Abstract
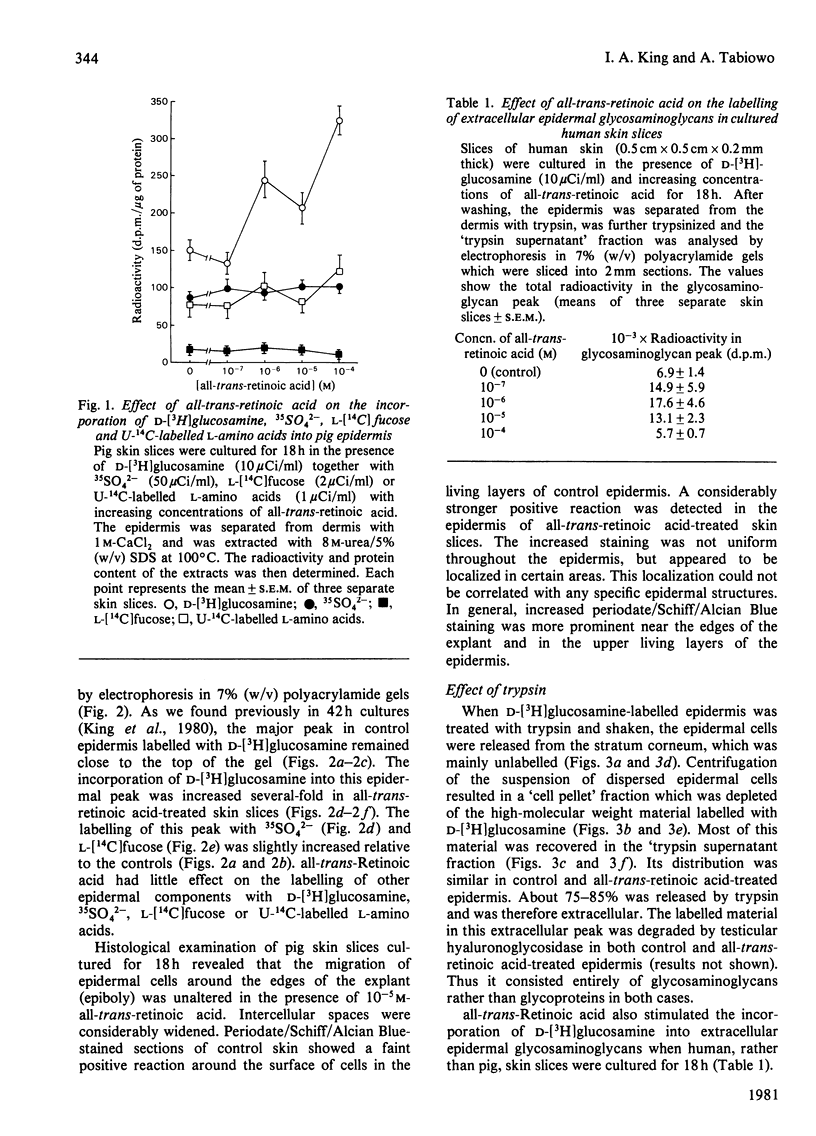
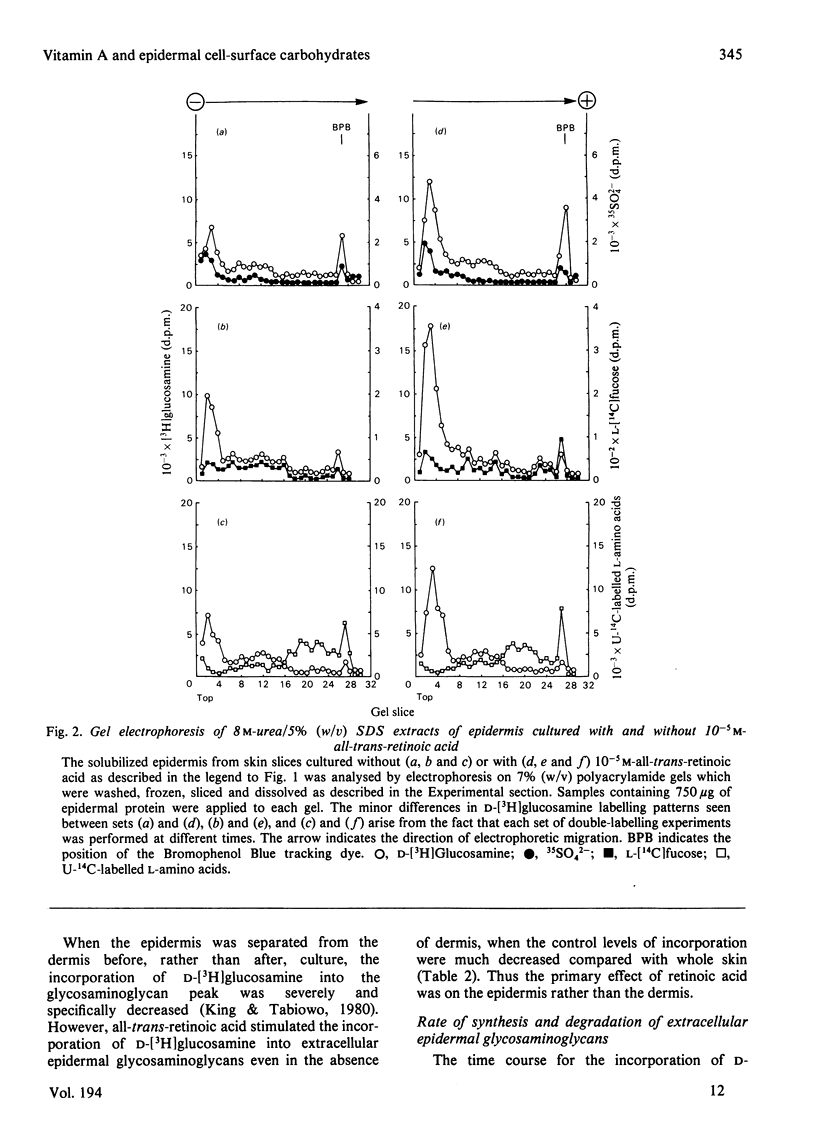
1. all-trans-Retinoic acid at concentrations greater than 10−7m stimulated the incorporation of d-[3H]glucosamine into 8m-urea/5% (w/v) sodium dodecyl sulphate extracts of 1m-CaCl2-separated epidermis from pig ear skin slices cultured for 18h. The incorporation of 35SO42−, l-[14C]fucose and U-14C-labelled l-amino acids was not significantly affected. 2. Electrophoresis of the solubilized epidermis showed increased incorporation of d-[3H]glucosamine into a high-molecular-weight glycosaminoglycan-containing peak when skin slices were cultured in the presence of 10−5m-all-trans-retinoic acid. The labelling of other epidermal components with d-[3H]glucosamine, 35SO42−, l-[14C]fucose and U-14C-labelled l-amino acids was not significantly affected by 10−5m-all-trans-retinoic acid. 3. Trypsinization dispersed the epidermal cells and released 75–85% of the total d-[3H]glucosamine-labelled material in the glycosaminoglycan peak. Thus most of this material was extracellular in both control and 10−5m-all-trans-retinoic acid-treated epidermis. 4. Increased labelling of extracellular epidermal glycosaminoglycans was also observed when human skin slices were treated with all-trans-retinoic acid, indicating a similar mechanism in both tissues. Increased labelling was also found when the epidermis was cultured in the absence of the dermis, suggesting a direct effect of all-trans-retinoic acid on the epidermis. 5. Increased incorporation of d-[3H]-glucosamine into extracellular epidermal glycosaminoglycans in 10−5m-all-trans-retinoic acid-treated skin slices was apparent after 4–8h in culture and continued up to 48h. all-trans-Retinoic acid (10−5m) did not affect the rate of degradation of this material in cultures `chased' with 5mm-unlabelled glucosamine after 4 or 18h. 6. Cellulose acetate electrophoresis at pH7.2 revealed that hyaluronic acid was the major labelled glycosaminoglycan (80–90%) in both control and 10−5m-all-trans-retinoic acid-treated epidermis. 7. The labelling of epidermal plasma membranes isolated from d-[3H]glucosamine-labelled skin slices by sucrose density gradient centrifugation was similar in control and 10−5m-all-trans-retinoic acid-treated tissue. 8. The results indicate that increased synthesis of mainly extracellular glycosaminoglycans (largely hyaluronic acid) may be the first response of the epidermis to excess all-trans-retinoic acid.
Full text
PDF


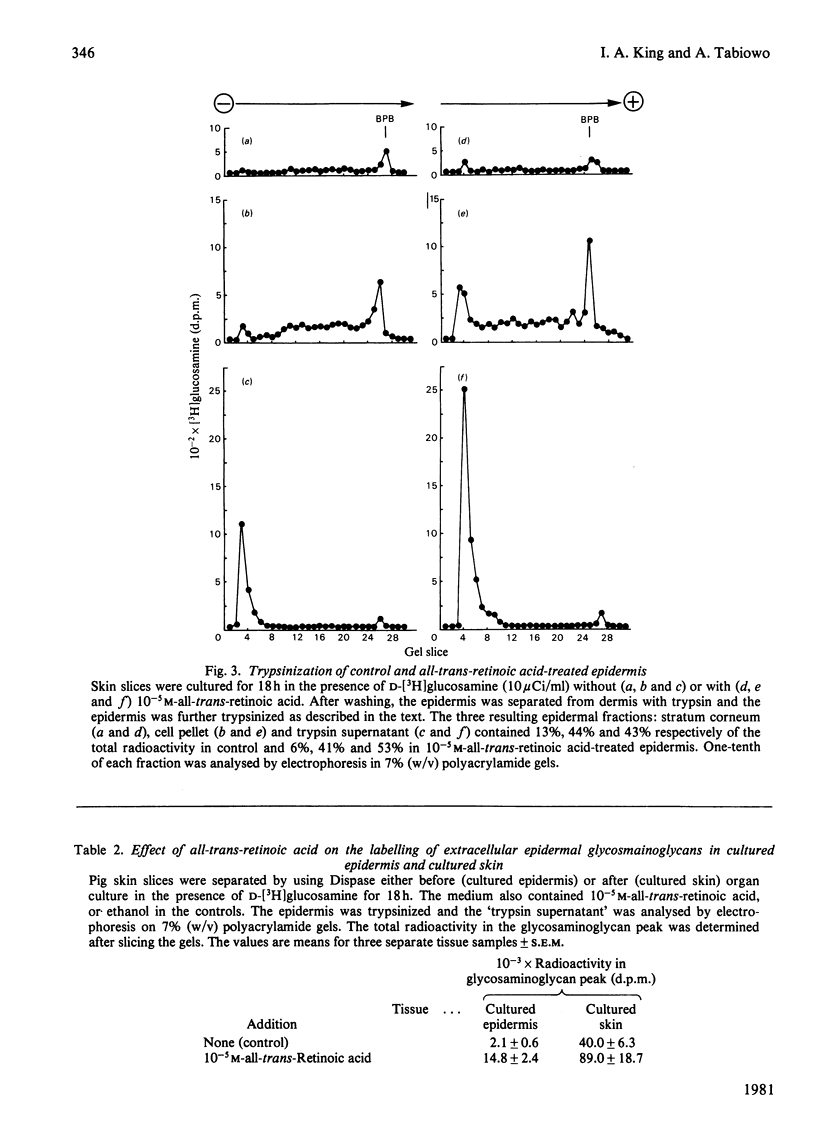


Selected References
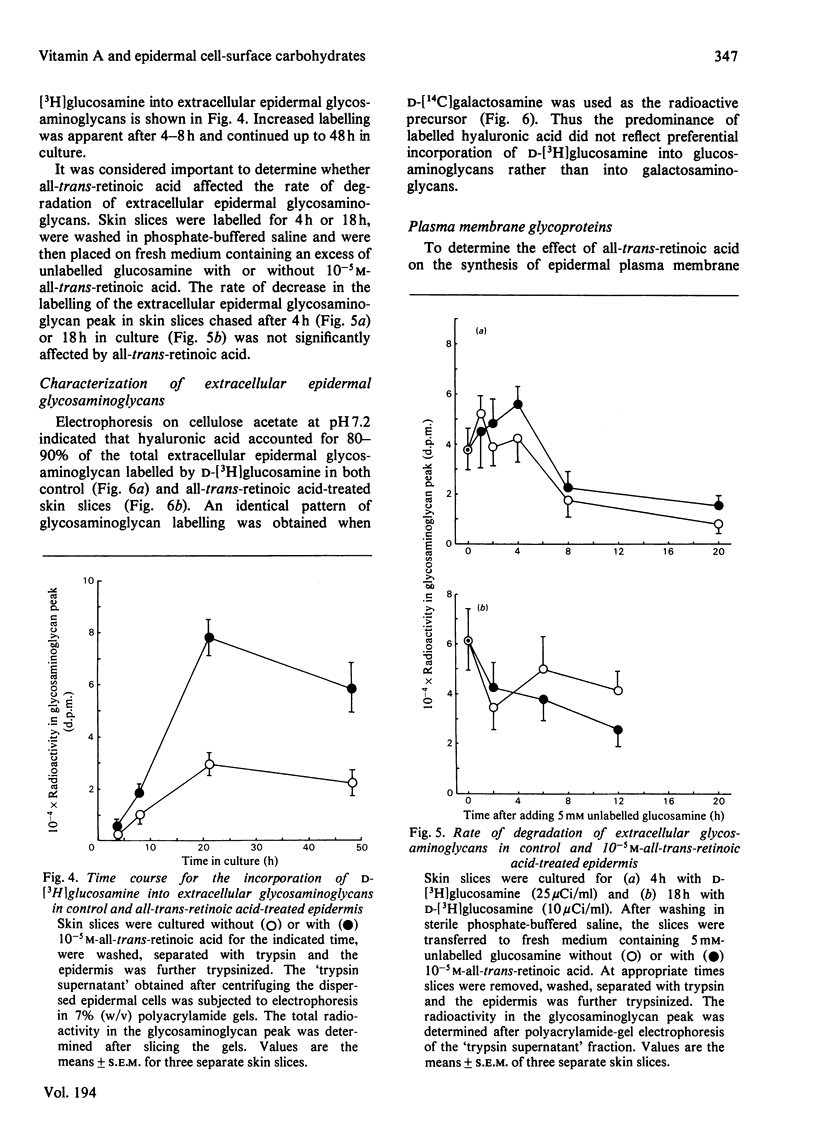
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo S., De Luca L. M., Silverman-Jones C. S., Yuspa S. H. Mode of action of retinol. Involvement in glycosylation reactions of cultured mouse epidermal cells. J Biol Chem. 1979 May 10;254(9):3279–3287. [PubMed] [Google Scholar]
- Barnett M. L., Szabo G. Effect of vitamin A on epithelial morphogenesis in vitro. Exp Cell Res. 1973 Jan;76(1):118–126. doi: 10.1016/0014-4827(73)90426-6. [DOI] [PubMed] [Google Scholar]
- Beitch I. The induction of keratinization in the corneal epithelium. A comparison of the "dry" and vitamin A-deficient eyes. Invest Ophthalmol. 1970 Nov;9(11):827–843. [PubMed] [Google Scholar]
- Billingham R. E., Silvers W. K. Studies on the conservation of epidermal specificies of skin and certain mucosas in adult mammals. J Exp Med. 1967 Mar 1;125(3):429–446. doi: 10.1084/jem.125.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M., Weinstein H. G., Johnson R. L., Veis A., Marshall R. T. Acidic glycosaminoglycans in human skin during fetal development and adult life. Biochim Biophys Acta. 1970 Jan 27;201(1):54–60. doi: 10.1016/0304-4165(70)90009-7. [DOI] [PubMed] [Google Scholar]
- De Luca L. M. The direct involvement of vitamin A in glycosyl transfer reactions of mammalian membranes. Vitam Horm. 1977;35:1–57. doi: 10.1016/s0083-6729(08)60520-8. [DOI] [PubMed] [Google Scholar]
- DeLuca L., Yuspa S. H. Altered glycoprotein synthesis in mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974 May;86(1):106–110. doi: 10.1016/0014-4827(74)90654-5. [DOI] [PubMed] [Google Scholar]
- Dion L. D., Blalock J. E., Gifford G. E. Vitamin A-induced density-dependent inhibition of L-cell proliferation. J Natl Cancer Inst. 1977 Mar;58(3):795–801. doi: 10.1093/jnci/58.3.795. [DOI] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E. Metaplasia produced in cultures of chick ectoderm by high vitamin A. J Physiol. 1953 Mar;119(4):470–488. doi: 10.1113/jphysiol.1953.sp004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hicks R. M. Hyperplasia and cornification of the transitional epithelium in the vitamin A-deficient rat. Changes in fine structure of the cells. J Ultrastruct Res. 1968 Feb;22(3):206–230. doi: 10.1016/s0022-5320(68)90016-6. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Shapiro S. S., Poon J. P. Characterization of the action of retinoids on mouse fibroblast cell lines. Exp Cell Res. 1979 Mar 15;119(2):289–299. doi: 10.1016/0014-4827(79)90356-2. [DOI] [PubMed] [Google Scholar]
- Jetten A. M. Retinoids specifically enhance the number of epidermal growth factor receptors. Nature. 1980 Apr 17;284(5757):626–629. doi: 10.1038/284626a0. [DOI] [PubMed] [Google Scholar]
- King I. A., Tabiowo A. The dermis is required for the synthesis of extracellular glycosaminoglycans in cultured pig epidermis. Biochim Biophys Acta. 1980 Oct 1;632(2):234–243. doi: 10.1016/0304-4165(80)90081-1. [DOI] [PubMed] [Google Scholar]
- King I. A., Tabiowo A., Williams R. H. Incorporation of l-[3H]fucose and d-[3H]glucosamine into cell-surface-associated glycoconjugates in epidermis of cultured pig skin slices. Biochem J. 1980 Jul 15;190(1):65–77. doi: 10.1042/bj1900065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarus G. S., Hatcher V. B., Levine N. Lysosomes and the skin. J Invest Dermatol. 1975 Sep;65(3):259–271. doi: 10.1111/1523-1747.ep12598332. [DOI] [PubMed] [Google Scholar]
- Lotan R., Giotta G., Nork E., Nicolson G. L. Characterization of the inhibitory effects of retinoids on the in vitro growth of two malignant murine melanomas. J Natl Cancer Inst. 1978 May;60(5):1035–1041. doi: 10.1093/jnci/60.5.1035. [DOI] [PubMed] [Google Scholar]
- Lotan R., Nicolson G. L. Inhibitory effects of retinoic acid or retinyl acetate on the growth of untransformed, transformed, and tumor cells in vitro. J Natl Cancer Inst. 1977 Dec;59(6):1717–1722. doi: 10.1093/jnci/59.6.1717. [DOI] [PubMed] [Google Scholar]
- Marchok A. C., Cone V., Nettesheim P. Induction of squamous metaplasia (vitamin A deficiency) and hypersecretory activity in tracheal organ cultures. Lab Invest. 1975 Oct;33(4):451–460. [PubMed] [Google Scholar]
- Mier P. D., Wood M. The acid mucopolysaccharides of mammalian skin. Br J Dermatol. 1969 Jul;81(7):528–533. doi: 10.1111/j.1365-2133.1969.tb16028.x. [DOI] [PubMed] [Google Scholar]
- Patt L. M., Itaya K., Hakomori S. I. Retinol induces density-dependent growth inhibition and changes in glycolipids and LETS. Nature. 1978 Jun 1;273(5661):379–381. doi: 10.1038/273379a0. [DOI] [PubMed] [Google Scholar]
- Roels O. A., Anderson O. R., Lui N. S., Shah D. O., Trout M. E. Vitamin A and membranes. Am J Clin Nutr. 1969 Aug;22(8):1020–1032. doi: 10.1093/ajcn/22.8.1020. [DOI] [PubMed] [Google Scholar]
- Rosso G. C., De Luca L., Warren C. D., Wolf G. Enzymatic synthesis of mannosyl retinyl phosphate from retinyl phosphate and guanosine diphosphate mannose. J Lipid Res. 1975 May;16(3):235–243. [PubMed] [Google Scholar]
- Sani B. P., Donovan M. K. Localization of retinoic acid-binding protein in nuclei and the nuclear uptake of retinoic acid. Cancer Res. 1979 Jul;39(7 Pt 1):2492–2496. [PubMed] [Google Scholar]
- Sani B. P., Hill D. L. Retinoic acid: a binding protein in chick embryo metatarsal skin. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1276–1282. doi: 10.1016/s0006-291x(74)80422-5. [DOI] [PubMed] [Google Scholar]
- Shapiro S. S., Poon J. P. Effect of retinyl acetate on sulfated glycosaminoglycan biosynthesis in dermal and epidermal cells in vitro. Connect Tissue Res. 1978;6(2):101–108. doi: 10.3109/03008207809152618. [DOI] [PubMed] [Google Scholar]
- Shapiro S. S., Poon J. P. Retinoic acid-induced alterations of growth and morphology in an established epithelial line. Exp Cell Res. 1979 Mar 15;119(2):349–357. doi: 10.1016/0014-4827(79)90363-x. [DOI] [PubMed] [Google Scholar]
- Tsambaos D., Mahrle G., Orfanos C. E. Epidermal changes induced by oral excess of aromatic retinoid in guinea pigs. Arch Dermatol Res. 1980;267(2):141–152. doi: 10.1007/BF00569100. [DOI] [PubMed] [Google Scholar]
- Ugel A. R., Chrambach A., Rodbard D. Fractionation and characterization of an oligomeric series of bovine keratohyalin by polyacrylamide gel electrophoresis. Anal Biochem. 1971 Oct;43(2):410–426. doi: 10.1016/0003-2697(71)90271-5. [DOI] [PubMed] [Google Scholar]
- Wolf G., Kiorpes T. C., Masushige S., Schreiber J. B., Smith M. J., Anderson R. S. Recent evidence for the participation of vitamin A in glycoprotein synthesis. Fed Proc. 1979 Oct;38(11):2540–2543. [PubMed] [Google Scholar]
- Yuspa S. H., Harris C. C. Altered differentiation of mouse epidermal cells treated with retinyl acetate in vitro. Exp Cell Res. 1974 May;86(1):95–105. doi: 10.1016/0014-4827(74)90653-3. [DOI] [PubMed] [Google Scholar]


