SUMMARY
Leprosy, a chronic infectious disease caused by Mycobacterium leprae complex, remains a significant global health concern despite being curable with multidrug therapy. Delayed diagnosis is common, particularly in non-endemic regions or when presenting with atypical symptoms. This can lead to missed opportunities for early intervention, potential disabilities, and increased transmission. Misdiagnosis is often compounded by leprosy’s ability to mimic other conditions, as illustrated in this case report.
We present a 43-year-old Filipino woman residing in Eastern Saudi Arabia, who presented to a dermatology clinic with a four-year history of recurrent skin rashes and a one-year history of painful, itchy nodules on her shins. She denied any systemic symptoms, recent travel, or known tuberculosis (TB) contact. Physical examination revealed multiple erythematous nodules on her shins with hyperpigmentation, but no lymphadenopathy or other skin lesions.
Initial laboratory tests, including blood counts, liver and kidney function, inflammatory markers, and HIV screening, were normal. Chest X-ray was unremarkable. The patient’s clinical presentation and laboratory results led to a provisional diagnosis of extrapulmonary TB, and she was started on anti-TB treatment.
However, her condition did not improve after several months of treatment. A skin biopsy was performed, and histopathological examination revealed granulomatous inflammation with acid-fast bacilli, raising suspicion for leprosy. Subsequent culture of the skin biopsy unexpectedly yielded Mycobacterium leprae, confirming the diagnosis of lepromatous leprosy.
The case study highlights the diagnostic challenges associated with leprosy, especially in non-endemic regions. The patient’s atypical presentation, lack of systemic symptoms, and the unexpected growth of M. leprae in cell-free culture media contributed to the initial misdiagnosis and delayed treatment. Early suspicion, prompt skin biopsy, and appropriate culture techniques are crucial for accurate diagnosis and timely initiation of effective therapy to prevent disability and transmission. This case also underscores the importance of considering leprosy as a differential diagnosis in patients presenting with atypical skin lesions, even in non-endemic areas. Continued awareness and education among healthcare providers are essential to improve early recognition and management of this treatable disease.
Keywords: Mycobacterium leprae, tuberculosis, skin nodules, culture, acid-fast bacilli
INTRODUCTION
Leprosy, a chronic infectious disease caused by Mycobacterium leprae complex, continues to pose a significant global health concern, with over 174,000 new cases reported in 2022, primarily in Africa and Southeast Asia [1]. This ancient disease, also known as Hansen’s disease, primarily affects cooler body regions and manifests diversely based on the patient’s immune response and bacterial load, ranging from tuberculoid leprosy (paucibacillary) to lepromatous leprosy (multibacillary) [2, 3]. In some cases, the clinical presentation of leprosy can resemble that of tuberculosis (TB), leading to potential misdiagnosis and delayed initiation of treatment [4, 5]. While leprosy is curable with multidrug therapy (MDT), late diagnosis is common, especially in non-endemic regions or when presenting with atypical symptoms [6]. This can lead to missed opportunities for early intervention and potential disabilities [7]. This is often compounded by the fact that leprosy can mimic other conditions, leading to misdiagnosis and delayed treatment. Early diagnosis and prompt initiation of MDT are crucial to prevent disability and transmission [8]. Transmission primarily occurs through prolonged household contact and inhalation of aerosols, with evidence suggesting a higher prevalence among household contacts than passive reporting [9]. In this case report, we present a patient with lepromatous leprosy who was initially misdiagnosed and treated for extrapulmonary TB due to the unexpected growth of Mycobacterium leprae in cell-free culture media, highlighting the challenges in diagnosing this disease.
CASE PRESENTATION
A 43-year-old Filipino woman presented to a dermatology clinic in Eastern Saudi Arabia with a four-year history of recurrent skin rashes and a one-year history of painful, itchy nodules on her shins. She denied systemic symptoms such as fever, weight loss, or cough and had no history of recent travel or any known TB contact. A history taking and physical examination relevant to leprosy were performed, including assessment for decreased skin sensation, motor examination, sensory examination, and nerve enlargement; these were all negative initially. The examination revealed multiple erythematous nodules on both shins with hyperpigmentation but no lymphadenopathy or other skin lesions. Initial laboratory tests showed normal blood counts, liver and kidney function, and inflammatory markers (C-reactive protein, procalcitonin, erythrocyte sedimentation rate). HIV screening was negative, and the chest X-ray was unremarkable.
A skin lower leg punch biopsy showed mixed vasculitis with septal and lobular panniculitis; obliterated vessels were demonstrated with histiocytic and plasmacytic infiltration; the picture was suggestive of erythema induratum (Nodular Vasculitis). Periodic acid Schiff (PAS) and Gomori methenamine silver (GMS) stains were negative, and the AFB Fite stain was positive. In addition, induced sputum samples tested positive for acid-fast bacilli (AFB) on both smear and routine mycobacterial culture, which were requested to consider the possibility of TB or other mycobacterial illness in the patient considering the ethnic group and chronicity of her symptoms (Figure 1). The patient was admitted under airborne isolation and subsequently started the standard anti-TB treatment. Despite repeated attempts of molecular identification for Mycobacterium tuberculosis complex (MTBC), the grown organism did not possess any of the gene targets included in two different nucleic acid amplification systems, the BD MAX MDRTB (BD, New Jersey, US) and Cepheid Xpert® MTB/RIF Ultra, Sunnyvale, US), or for common non-tuberculous mycobacteria (NTM) using line probe assay (Hain Lifescience GMBH, Nehren, Germany). After two months of treatment, the patient’s condition did not improve, and continued to shed acid-fast bacilli on direct microscopy with evidence of foamy macrophages (Figure 2), and continuously grew acid-fast bacilli in serial 13 cultures over 3 months. A subsequent skin biopsy taken in two-month intervals showed poorly circumscribed nodules of macrophages distended with large numbers of AFB. The nodules extended to the hypodermis to form erythema nodosum. PAS and GMS stains were also negative, but the AFB stain was still positive. These findings, in conjunction with the patient’s clinical presentation and non-response to anti-TB therapy, prompted a reevaluation of the diagnosis. After careful consideration, a diagnosis of lepromatous leprosy (LL) with type 2 reaction (T2R) was clinically established, and the liquid unidentified culture broth material was sent for 16S RNA amplicon-based sequencing, which confirmed speciation of M. leprae (Washington University in St. Louis, USA). One month after initiating a combination of dapsone100 mg PO once daily, rifampicin 600 mg PO once daily, and clofazimine 50 mg PO once daily, the patient’s symptoms started to subside, and her inflamed skin lesions dramatically improved, while the prescription was planned to continue for 24 months (Figure 3).
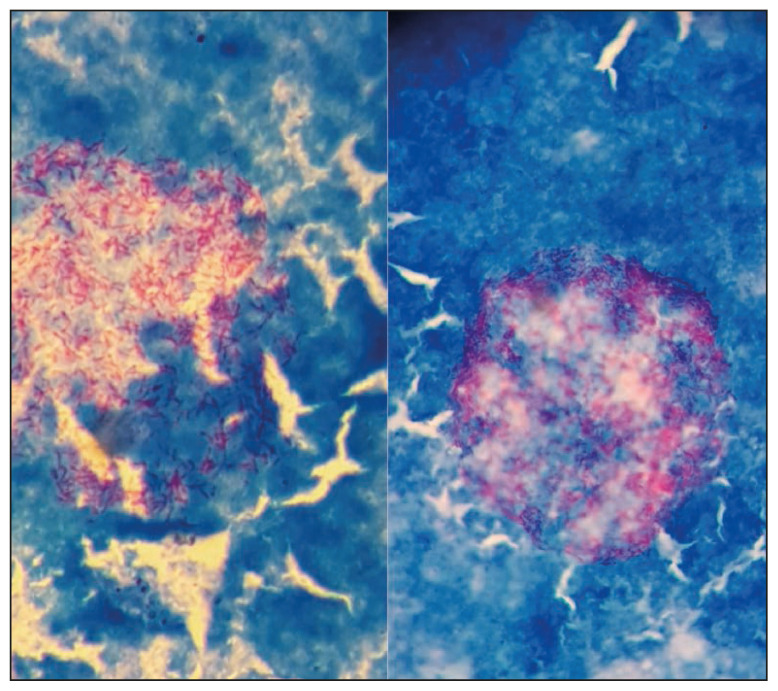
Figure 1.
Acid fast bacilli seen on stained films from flagging positive MGIT tubes belonging to the leprosy case confirming the growth of Mycobacterium leprae in cell-free laboratory media.
Figure 2.
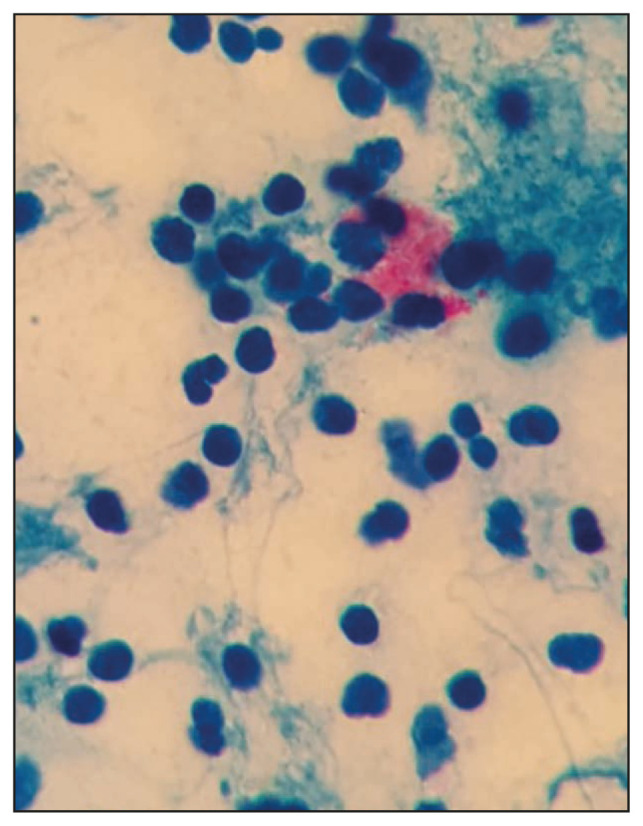
Acid fast bacilli seen on direct smears of induced sputum from a leprosy case showing foamy cells representing macrophages.
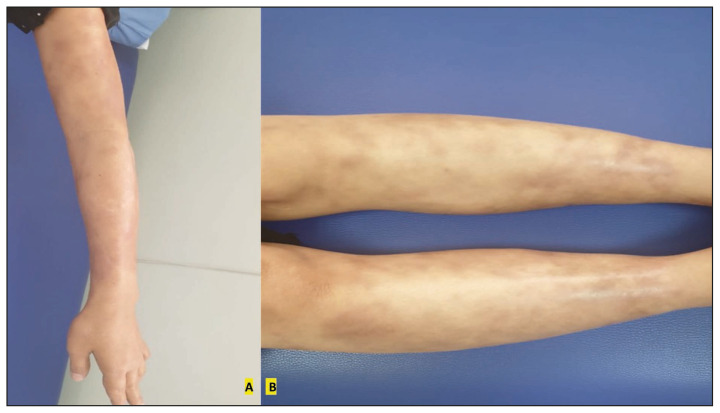
Figure 3.
Significant improvement of leprosy upper limbs (A) and lower limb (B) skin lesions after one month of leprosy treatment.
DISCUSSION
Leprosy and TB are both chronic granulomatous infections caused by different types of mycobacteria and may, in certain cases, overlap in their clinical or histopathological features [4, 10]. The clinical similarities between TB and leprosy that led to consider leprosy in the differential diagnosis include the presence of chronic skin lesions with granulomatous inflammation and positive acid-fast staining on biopsy. The working diagnosis of TB was initially favored due to the positive AFB smear and culture from sputum, along with the patient’s lack of typical leprosy symptoms like sensory loss or nerve thickening. While leprosy predominantly affects the skin and peripheral nerves, cutaneous manifestations of TB can mimic leprosy, further complicating the diagnostic process [5, 11, 12]. Lupus vulgaris, the most common chronic form of cutaneous TB, can closely resemble leprosy, leading to potential misdiagnosis [11]. Furthermore, the histopathological similarities between cutaneous TB and neural leprosy can also contribute to diagnostic errors [12]. The initial misdiagnosis of TB in this report was primarily based on the clinical presentation of skin nodules, a positive AFB smear and culture from sputum, and the absence of typical leprosy symptoms such as sensory loss or nerve thickening. However, the lack of response to anti-TB treatment, persistent AFB shedding, and the presence of foamy macrophages on microscopy prompted a reevaluation. The subsequent negative results for MTBC on molecular testing and the eventual identification of M. leprae through 16S RNA sequencing definitively ruled out TB.
The misdiagnosis of leprosy is common, particularly in non-endemic regions or when patients present with atypical symptoms. In this case report, a patient with lepromatous leprosy and T2R experienced a delayed diagnosis due to the initial neglect of leprosy as a possibility based on negative travel history and nonspecific skin presentations. Further confusion was mainly caused by the unexpected growth of an obligate intracellular bacterium, M. leprae complex, on routine lab media. This case emphasizes the importance of considering leprosy in the differential diagnosis, even without classic presentation in non-endemic areas. Other differential diagnoses that were considered include cutaneous sarcoidosis, erythema induratum, and cutaneous lymphoma. Sarcoidosis was less likely due to the absence of systemic symptoms and the lack of typical non-caseating granulomas on biopsy. Erythema induratum was considered due to the presence of panniculitis on the initial biopsy, but the lack of response to standard treatment and the positive AFB staining argued against it. Cutaneous lymphoma was also less likely given the absence of lymphadenopathy and the atypical histopathological findings. This patient presented with a four-year history of recurrent, atypical skin lesions that progressed to painful, hyperpigmented nodules (T2R). The initial skin biopsy showed positive acid-fast staining, which raised the suspicion of cutaneous tuberculosis, as the test cannot differentiate between leprosy and TB [12, 13]. Additionally, the patient’s positive sputum smear and culture for AFB further supported the initial misdiagnosis of TB, as a variable proportion (10–50%) of extrapulmonary TB cases can have concomitant pulmonary involvement [14]. It also highlights the need for increased awareness among clinicians in non-endemic areas about the overlapping features of leprosy and cutaneous TB. The misdiagnosis of leprosy as TB, as in this case, can lead to delayed treatment and potential long-term complications. Timely and accurate diagnosis is crucial for initiating effective treatment and preventing transmission [15]. Other rare presentations of leprosy include Erythema nodosum leprosum necroticans (ENe), a severe form of type 2 leprosy reaction. It typically occurs in patients with lepromatous or borderline lepromatous leprosy and is characterized by painful, ulcerated, and necrotic skin lesions resulting from an immune complex-mediated reaction, often triggered by infections or medications [16].
Routine laboratory tests, such as AFB smear and culture, cannot reliably distinguish M. leprae from other mycobacterial species, even though the latter is described as typically not able to grow in vitro [3]. This can lead to misidentification, especially in cases where clinical suspicion for leprosy is low or when the patient presents with atypical manifestations [15]. For instance, the growth of AFB on culture media, as observed in this case, could be misleading, particularly in regions where tuberculosis is more prevalent [17]. While molecular techniques like commercial nucleic acid amplification assays can accurately identify MTBC, these are not always readily available or routinely performed in resource-limited settings. Further, the commercial kits in diagnostic units don’t identify M. leprae complex [18]. Misidentification of M. leprae as NTM by certain molecular diagnostic kits has been also described [19]. Therefore, relying solely on routine laboratory tests can result in misdiagnosis and delayed initiation of appropriate treatment for leprosy. At the end of the 19th century, tracheobronchial involvement of leprosy was described along with nasopharynx, larynx, and nose with rich leprosy bacilli in nasal discharge [19]. This may explain positive sputum AFB staining from upper airways as demonstrated in this report. However, frequent positive AFB smears and cultures are extremely rare as the bacterium is known to be unable to grow on artificial media [19–21]. Amplicon-based sequencing of genes such as 16S rRNA remains the most accurate confirmatory tool for detecting M. leprae isolated from suspected leprosy patients. Validating this method has been also proposed for epidemiological studies to identify asymptomatic carriers of leprosy among household contacts or within populations in an endemic area [22]. This method also has the potential to assess the effectiveness of treatments which is currently under investigation [23]. Alternative targets within the M. leprae genome, such as rpoT, sodA, 36-kDa antigen, Complex 85, and repetitive sequences used in RLEP, have been effectively utilized for accurate detection of the bacterium. Notably, the RLEP region has consistently exhibited superior sensitivity and specificity compared to other targets [24]. The use of these molecular diagnostic tools not only streamlines leprosy diagnosis but also plays a crucial role in improving treatment outcomes, particularly in non-endemic regions where diagnostic uncertainty may be encountered.
CONCLUSION
This case underscores the critical importance of maintaining a high index of suspicion for leprosy, even in non-endemic regions and when faced with atypical presentations or unexpected laboratory findings. A combination of comprehensive clinical evaluation, including a detailed history and thorough physical examination, and appropriate diagnostic testing, is essential to differentiate leprosy from other conditions with similar manifestations, such as cutaneous tuberculosis. Early diagnosis and prompt initiation of therapy are crucial to prevent disability, transmission, and ultimately improve patient outcomes. This case also highlights the potential for misdiagnosis due to the unexpected growth of M. leprae in culture, emphasizing the need for advanced molecular techniques for definitive identification.
Acknowledgments
The authors would like to thank all healthcare workers who were involved in this patient’s care, and the patient for her permission to publish the case for dissemination of knowledge.
Footnotes
Authors contribution: All authors were involved in the conception and design data collection, analysis, or interpretation of results, and manuscript preparation or review.
Conflicts of interest: No conflict of interest is declared by the authors.
Ethical statement: The Institutional Review Board approval was obtained from the Research Bioethics Committee at Imam Abdulrahman bin Faisal University (IRB-2024-01-261) and the patient’s informed consent was obtained. The manuscript complies with the Helsinki Declaration of 1964 and all its subsequent amendments.
Funding: No fund was obtained in this work.
Data sharing statement: Data supporting the findings and conclusions are available upon reasonable request from the author.
REFERENCES
- 1.World Health Organization. Global leprosy update: Time for action, accountability and inclusion. Wkly Epidemiol Rec. 2023;98(13):105–120. [Google Scholar]
- 2.Santacroce L, Del Prete R, Charitos IA, Bottalico L. Mycobacterium leprae: A historical study on the origins of leprosy and its social stigma. Infez Med. 2021;29(4):623–632. doi: 10.53854/liim-2904-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scollard DM, Dacso MM, Abad-Venida ML. Tuberculosis and Leprosy: Classical Granulomatous Diseases in the Twenty-First Century. Dermatol Clin. 2015;33(3):541–62. doi: 10.1016/j.det.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz S, Vatanchi M, Moussavi F, DeLury J, Heilman E. When amplified Mycobacterium tuberculosis direct (MTD) testing confuses leprosy diagnosis: A case report. JAAD Case Rep. 2019;5(2):131–133. doi: 10.1016/j.jdcr.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche PW, Britton WJ, Lockwood DNJ, Lucas SB. The neglected role of the household in leprosy transmission. Lancet Infect Dis. 2011;11(4):299–306. doi: 10.1016/S1473-3099(06)70471-9. [DOI] [Google Scholar]
- 7.Richardus JH, Habbema JDF, van der Meulen J. The impact of leprosy control on the transmission of M. leprae: is elimination being attained? Lepr Rev. 2004;75(2):102–116. [PubMed] [Google Scholar]
- 8.World Health Organization. Global leprosy update, 2023: Time for action, accountability and inclusion. Wkly Epidemiol Rec. 2023;98(13):105–120. [Google Scholar]
- 9.Moet FJ, Pahan D, Schuring RP, Oskam L, Richardus JH. Physical distance, genetic relationship, age, and leprosy classification are independent risk factors for leprosy in contacts of patients with leprosy. J Infect Dis. 2006 Jan;193(3):346–353. doi: 10.1086/499278. [DOI] [PubMed] [Google Scholar]
- 10.Mandal BC, Bandyopadhyay G. Leprosy mimicry of lupus vulgaris and misdiagnosis of leprosy-a case report. Indian J Lepr. 2012;84(1):23–25. [PubMed] [Google Scholar]
- 11.Santos JB, Figueiredo AR, Ferraz CE, Oliveira MH, Silva PG, Medeiros VL. Cutaneous tuberculosis: epidemiologic, etiopathogenic and clinical aspects - part I. An Bras Dermatol. 2014;89(2):219–228. doi: 10.1590/abd1806-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty S, Umakanth S, Manandhar B, Nepali PB. Coinfection of leprosy and tuberculosis. BMJ Case Rep. 2018;15:bcr2017222352. doi: 10.1136/bcr-2017-222352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Zha S, Shui TJ. Presenting symptoms of leprosy at diagnosis: Clinical evidence from a cross-sectional, population-based study. PLoS Negl Trop Dis. 2021;15(11):e0009913. doi: 10.1371/journal.pntd.0009913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2015;78(2):47–55. doi: 10.4046/trd.2015.78.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockwood DNJ, Suneetha S. Leprosy: too complex a disease for a simple elimination paradigm. Bull World Health Organ. 2005;83(5):391–397. doi: 10.1097/OLQ.0000000000001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfieri A, Dewi ST, Siswati AS, Pudjiati SR, Soebono H. Erythema nodosum leprosum necroticans: a case report of an atypical severe type 2 leprosy reaction and literature review. Infez Med. 2024;32(2):248–253. doi: 10.53854/liim-3202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerr-Pontes LR, Montenegro A, Barreto ML, Feldmeier H. Inequality and leprosy in the city of Salvador, Brazil. Int J Epidemiol. 2006;35(3):619–626. doi: 10.1093/ije/dyi319. [DOI] [Google Scholar]
- 18.Mendes MF, Silva JS, Nery JA, Setubal S, Sarno EN, Suffys PN. PCR-based identification of Mycobacterium leprae DNA in tissue sections of cutaneous lesions. J Clin Microbiol. 2001;39(3):1064–1067. [Google Scholar]
- 19.Lefmann M, Moter A, Schweickert B, Göbel UB. Misidentification of Mycobacterium leprae as Mycobacterium intracellulare by the COBAS AMPLICOR M. intracellulare test. J Clin Microbiol. 2005;43(4):1928–1929. doi: 10.1128/JCM.43.4.1981-1985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azad Kamran N, Agapito Annaflor G, Batterman Hollis J, Schwab Dale A. Mycobacterium leprae Growth in an MGIT Broth Culture Tube. Clin Microbiol Newsl. 2019;41(18):164–166. doi: 10.1016/j.clinmicnews.2019.02.001. [DOI] [Google Scholar]
- 21.Ghielmetti G, Schmitt S, Friedel U, Guscetti F, Walser-Reinhardt L. Unusual Presentation of Feline Leprosy Caused by Mycobacterium lepraemurium in the Alpine Region. Pathogens. 2021;1,10(6):687. doi: 10.3390/pathogens10060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques LÉC, Frota CC, Quetz JDS, Bindá AH, Mota RMS, Pontes MAA, et al. Evaluation of 16S rRNA qPCR for detection of Mycobacterium leprae DNA in nasal secretion and skin biopsy samples from multibacillary and paucibacillary leprosy cases. Pathog Glob Health. 2018;112(2):72–78. doi: 10.1080/20477724.2017.1394373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann ADS, Fontes ANB, Lopes MQP, Suffys PN, Moraes MO, Lara FA. Heterogeneous persistence of Mycobacterium leprae in oral and nasal mucosa of multibacillary patients during multidrug therapy. Mem Inst Oswaldo Cruz. 2022;17(117):e220058. doi: 10.1590/0074-02760220058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma M, Singh P. Advances in the diagnosis of leprosy. Front Trop Dis. 2022:3. doi: 10.3389/fitd.2022.893653. [DOI] [Google Scholar]