160. 10.1002/biof.2062
David Castro‐Vázquez and Paula Arribas‐Castaño contributed equally to this study.
Yasmina Juarranz and Mar Carrión jointly supervised this work.
DATA AVAILABILITY STATEMENT
Data and further details regarding the manuscript will be made available by request to the corresponding author.
REFERENCES
- 1. Bolamperti S, Villa I, Rubinacci A. Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 2022;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakthiswary R, Uma Veshaaliini R, Chin KY, Das S, Sirasanagandla SR. Pathomechanisms of bone loss in rheumatoid arthritis. Front Med. 2022;9:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song S, Guo Y, Yang Y, Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. 2022;237:108–168. [DOI] [PubMed] [Google Scholar]
- 4. Rutkovskiy A, Stenslokken KO, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res. 2016;22:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ponzetti M, Rucci N. Osteoblast differentiation and signaling: established concepts and emerging topics. Int J Mol Sci. 2021;22(13):6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Florencio‐Silva R, Sasso GR, Sasso‐Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015;2015:421–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells. 2013;5:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bays JL, DeMali KA. Vinculin in cell‐cell and cell‐matrix adhesions. Cell Mol Life Sci. 2017;74:2999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao Y, Sun Q, Huo B. Focal adhesion regulates osteogenic differentiation of mesenchymal stem cells and osteoblasts. Biomater Transl. 2021;2:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu MC, Yu HW, Chen YQ, Ou MH, Serrano R, Huang GL, et al. Early committed polarization of intracellular tension in response to cell shape determines the osteogenic differentiation of mesenchymal stromal cells. Acta Biomater. 2023;163:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakahama K. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010;67:4001–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast‐osteoclast communication and bone homeostasis. Cells. 2020;9:2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. [DOI] [PubMed] [Google Scholar]
- 15. Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia‐Castellano JM, Diaz‐Herrera P, Morcuende JA. Is bone a target‐tissue for the nervous system? New advances on the understanding of their interactions. Iowa Orthop J. 2000;20:49–58. [PMC free article] [PubMed] [Google Scholar]
- 17. Lerner UH, Persson E. Osteotropic effects by the neuropeptides calcitonin gene‐related peptide, substance P and vasoactive intestinal peptide. J Musculoskelet Neuronal Interact. 2008;8:154–165. [PubMed] [Google Scholar]
- 18. Igwe JC, Jiang X, Paic F, Ma L, Adams DJ, Baldock PA, et al. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J Cell Biochem. 2009;108:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanemitsu M, Nakasa T, Shirakawa Y, Ishikawa M, Miyaki S, Adachi N. Role of vasoactive intestinal peptide in the progression of osteoarthritis through bone sclerosis and angiogenesis in subchondral bone. J Orthop Sci. 2020;25:897–906. [DOI] [PubMed] [Google Scholar]
- 20. Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide‐containing nerve fibers. Science. 1986;232:868–871. [DOI] [PubMed] [Google Scholar]
- 21. Lerner UH. Neuropeptidergic regulation of bone resorption and bone formation. J Musculoskelet Neuronal Interact. 2002;2:440–447. [PubMed] [Google Scholar]
- 22. Martinez C, Juarranz Y, Gutierrez‐Cañas I, Carrion M, Perez‐Garcia S, Villanueva‐Romero R, et al. A clinical approach for the use of VIP axis in inflammatory and autoimmune diseases. Int J Mol Sci. 2019;21(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juarranz Y, Abad C, Martinez C, Arranz A, Gutierrez‐Cañas I, Rosignoli F, et al. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen‐induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2005;7:1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muschter D, Schafer N, Stangl H, Straub RH, Grassel S. Sympathetic neurotransmitters modulate osteoclastogenesis and osteoclast activity in the context of collagen‐induced arthritis. PLoS One. 2015;10:139–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castro‐Vazquez D, Lamana A, Arribas‐Castaño P, Gutierrez‐Cañas I, Villanueva‐Romero R, Perez‐Garcia S, et al. The neuropeptide VIP limits human Osteoclastogenesis: clinical associations with bone metabolism markers in patients with early arthritis. Biomedicine. 2021;9:1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lundberg P, Lie A, Bjurholm A, Lehenkari PP, Horton MA, Lerner UH, et al. Vasoactive intestinal peptide regulates osteoclast activity via specific binding sites on both osteoclasts and osteoblasts. Bone. 2000;27:803–810. [DOI] [PubMed] [Google Scholar]
- 27. Mukohyama H, Ransjo M, Taniguchi H, Ohyama T, Lerner UH. The inhibitory effects of vasoactive intestinal peptide and pituitary adenylate cyclase‐activating polypeptide on osteoclast formation are associated with upregulation of osteoprotegerin and downregulation of RANKL and RANK. Biochem Biophys Res Commun. 2000;271:158–163. [DOI] [PubMed] [Google Scholar]
- 28. Lundberg P, Lerner UH. Expression and regulatory role of receptors for vasoactive intestinal peptide in bone cells. Microsc Res Tech. 2002;58:98–103. [DOI] [PubMed] [Google Scholar]
- 29. Qu H, Zhuang Y, Zhu L, Zhao Z, Wang K. The effects of vasoactive intestinal peptide on RANKL‐induced osteoclast formation. Ann Transl Med. 2021;9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hohmann EL, Tashjian AH Jr. Functional receptors for vasoactive intestinal peptide on human osteosarcoma cells. Endocrinology. 1984;114:1321–1327. [DOI] [PubMed] [Google Scholar]
- 31. Bjurholm A, Kreicbergs A, Schultzberg M, Lerner UH. Neuroendocrine regulation of cyclic AMP formation in osteoblastic cell lines (UMR‐106‐01, ROS 17/2.8, MC3T3‐E1, and Saos‐2) and primary bone cells. J Bone Miner Res. 1992;7:1011–1019. [DOI] [PubMed] [Google Scholar]
- 32. Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T. Expression of mRNAs for neuropeptide receptors and beta‐adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett. 1997;233:125–128. [DOI] [PubMed] [Google Scholar]
- 33. Lundberg P, Lundgren I, Mukohyama H, Lehenkari PP, Horton MA, Lerner UH. Vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase‐activating peptide receptor subtypes in mouse calvarial osteoblasts: presence of VIP‐2 receptors and differentiation‐induced expression of VIP‐1 receptors. Endocrinology. 2001;142:339–347. [DOI] [PubMed] [Google Scholar]
- 34. Ciuffreda MC, Malpasso G, Musarò P, Turco V, Gnecchi M. Protocols for in vitro differentiation of human mesenchymal stem cells into osteogenic, chondrogenic and adipogenic lineages. In: Gnecchi M, editor. Mesenchymal stem cells: methods and protocols. New York: Springer; 2016. p. 149–158. [DOI] [PubMed] [Google Scholar]
- 35. Berberi A, Al‐Nemer F, Hamade E, Noujeim Z, Badran B, Zibara K. Mesenchymal stem cells with osteogenic potential in human maxillary sinus membrane: an in vitro study. Clin Oral Investig. 2017;21:1599–1609. [DOI] [PubMed] [Google Scholar]
- 36. Bou Assaf R, Fayyad‐Kazan M, Al‐Nemer F, Makki R, Fayyad‐Kazan H, Badran B, et al. Evaluation of the osteogenic potential of different scaffolds embedded with human stem cells originated from Schneiderian membrane: an in vitro study. Biomed Res Int. 2019;2019:2868673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldring SR, Goldring MB. Cytokines and skeletal physiology. Clin Orthop Relat Res. 1996;324:13–23. [DOI] [PubMed] [Google Scholar]
- 38. Konttinen Y, Imai S, Suda A. Neuropeptides and the puzzle of bone remodeling. State of the art. Acta Orthop Scand. 1996;67:632–639. [DOI] [PubMed] [Google Scholar]
- 39. Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M. Neuropeptide Y‐, tyrosine hydroxylase‐ and vasoactive intestinal polypeptide‐immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988;25:119–125. [DOI] [PubMed] [Google Scholar]
- 40. Hill EL, Elde R. Distribution of CGRP‐, VIP‐, D beta H‐, SP‐, and NPY‐immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–480. [DOI] [PubMed] [Google Scholar]
- 41. Xiao J, Yu W, Wang X, Wang B, Chen J, Liu Y, et al. Correlation between neuropeptide distribution, cancellous bone microstructure and joint pain in postmenopausal women with osteoarthritis and osteoporosis. Neuropeptides. 2016;56:97–104. [DOI] [PubMed] [Google Scholar]
- 42. Hohmann EL, Levine L, Tashjian AH Jr. Vasoactive intestinal peptide stimulates bone resorption via a cyclic adenosine 3′,5′‐monophosphate‐dependent mechanism. Endocrinology. 1983;112:1233–1239. [DOI] [PubMed] [Google Scholar]
- 43. Rahman S, Dobson PR, Bunning RA, Russell RG, Brown BL. The regulation of connective tissue metabolism by vasoactive intestinal polypeptide. Regul Pept. 1992;37:111–121. [DOI] [PubMed] [Google Scholar]
- 44. Lundberg P, Bostrom I, Mukohyama H, Bjurholm A, Smans K, Lerner UH. Neuro‐hormonal control of bone metabolism: vasoactive intestinal peptide stimulates alkaline phosphatase activity and mRNA expression in mouse calvarial osteoblasts as well as calcium accumulation mineralized bone nodules. Regul Pept. 1999;85:47–58. [DOI] [PubMed] [Google Scholar]
- 45. Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. [DOI] [PubMed] [Google Scholar]
- 46. Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12:247–253. [DOI] [PubMed] [Google Scholar]
- 47. Komori T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int J Mol Sci. 2019;20:1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shi L, Feng L, Zhu ML, Yang ZM, Wu TY, Xu J, et al. Vasoactive intestinal peptide stimulates bone marrow‐mesenchymal stem cells osteogenesis differentiation by activating Wnt/beta‐catenin signaling pathway and promotes rat skull defect repair. Stem Cells Dev. 2020;29:655–666. [DOI] [PubMed] [Google Scholar]
- 49. Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. [DOI] [PubMed] [Google Scholar]
- 50. Sonowal H, Kumar A, Bhattacharyya J, Gogoi PK, Jaganathan BG. Inhibition of actin polymerization decreases osteogeneic differentiation of mesenchymal stem cells through p38 MAPK pathway. J Biomed Sci. 2013;20:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suzuki H, Tatei K, Ohshima N, Sato S, Izumi T. Regulation of MC3T3‐E1 differentiation by actin cytoskeleton through lipid mediators reflecting the cell differentiation stage. Biochem Biophys Res Commun. 2019;514:393–400. [DOI] [PubMed] [Google Scholar]
- 52. Khan AU, Qu R, Fan T, Ouyang J, Dai J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res Ther. 2020;11:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen K, Obinata H, Izumi T. Detection of G protein‐coupled receptor‐mediated cellular response involved in cytoskeletal rearrangement using surface plasmon resonance. Biosens Bioelectron. 2010;25:1675–1680. [DOI] [PubMed] [Google Scholar]
- 54. Romanello M, Moro L, Pirulli D, Crovella S, D'Andrea P. Effects of cAMP on intercellular coupling and osteoblast differentiation. Biochem Biophys Res Commun. 2001;282:1138–1144. [DOI] [PubMed] [Google Scholar]
- 55. Luo J, Zhou W, Zhou X, Li D, Weng J, Yi Z, et al. Regulation of bone formation and remodeling by G‐protein‐coupled receptor 48. Development. 2009;136:2747–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ma W, Zhang X, Shi S, Zhang Y. Neuropeptides stimulate human osteoblast activity and promote gap junctional intercellular communication. Neuropeptides. 2013;47:179–186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. VIP levels at Day 10 of osteogenic differentiation were determined by ELISA in cell culture supernatants. Data are presented as mean ± SEM for each donor.
Data Availability Statement
Data and further details regarding the manuscript will be made available by request to the corresponding author.


