Abstract
OBJECTIVE
Determine whether early administration (EA) of long-acting insulin in pediatric diabetic ketoacidosis (DKA) reduces time to acidosis resolution while maintaining safety when compared with late administration (LA).
METHODS
This retrospective review compared EA (within 4 hours) to LA (4 to 24 hours) of long-acting insulin in DKA management in the pediatric intensive care unit between 2015 and 2022. Admissions were excluded for patients ≥18 years of age, without type 1 diabetes, with insufficient laboratory data, or who did not receive insulin glargine within 24 hours of starting treatment. Primary outcome was resolution of acidosis, measured as time to normalization of serum sodium bicarbonate concentration (>15 mEq/L). Secondary outcomes included hospital and intensive care lengths of stay, and insulin infusion duration. Safety outcomes were hypokalemia, hypoglycemia, and cerebral edema.
RESULTS
Of the 233 admissions evaluated, 51 met inclusion for each group. The median patient age was 11 years, 42% female, and 59% had new-onset diabetes. No difference was found in the median time to acidosis resolution (8.13 hours [EA] and 8.02 hours [LA]; p = 0.4161). Median insulin infusion durations were 16.2 and 17.6 hours for EA and LA, respectively (p = 0.8750). Median hospital stay was 2 days for both groups (p = 0.9068). Hypoglycemia and hypokalemia rates were not significantly different but occurred more often than previously reported.
CONCLUSIONS
Early administration of long-acting insulin in pediatric DKA did not affect acidosis duration or treatment length when compared with late administration. Incidence of hypoglycemia and hypokalemia were similar between groups.
Keywords: diabetes mellitus type 1, diabetic ketoacidosis, hypoglycemia, hypokalemia, insulin glargine, insulin, long-acting, intensive care units, pediatric
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening complication frequently observed in children with type 1 diabetes mellitus (T1DM), contributing significantly to morbidity and mortality rates in this population.1,2 Patients with T1DM can have a substantial impact on the health care system. About one quarter of all newly diagnosed children present with DKA and up to 10% of all established patients are hospitalized for DKA each year.3–5
Standard treatment strategies for DKA involve fluid and electrolyte replacement followed by an insulin infusion, which is continued until acidosis resolution, measured by venous pH or serum bicarbonate concentration.6 The 2024 American Diabetes Association (ADA) Standards of Care recommend insulin transition protocols that include subcutaneous long-acting insulin 2 to 4 hours prior to discontinuing intravenous insulin infusions.7 This recommendation is based on findings that long-acting insulin contributes to decreased time to acidosis resolution, rate of rebound hyperglycemia, and length of hospital stay, without increased incidence of hypoglycemia.8–10 The updated 2022 International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines are now consistent with adult recommendations to begin long-acting insulin while the patient is still receiving an insulin infusion.6 Unlike the ADA, ISPAD guidelines do not specify timing for the initiation of long-acting insulin. However, they do suggest an example of administering long-acting insulin in the evening and stopping the insulin infusion the next morning.6
When compared against late administration (LA) or an absence of long-acting insulin, administering long-acting insulin early (early administration, EA) in the DKA process may result in faster resolution, but safety data are mixed.9–14 Available information in the pediatric population suggests early use of long-acting insulin within the first 6 hours of treatment or admission safely reduces insulin requirements and causes faster resolution of acidosis, consistent with results in adults.11,14–16 Other findings caution against EA of long-acting insulin more than 3 hours before discontinuing the insulin infusion owing to an observed increase in hypokalemia.12 The considerable pharmacokinetic variability observed in severely ill pediatric populations, discrepancies in adverse event outcomes, and ambiguity in recommended timing of long-acting insulin prior to stopping the insulin infusion highlight the necessity for further research on medication usage in this context.6,7,15
This study aims to evaluate the safety and efficacy implications of initiating long-acting insulin early in the management of pediatric patients with DKA. In 2015, the study site’s pediatric DKA order set was updated to include options to administer insulin glargine immediately upon initiation of DKA management, in the evening of the same day, or not at all. Following this practice change, patients who received insulin glargine within 4 hours of starting an insulin infusion (EA) were compared with those who received it between 4 and 24 hours after starting the infusion (LA). The 4-hour time frame was selected because the study site does more frequent laboratory monitoring in the first 4 hours of treatment, and thus was considered the early stage of management. The primary objective was to assess whether EA will reduce the time to DKA resolution, compared with LA. The secondary objective was to evaluate the impact of EA on length of stay (LOS) and insulin duration as well as the rate of adverse effects, such as hypoglycemia, hypokalemia, and cerebral edema.
Materials and Methods
This retrospective electronic medical chart review was conducted at Stormont Vail Hospital, a large community hospital with a 6-bed pediatric intensive care unit (PICU) in Topeka, Kansas. The sample population was identified through a comprehensive record of patients admitted to the PICU with DKA that was maintained by hospital staff between August 1, 2015, and August 31, 2022. This record was further supplemented by an electronic medical record report of patients admitted to the PICU for treatment of DKA based on primary coded diagnosis that included DKA in T1DM. Patients were excluded from the study if they were older than 18 years at admission, lacked a diagnosis of T1DM, had insufficient laboratory data, or did not receive at least 1 dose of insulin glargine within 24 hours of initiating DKA treatment. Data collection discovered some patients who received DKA treatment but whose acidosis resolved prior to initiation of treatment. These patients were also excluded. Admissions were then categorized as EA (insulin glargine administered within 4 hours of starting the insulin infusion) or LA (insulin glargine administered 4–24 hours after starting the insulin infusion). All admissions were considered as independent admissions during outcome analysis, and patients with multiple admissions were evaluated multiple times. Demographic data were evaluated on unique patients during the index admission (first qualifying admission within the study period) only, excluding data from repeated admissions.
Throughout the study time frame, a 2-bag fluid and electrolyte management system was used for the treatment of pediatric DKA (Table 1). The fluid infusion ratios were determined from the patient’s blood glucose (BG) concentrations, as indicated in Table 1. Regular insulin infusion was initiated at 0.1 units/kg/hr (maximum 7 units/hr) and was adjusted to 0.05 units/kg/hr once BG concentrations reached 80 to 100 mg/dL. Per the institutional protocol, basic metabolic panel, magnesium, and phosphorous measurements were to be obtained every 2 hours for 2 occurrences and subsequently changed to every 4 hours for the duration of DKA treatment.
Table 1.
Intravenous Fluid Titration Protocol for Pediatric DKA Management at Stormont Vail Hospital
| BG, mg/dL | Percentage of Intravenous Fluid Hourly Rate | Final Dextrose Concentration | |
|---|---|---|---|
| Bag 1: NS, 20 mEq KAc, 20 mEq KPO4 | Bag 2: D10NS, 20 mEq KAc, 20 mEq KPO4 | ||
| >250 | 100% | 0% | 0% |
| 200–249 | 50% | 50% | 5% |
| 150–199 | 25% | 75% | 7.5% |
| <150 | 0% | 100% | 10% |
BG, blood glucose; DKA, diabetic ketoacidosis; KAc, potassium acetate; KPO4, potassium phosphate; NS, “normal saline” or 0.9% sodium chloride solution
The primary outcome of time to resolution of acidosis was defined as a serum bicarbonate (CO2 measured by metabolic panel) concentration greater than 15 mEq/L.6 Secondary outcomes were the LOS in days in both the hospital and PICU, and the duration of insulin infusion in hours. LOS was recorded in full hospital day increments, starting from the date of patient admission to the date of discharge. If a patient was admitted and discharged on the same day, the LOS was set as 1 day. Infusion start time and insulin glargine administration times were determined by medication administration times from bar code scans. Safety outcomes were any occurrence of hypoglycemia (BG concentration <70 mg/dL), hypokalemia (serum potassium concentration <3.5 mEq/L), and cerebral edema. In cases where data were higher or lower than reportable values, the values were converted to the next unit (e.g., serum bicarbonate reported as <10 mEq/L converted to 9 mEq/L; glycated hemoglobin A1C [A1C)] >16.9% was converted to 17.0%). Patient demographic information, laboratory data, and outcomes were collected from the electronic medical record and stored in a secured electronic database. All data were independently collected as objective measures, with the exception that identification of cerebral edema required review of progress notes.
Chi-squared test was used to analyze categorical variables, specifically the number of admissions presenting with new-onset T1DM, unique patient sex, and occurrences of hypokalemia and hypoglycemia per admission. Fisher exact test was used to evaluate the incidence of cerebral edema. Continuous data, including number of admissions, age, weight, initial values of A1C, pH, serum bicarbonate concentration, BG concentration, and potassium concentration per patient, as well as LOS, hours to acidosis resolution, and duration of insulin infusion for each admission were analyzed by using Mann-Whitney U test. Linear regression was used to explore correlation between timing of insulin glargine administration with time to acidosis resolution. The statistical significance was determined by using an alpha level of 0.05. Subgroup analyses were completed for 1) admissions when insulin glargine was given prior to resolution of acidosis; 2) admissions when insulin glargine was given prior to the insulin infusion; and 3) admissions with new-onset T1DM. Each subgroup was analyzed in the same method as the entire sample population.
Results
During the study period, there was a total of 233 admissions to the PICU for treatment of DKA, see Supplemental Figure. After exclusions, including 3 admissions where acidosis resolved prior to starting treatment, the final sample included 51 admissions in the LA group and 51 admissions in the EA group (see Supplemental Figure). The number of admissions when long-acting insulin was given between 4 and 6 hours after starting DKA treatment was 5. No significant differences were observed in patient demographics or initial laboratory values, as indicated in Table 2. Among the 79 unique patients included in the study, the median age was 11 years (range, 1.4–17), with 42% (n = 33) female, and 59% (n = 47) presenting with new-onset T1DM. Median admissions per patient was 1 (IQR, 1–1); 77% (n = 61) with 1 eligible admission, 19% (n = 15) with 2, and 1% (n = 1) each with 3, 4, and 6 eligible admissions. Per admission, the median initial A1C was 11.4% (IQR, 10.3–13.0), median BG concentration was 446 mg/dL (IQR, 359–539), and median blood pH was 7.12 (IQR, 7.00–7.20). The median daily dose of insulin glargine was 18 units (IQR, 9–31), and the insulin infusion was started at 0.1 units/kg/hr in 94 of 102 admissions (92%; range, 0.05–0.7 units/kg/hr).
Table 2.
Baseline Clinical Characteristics at Admission
| Early Administration | Late Administration | p value | |
|---|---|---|---|
| Patients per group, n* | 41 | 38 | |
| Female, n (%) | 16 (39) | 17 (45) | 0.6070 |
| Age, median (IQR), yr | 11 (8–14) | 11 (6–13) | 0.4984 |
| New-onset T1DM, n (%) | 26 (63) | 21 (55) | 0.4609 |
| Number of admissions per patient, median (IQR), n | 1 (1–1) | 1 (1–1) | 0.9413 |
| All admissions per group, n † | 51 | 51 | |
| Weight, median (IQR), kg | 36.3 (25.9–57.9) | 39.0 (21.1–57.0) | 0.9920 |
| A1C, median (IQR), % | 11.1 (10.3–13.2) | 11.9 (10.3–12.9) | 0.4948 |
| Blood glucose, median (IQR), mg/dL | 446 (381–552) | 445 (325–533) |
0.7128 |
| pH, median (IQR) | 7.1 (7.0–7.2) | 7.1 (7.0–7.2) | 0.9253 |
| CO2, median (IQR), mEq/L | 9 (9–13) | 9 (9–13) | 0.4028 |
| Potassium, median (IQR), mEq/L | 4.7 (4.1–5.2) | 4.6 (4.1–5.3) | 0.8383 |
A1C, glycated hemoglobin A1C; CO2, serum sodium bicarbonate concentration; T1DM, type 1 diabetes mellitus
Baseline demographic data were evaluated only for unique patients during their initial qualifying admission; repeated admissions were not evaluated for these baseline characteristics.
All admissions were included.
The administration of early insulin glargine did not have any significant impact on outcomes (Table 3). The hours elapsed from the initiation of insulin infusion until the resolution of acidosis were not significantly different between EA and LA groups, with a median time of 8.13 hours (IQR, 5.75–11.12) and 8.02 hours (IQR, 3.83–14.33), respectively (p = 0.4161). The Figure illustrates the relationship between the time insulin glargine was administered and the time acidosis resolved, showing high variability regardless of administration time (R2 = 0.0032) and a lack of correlation (β = −0.0489, p = 0.5721). The insulin infusion duration was comparable, with a median of 16.2 hours (IQR, 13.2–21.5) in the EA group and 17.6 hours (IQR, 10.6–22.3) in the LA group (p = 0.8750). Lengths of stay did not differ significantly, with overall medians for hospital LOS of 2 days (IQR, 1–3; p = 0.9068), and PICU LOS of 1 day (IQR, 1–1; p = 0.8174).
Table 3.
Efficacy and Safety Outcomes of Early and Late Insulin Glargine Administration
| Efficacy Outcome | Early Administration (n = 51) | Late Administration (n = 51) | p value | |
|---|---|---|---|---|
| Time until CO2 >15 mEq/L, median (IQR), hr |
8.13 (5.75–11.12) |
8.02 (3.83–14.33) |
0.4161 | |
| Insulin infusion duration, median (IQR), hr | 16.2 (13.2–21.5) |
17.6 (10.6–22.3) |
0.8750 | |
| Insulin glargine dose, median (IQR), units/kg | 0.5 (0.3–0.5) | 0.5 (0.3–0.5) | 0.9546 | |
| Hospital LOS, median (IQR), days | 2 (1–3) | 2 (1–3) | 0.9068 | |
| PICU LOS, median (IQR), days | 1 (1–1) | 1 (1–1) | 0.8174 | |
| Safety Outcome | Early Administration (n = 51) | Late Administration (n = 51) | Relative Risk (95% CI) | p value |
| Any adverse event, n (%) | 33 (65) | 36 (71) | 1.20 (0.68–2.11) | 0.5255 |
| Hypoglycemia, n (%) |
23 (45) | 22 (43) | 1.05 (0.68–1.62) | 0.8419 |
| Hypokalemia, n (%) |
21 (41) | 21 (41) | 1.00 (0.63–1.59) | 1.0000 |
| Cerebral edema, n (%) | 1 (1) | 0 (0) | ||
| Hypoglycemia and hypokalemia within the same admission, n (%) | 11 (22) | 7 (14) | 1.57 (0.66–3.73) | 0.2988 |
CO2, serum sodium bicarbonate concentration; LOS, length of stay; PICU, pediatric intensive care unit
Figure.
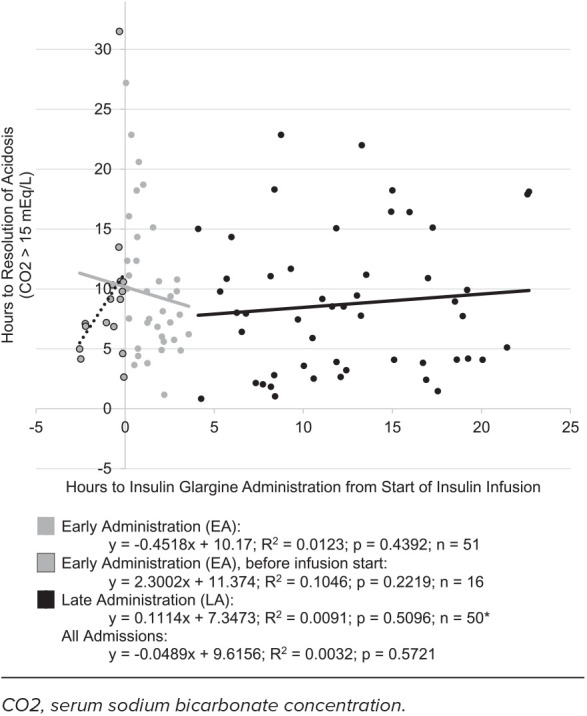
Hours to insulin glargine versus hours to resolution of acidosis.
Adverse events of hypokalemia, hypoglycemia, or cerebral edema occurred in 68% of admissions (n = 69), described in Table 3. Hypokalemia and hypoglycemia occurred during the same admission in 18% of admissions (n = 18). The RR of both hypoglycemia and hypokalemia between EA and LA groups was 1.57 (95% CI, 0.66–3.73), slightly favoring LA, but not statistically significant (p = 0.2988). One case of suspected cerebral edema was documented in the EA group, although it was not confirmed through imaging. Hypoglycemia also occurred in this admission.
Admissions with hypokalemia and administration of insulin glargine prior to resolution of acidosis (n = 29, 44%) had a significantly lower median baseline potassium serum concentration (4.1 mEq/L; IQR, 3.6–4.7) than those without hypokalemia (4.8 mEq/L; IQR, 4.5–5.5; p = 0.0002). Admissions with hypokalemia also had a lower median baseline pH (7.0; IQR, 6.9–7.1) and were more likely to have new-onset T1DM (n = 20, 69%) than admissions without hypokalemia (median pH, 7.1; IQR, 7.0–7.2; p = 0.0047; 41% new-onset T1DM; n = 15; p = 0.0217). The median time to acidosis resolution was nearly twice as long during admissions with hypokalemia (13.48 hours; IQR, 9.91–18.46) compared with those without (7.20 hours; IQR, 5.31–10.63; p < 0.0001), along with a longer median insulin infusion duration (21.4 hours; IQR, 17.1 − 27.7) compared with those without hypokalemia (14.5 hours, IQR,12.8–19.5; p = 0.0006). Some hospital LOSs were longer when hypokalemia occurred, though the median LOS regardless of hypokalemia was 2 days (p = 0.0136).
Nearly all admissions in the EA group (98%, n = 50) received insulin glargine before resolution of acidosis, compared with 31% in the LA group (n = 16) (p < 0.0001). When excluding admissions when long-acting insulin was administered after acidosis resolution, there were significantly more females in the LA group than in the EA group (71%, n = 10 and 36%, n = 16, respectively; p = 0.0313). Both median blood pH and CO2 were significantly lower in the LA group. Median blood pH was 7.0 (IQR, 6.9–7.1) for LA and 7.1 (IQR, 7.0–7.2) for EA (p = 0.0154). Median CO2 was 9 mEq/L (IQR, 9–9) for LA and also 9 mEq/L for LA, though LA had more variability with at least 25% of patients with values of 13 mEq/L or higher (IQR, 9–13) (p = 0.0362). CO2 normalized significantly earlier in the EA group (median, 8.63 hours; IQR, 5.95–11.42) than in the LA group (median, 15.04 hours; IQR, 10.90–18.28; p = 0.0004). Insulin infusion duration was significantly longer in the LA group (median, 22.1 hours; IQR, 17.4–28.7) than in the EA group (median, 16.3 hours; IQR, 13.3–21.8; p = 0.0208). Other outcome measures and adverse events were similar between the 2 subgroups of admissions when insulin glargine is administered before acidosis resolution.
Sixteen admissions involved administration of insulin glargine before initiation of the insulin infusion. A subgroup analysis of these admissions within the EA group is detailed in Table 4. More patients with new-onset T1DM received insulin glargine after infusion initiation compared with before (31% versus 72%, p = 0.0118). Admissions with insulin glargine administered after the infusion started had a higher median baseline BG concentration (502 mg/dL, IQR, 411–597) than before the infusion initiation (360 mg/dL; IQR, 273–427; p = 0.0005). No other significant differences were observed in the baseline characteristics or study outcomes. The only reported case of cerebral edema occurred during an admission where insulin glargine was administered before infusion initiation (6% versus 0%, p = 0.3200).
Table 4.
Comparison of Administration of Insulin Glargine Before Versus After Initiation of Insulin Infusion in the Early Administration Group
| Index Admission* | Before Infusion (n = 16) | After Infusion (n = 29) | p value |
|---|---|---|---|
| Age, median (IQR), yr | 11 (9–14) | 10 (8–13) | 0.6438 |
| Female, n (%) | 5 (31) | 11 (38) | 0.7519 |
| New-onset T1DM, n (%) | 5 (31) | 21 (72) | 0.0118 |
| All Admissions † | Before Infusion (n = 16) | After Infusion (n = 34) | p value |
| Blood glucose, median (IQR), mg/dL | 360 (273–427) | 502 (411–597) | 0.0005 |
| CO2, median (IQR), mEq/L | 12 (9–14) | 9 (9–12) | 0.1116 |
| Time until CO2 >15 mEq/L, median (IQR), hr |
8.16 (5.45–10.52) | 8.76 (5.95–12.85) | 0.3938 |
| Insulin infusion duration, median (IQR), hr | 16.6 (10.3–25.3) | 16.3 (13.4–21.8) | 0.7869 |
| Any adverse event, n (%) | 11 (69) | 21 (62) | 0.7568 |
| Hypoglycemia, n (%) |
8 (50) | 14 (41) | 0.5577 |
| Hypokalemia, n (%) |
5 (31) | 16 (47) | 0.3653 |
| Cerebral edema, n (%) | 1 (6) | 0 | 0.3200 |
| Both hypoglycemia and hypokalemia, n (%) | 2 (13) | 9 (26) | 0.4657 |
CO2, serum sodium bicarbonate concentration; T1DM, type 1 diabetes mellitus
Baseline demographic data were evaluated only for unique patients during their initial qualifying admission; repeated admissions were not evaluated for these baseline characteristics.
All admissions were included.
Most index admissions were for new-onset T1DM (59%, n = 47), which increases to 64% (n = 35) when patients who received insulin glargine after acidosis resolution are excluded (Table 5). While the median age trended lower in patients with new-onset T1DM (9 years, IQR, 6–12) than in patients with established T1DM (12 years; IQR, 9–14), this difference was not statistically significant. Patients with new-onset T1DM had lower median baseline weight and serum potassium concentrations, and higher A1C and BG concentrations than patients with established T1DM. Regarding outcomes, no significant differences were found in the time to CO2 normalization or insulin infusion duration between the 2 groups. However, patients with new-onset T1DM experienced significantly longer hospital LOS and higher incidence of hypokalemia than patients with established T1DM.
Table 5.
Comparison of Patients With New-Onset and Established Type 1 Diabetes Mellitus, Excluding Those Administered Insulin Glargine Before Acidosis Resolution
| Baseline Characteristic | New-Onset T1DM (n = 35) | Established T1DM (n = 20) | p value | |
|---|---|---|---|---|
| Age, median (IQR), yr | 9 (6–12) | 12 (9–14) | 0.0702 | |
| Female, n (%) | 14 (40) | 12 (60) | 0.1530 | |
| Weight, median (IQR), kg | 32.7 (18.9–40.0) | 49.5 (30.8–61.2) | 0.0133 | |
| A1C, median (IQR), % | 12.1 (11.0–14.4) | 10.5 (10.0–13.0) | 0.0275 | |
| Blood glucose, median (IQR), mg/dL | 514 (446–598) | 399 (333–465) | 0.0019 | |
| CO2, median (IQR), mEq/L | 9 (9–12) | 9 (9–12) | 0.8269 | |
| Potassium, median (IQR), mEq/L | 4.2 (3.7–4.8) | 4.9 (4.4–5.2) | 0.0127 | |
| Outcome | Relative Risk (95% CI) | p value | ||
| Time until CO2 >15 mEq/L, median (IQR), hr |
10.78 (7.50–15.02) | 9.47 (6.86–14.93) | 0.4950 | |
| Insulin infusion duration, median (IQR), hr | 18.1 (13.9–23.3) | 15.0 (12.8–21.7) | 0.1416 | |
| Hospital LOS, median (IQR), days | 3 (2–3) | 1 (1–2) | 0.0024 | |
| PICU LOS, median (IQR), days | 1 (1–1) | 1 (1–1) | 0.6492 | |
| Any adverse event, n (%) | 25 (71) | 11 (55) | 1.30 (0.83–2.03) | 0.2177 |
| Hypoglycemia, n (%) |
14 (40) | 8 (40) | 1.00 (0.51–1.96) | 1.0000 |
| Hypokalemia, n (%) |
20 (57) | 5 (25) | 2.29 (1.02–5.15) | 0.0213 |
| Both hypoglycemia and hypokalemia, n (%) | 9 (26) | 2 (10) | 2.57 (0.62–10.75) | 0.1697 |
| Cerebral edema, n (%) | 0 | 1 (5) | 0.3636 | |
A1C, glycated hemoglobin A1C; CO2, serum sodium bicarbonate concentration; LOS, length of stay; PICU, pediatric intensive care unit; T1DM, type 1 diabetes mellitus
Discussion
This study of pediatric patients with T1DM treated in the PICU for DKA did not reveal any significant differences in the clinical resolution of DKA based on the timing of long-acting insulin administration. ADA recommends to administer long-acting insulin “late” (2 to 4 hours before stopping the insulin infusion) to prioritize a reduction in rebound hyperglycemia.7 Methods used in children to improve transition include long-acting insulin administration beginning as early as possible from hospital admission or initiation of the insulin infusion compared against either later administration or absence of long-acting insulin.8–14
Prior studies evaluating administration timing in pediatric patients have mixed results. A reduction in time to acidosis resolution has been reported with long-acting insulin administration within 6 hours of admission or insulin infusion start time, compared with either no long-acting insulin or later administration (roughly 24 ±14 hours from infusion start time).11,14 The EA and LA determination in our study was set at 4 hours, rather than the 6-hour threshold in previous studies. This adjustment aligns with the more frequent laboratory collections during the first 4 hours of treatment at our site, reflecting a more acute stage of treatment. Only 5 admissions received insulin glargine between 4 and 6 hours in our study, which would minimally affect our results given the low number of admissions who could have been reassigned to EA. Another study found that administering long-acting insulin more than 4 hours before discontinuing the insulin infusion resulted in no difference in the duration of DKA, when compared with administration within 2 hours of the infusion stop time or none at all.12 Our study found no difference in the duration of acidosis or insulin infusion when insulin glargine was administered within 4 hours of the insulin infusion start time compared with administration during a range of 4 to 24 hours. Taking these previous studies into consideration, our research indicates that administration of long-acting insulin in the treatment of DKA may be used to improve efficacy outcomes regardless of whether early or late in the treatment.11–14
Administering long-acting insulin outside of regular dosing intervals may pose challenges during the patient’s transition to a lower level of care, particularly with regard to scheduling subsequent doses. The 2022 ISPAD guidelines recommend administration of long-acting insulin prior to stopping the insulin infusion but give no preference regarding timing.6 Ideally, if the patient has a known, pre-admission long-acting insulin regimen, the dose could be administered at their usual time. However, for patients presenting with new-onset T1DM, aligning the administration of insulin glargine with a conventional dosing schedule, such as 0900 or 2100, could facilitate smooth transitions in patient care.
The definition of acidosis resolution used in this study aligns with the DKA definition outlined in the 2018 ISPAD guidelines and previous pediatric studies.6,11,12 After the initiation of this study, newer guidelines have expanded the diagnosis criteria to include patients with a serum bicarbonate concentration below 18 mEq/mL, as opposed to the previous threshold of 15 mEq/mL. Had our criteria been adjusted, the time to resolution could have been extended substantially, allowing for potential differences between early and late insulin glargine.
The findings of this study align with the typical short LOSs experienced by children admitted with DKA.16 Although we found no differences in LOS when comparing EA and LA, measurement by hours instead of days could have produced significant results. The overall median time to DKA resolution was 8.08 hours and the median duration of the insulin infusion was considerably later at 16.6 hours, suggesting opportunities to optimize care. Institutions that have the capability to provide meals outside of regular mealtimes may be able to discontinue insulin infusions earlier, leading to a reduction in LOS.
The observed rates of hypoglycemia and hypokalemia in this study exceeded the anticipated rate, with 44% of all admissions experiencing hypoglycemic events and 41% experiencing hypokalemia, compared with a previous study that reported rates of approximately 22% and 34%, respectively.12 A more recent study of EA reported different rates of adverse events, with only 7% hypoglycemia and 65% hypokalemia.14 In our investigation, the median baseline serum potassium concentration in admitted patients who experienced hypokalemia was 0.7 mEq/L lower than for those who did not, and a similar trend was seen by Harrison et al.12 Admissions with hypokalemia had significantly lower baseline pH and required double the amount of time to resolve out of acidosis. This suggests that lower baseline potassium concentrations serve as a predisposing factor for the development of hypokalemia, but are unrelated to timing of long-acting insulin administration. The lower risk of developing hypokalemia identified by Harrison et al12 could be associated with the differences in comparison groups, as they included patients in their LA group that did not receive insulin glargine.
Protocol and treatment differences affect direct comparisons for hypoglycemia, with notable differences in definitions of hypoglycemia, when to add dextrose to treatment, and dextrose concentrations used in titrations. Prior pediatric studies have defined hypoglycemia as BG concentrations lower than 60 mg/dL, while our study used a threshold of lower than 70 mg/dL per institutional policy.11,12,14 If our hypoglycemia threshold was decreased to 60 mg/dL, the percentage of admissions with hypoglycemia would decrease from 44% (n = 45) to 25% (n = 26), closer to the expected rate of 7% to 22%.12,14 The protocol published by Harrison et al12 added dextrose earlier in treatment, when BG concentration went below 300 mg/dL. Their protocol also used higher concentrations of dextrose to titrate (5%, 10%, and 12%) compared with 5%, 7.5%, and 10% used in our study. Collectively, these may explain the higher relative incidence of hypoglycemia observed in the current study.11,12
While cerebral edema accounts for 60% to 90% of all deaths associated with DKA, the national incidence is relatively low, ranging from 0.5% to 0.9%.5 In this study, only 1 case of suspected cerebral edema was reported among the 102 admissions, and this case was not confirmed by imaging. With only 1 occurrence, a conclusion regarding a potential number needed to harm cannot be established for this clinically significant outcome.
Only 16 admissions (31%) in the LA group received long-acting insulin prior to resolution of acidosis. The remaining received insulin glargine after resolution, but within 24 hours of insulin infusion initiation. If the dose was not given at all during treatment of DKA, it was often ordered after DKA resolution following consultation with the endocrinology department. A subgroup analysis assessed EA and LA admissions that received long-acting insulin before acidosis resolution. Notably, the LA group exhibited significantly lower median blood pH and CO2 levels upon admission than the EA group. The median time to acidosis resolution (EA: 8.63 hours, LA: 15.04 hours; p = 0.0004), and median insulin infusion duration (EA: 16.3 hours, LA: 22.1 hours; p = 0.0208) were both significantly shorter with early administration. Hyperkalemia and hypoglycemia were not different between groups. These findings suggest that administering long-acting insulin within 4 hours may reduce the time to DKA resolution if given before acidosis resolution, without increasing adverse effects. However, the significantly worse baseline acidosis in the LA group warrants further investigation to validate this conclusion.
A subgroup of 16 admissions received insulin glargine prior to the initiation of the insulin infusion, which is not common practice. Because the institutional protocol did not clarify which insulin should be initiated first, insulin glargine was occasionally started more quickly than the insulin infusion. The significant differences in baseline characteristics among this subgroup compared with the rest of the EA group include a lower initial median BG concentration and increased incidence of new-onset T1DM. Importantly, there were no statistically significant differences observed in efficacy outcomes or adverse effects when compared with the rest of the population. The onset of action for insulin glargine, approximately 1.5 hours, would occur earlier in the course of DKA treatment in this particular population.17 Introducing a secondary insulin to the insulin infusion at an earlier stage in the treatment process warrants further research.
Considering that about two-thirds (64%) of included index admissions involved patients with new-onset T1DM, we opted to conduct an additional analysis to explore potential implications of a new T1DM diagnosis on clinical and safety outcomes. While this percentage is greater than some previously reported figures,11,12 it is similar to the approximately 53% of patients with new-onset T1DM in the study by Welter et al.14 No significant differences were observed in the duration of insulin infusion or PICU LOS between new-onset and previously established T1DM. Newly diagnosed patients had a longer median total hospital LOS of 3 days (IQR, 2–3) compared with 1 day (IQR, 1–2) for patients with established T1DM (p = 0.0024). This extended duration can likely be attributed to increased education requirements prior to discharge for newly diagnosed patients. While hypoglycemia did not occur more often in new-onset T1DM, these patients were 2.3 times more likely to develop hypokalemia. This finding correlates with the higher baseline BG and lower potassium serum concentrations seen in the new-onset T1DM group. This information suggests that BG and potassium concentrations at presentation in the context of a new T1DM diagnosis may serve as better predictors of the risk of adverse effects than the timing of the insulin glargine dose.
Although this study lacked randomization, the baseline characteristics and initial clinical values of the 2 groups were comparable. This strengthens the reliability of the findings and suggests that any observed differences between the groups are more likely to be attributed to the intervention rather than baseline disparities. Furthermore, the results of this study in conjunction with Welter et al14 help support a lack of definitive time frame to start long-acting insulin because administration early in admission or treatment phase may have benefits and it does not appear to increase adverse events.6,14 This differs from the ADA recommendation to begin long-acting insulin 2 to 4 hours prior to the infusion stop time.7
This study is subject to several limitations that merit discussion. As a retrospective analysis, this study depended on existing medical records, which may have inconsistencies in documentation that could affect data accuracy. There is also a potential for selection bias, because the timing of insulin glargine administration was determined by clinicians. However, given the absence of differences in time to clinical resolution and the substantial variability observed in both groups, it is unlikely that intentional selection of dosing time would have significantly influenced outcomes.
It is important to note that the start time of this study was based on the administration of the insulin infusion rather than the actual onset of DKA. While this approach is consistent with previous studies,10,11 it poses a limitation in precisely determining the timing of events and in direct comparison to studies using admission time as reference. The assessment of the exact timing of acidosis resolution is further complicated by routine laboratory checks conducted at 4-hour intervals after the first 4 hours of treatment. This is substantiated by the median time to acidosis resolution of approximately 8 hours, falling on the 4-hour interval schedule. This approach is implemented to minimize blood draws in pediatric patients but hinders the precise determination of DKA resolution timing. Approximately half of index admissions of unique patients were for new-onset T1DM, and each admission was considered independently with the goal of reducing demographic skew from patients with multiple readmissions; however, this does make it difficult to draw conclusions about age-related, disease-related, or prior treatment–related effects on outcomes. Additionally, adverse event timing and incidence per admission were not recorded. This information would contribute to a more comprehensive understanding of the relationship between insulin glargine timing and study outcomes.
Based on the findings in this study, our institutional practices for the treatment of pediatric patients with DKA will be updated to recommend dosing insulin glargine every evening at 2100 for new diagnosis patients, removing the option to administer a dose as soon as possible. For patients who use a long-acting insulin prior to admission, their home schedule should be continued. These revisions establish routine dosing schedules that are less likely to require timing adjustments later in the hospital stay and facilitate smoother transitions of care. To reduce the risk of hypoglycemia, the threshold to decrease the rate of the insulin infusion will be increased from 80 to 100 mg/dL to 100 to 130 mg/dL and dextrose will be supplemented earlier, at BG concentrations <300 mg/dL instead of BG <250 mg/dL. Future investigations should focus on exploring the effects of insulin glargine in the context of updated thresholds for clinical resolution of acidosis and in subpopulations, such as individuals with insulin pumps. By addressing these areas, comprehension of the therapeutic potential of insulin glargine in DKA treatment can be expanded to optimize patient outcomes.
Conclusion
Early administration of long-acting insulin in treatment of pediatric DKA did not affect the time to DKA resolution or rates of adverse effects when compared with later administration.
Supplementary Material
Acknowledgments.
Pediatric critical care medical team at Stormont Vail Health for their assistance. Preliminary results were presented at the ASHP Midyear Clinical Meeting in Las Vegas, NV, on December 7, 2022; PPAG Annual Meeting Resident Project Presentation in Dallas, TX, on May 5, 2023; and Stormont Vail Health Resident Grand Rounds on May 31, 2023.
ABBREVIATIONS
- ADA
American Diabetes Association;
- A1C
glycated hemoglobin A1C;
- BG
blood glucose;
- CO2
serum bicarbonate concentration;
- DKA
diabetic ketoacidosis;
- EA
early administration;
- ISPAD
International Society for Pediatric and Adolescent Diabetes;
- LA
late administration;
- LOS
length of stay;
- PICU
pediatric intensive care unit;
- T1DM
type 1 diabetes mellitus
Footnotes
Disclosure. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent. Exemption from Stormont Vail Health Institutional Review Board; IRB00008059, October 13, 2022.
Supplemental Material. DOI: 10.5683/1551-6776-29.6.614.S1
References
- 1.Smith CP, Firth D, Bennett S, et al. Ketoacidosis occurring in newly diagnosed and established diabetic children. Acta Paediatr . 1998;87(5):537–541. doi: 10.1080/08035259850158245. [DOI] [PubMed] [Google Scholar]
- 2.Ramphul K, Joynauth J. An update on the incidence and burden of diabetic ketoacidosis in the US [published correction appears in Diabetes Care. 2022;45(7):1698] Diabetes Care . 2020;43(12):e196–e197. doi: 10.2337/dc20-1258. [DOI] [PubMed] [Google Scholar]
- 3.Batwa M, Alharthi L, Ghazal R, et al. Diabetic ketoacidosis at the onset of type 1 diabetes mellitus among children and adolescents in Jeddah, Saudi Arabia: a study from the emergency department. Cureus . 2022;14(4):e24456. doi: 10.7759/cureus.24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi Z. Frequency of ketoacidosis in newly diagnosed type 1 diabetic children. Oman Med J . 2010;25(2):114–117. doi: 10.5001/omj.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes . 2018;19((suppl 27)):155–177. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 6.Glaser N, Fritsch M, Priyambada L, et al. ISPAD clinical practice consensus guidelines 2022: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes . 2022;23(7):835–856. doi: 10.1111/pedi.13406. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Professional Practice Committee. 16—Diabetes Care in the Hospital: Standards of Care in Diabetes-2024. Diabetes Care . 2024;47((suppl.1)):S295–S306. doi: 10.2337/dc24-S016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed A, Ploetz J, Hamarshi MS. Evaluation of early administration of insulin glargine in the acute management of diabetic ketoacidosis. Curr Diabetes Rev . 2021;17(8):e030221191986. doi: 10.2174/1573399817666210303095633. [DOI] [PubMed] [Google Scholar]
- 9.Lim Y, Ohn JH, Jeong J, et al. Effect of the concomitant use of subcutaneous basal insulin and intravenous insulin infusion in the treatment of severe hyperglycemic patients. Endocrinol Metab (Seoul) . 2022;37(3):444–454. doi: 10.3803/EnM.2021.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsia E, Seggelke S, Gibbs J, et al. Subcutaneous administration of glargine to diabetic patients receiving insulin infusion prevents rebound hyperglycemia. J Clin Endocrinol Metab . 2012;97(9):3132–3137. doi: 10.1210/jc.2012-1244. [DOI] [PubMed] [Google Scholar]
- 11.Shankar V, Haque A, Churchwell KB, Russell W. Insulin glargine supplementation during early management phase of diabetic ketoacidosis in children. Intensive Care Med . 2007;33(7):1173–1178. doi: 10.1007/s00134-007-0674-3. [DOI] [PubMed] [Google Scholar]
- 12.Harrison VS, Rustico S, Palladino AA, et al. Glargine co-administration with intravenous insulin in pediatric diabetic ketoacidosis is safe and facilitates transition to a subcutaneous regimen. Pediatr Diabetes . 2017;18(8):742–748. doi: 10.1111/pedi.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thammakosol K, Sriphrapradang C. Effectiveness and safety of early insulin glargine administration in combination with continuous intravenous insulin infusion in the management of diabetic ketoacidosis: a randomized controlled trial. Diabetes Obes Metab . 2023;25(3):815–822. doi: 10.1111/dom.14929. [DOI] [PubMed] [Google Scholar]
- 14.Welter KJ, Marquez JL, Marshik PL, et al. Evaluation of early insulin glargine administration in the treatment of pediatric diabetic ketoacidosis. J Pediatr Pharmacol Ther . 2023;28(2):149–155. doi: 10.5863/1551-6776-28.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakkar N, Salerno S, Hornik CP, Gonzalez D. Clinical pharmacology studies in critically ill children. Pharm Res . 2017;34(1):7–24. doi: 10.1007/s11095-016-2033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tieder JS, McLeod L, Keren R, et al. Variation in resource use and readmission for diabetic ketoacidosis in children's hospitals. Pediatrics . 2013;132(2):229–236. doi: 10.1542/peds.2013-0359. [DOI] [PubMed] [Google Scholar]
- 17.Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes . 2000;49(12):2142–2148. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


