Abstract
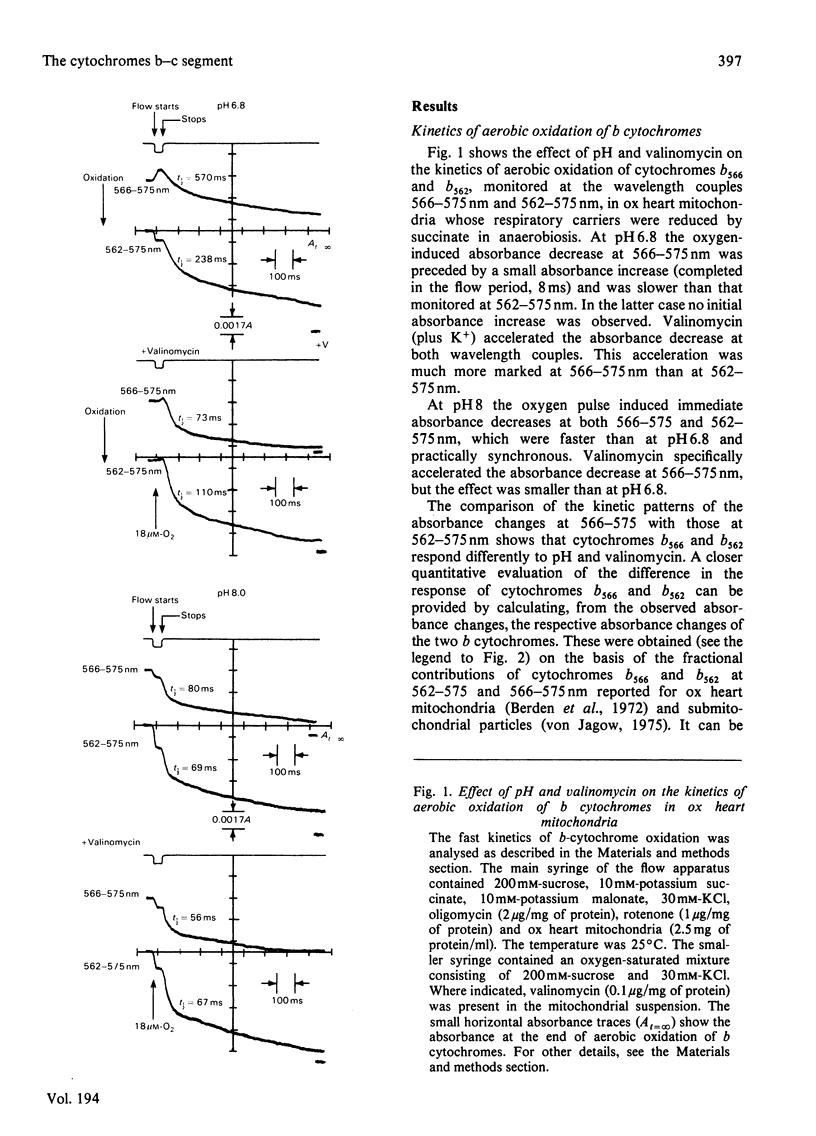
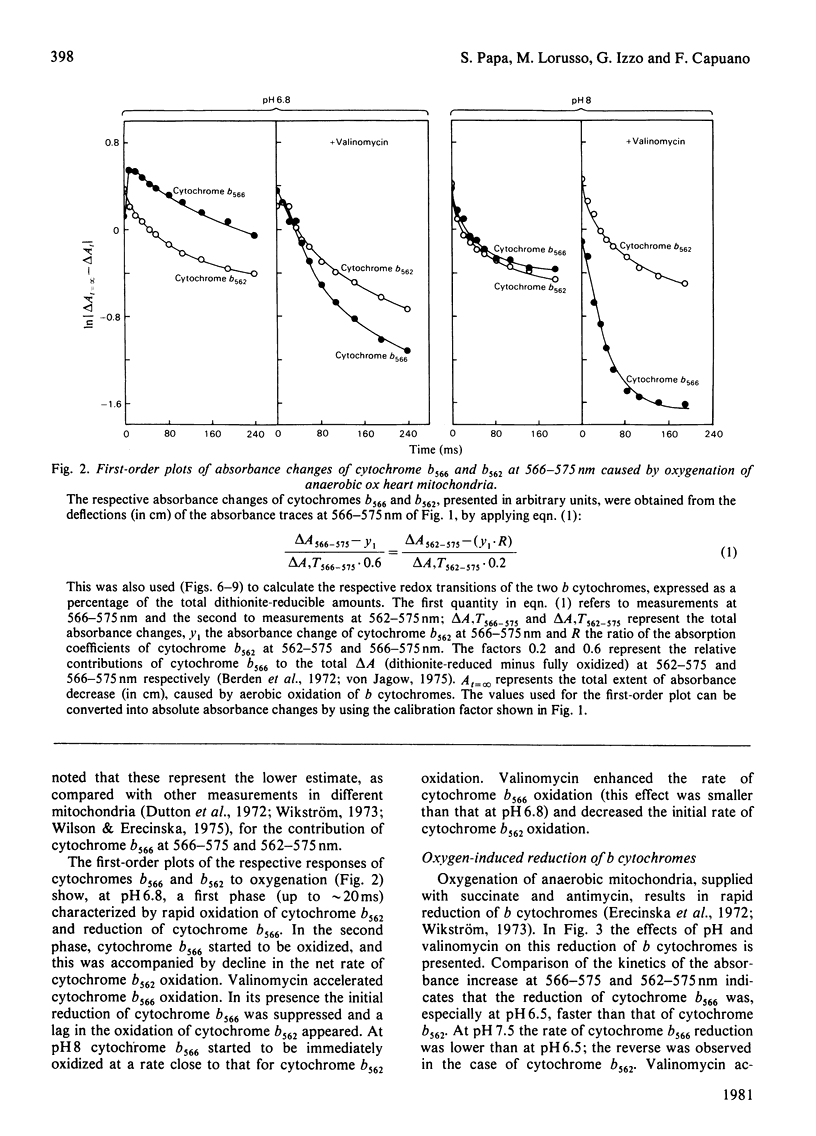
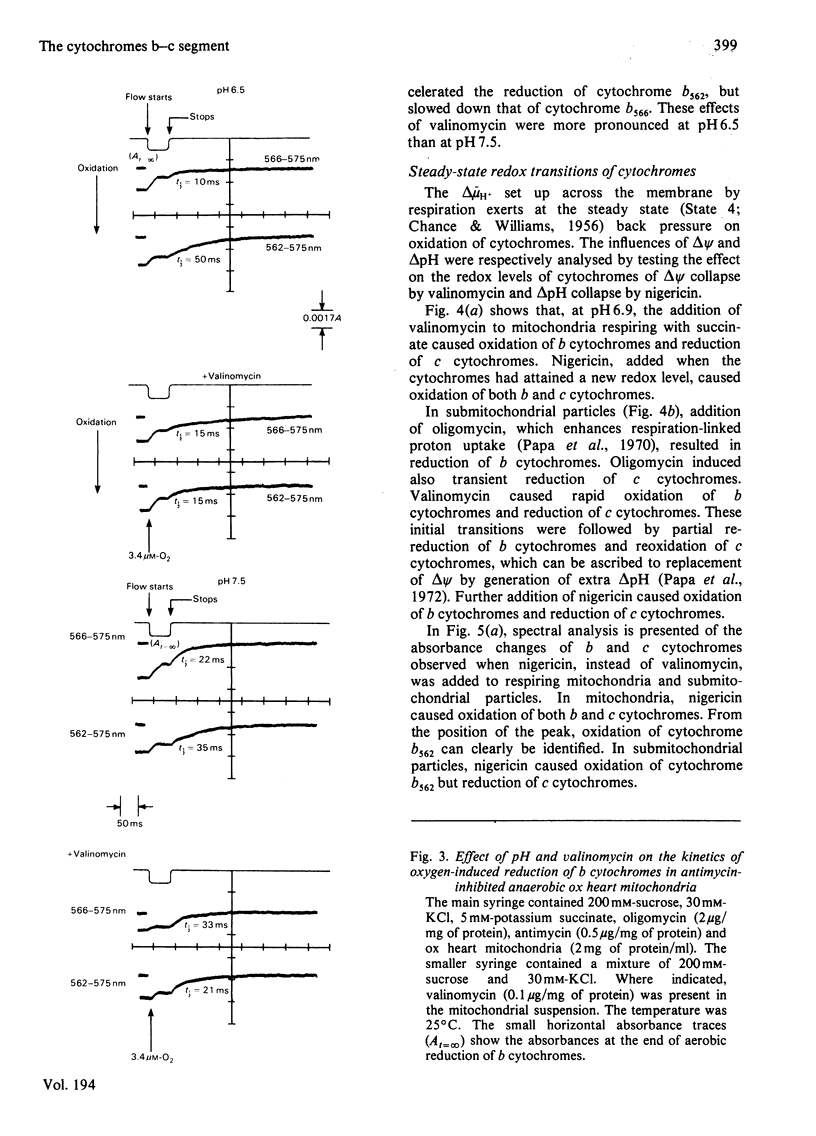
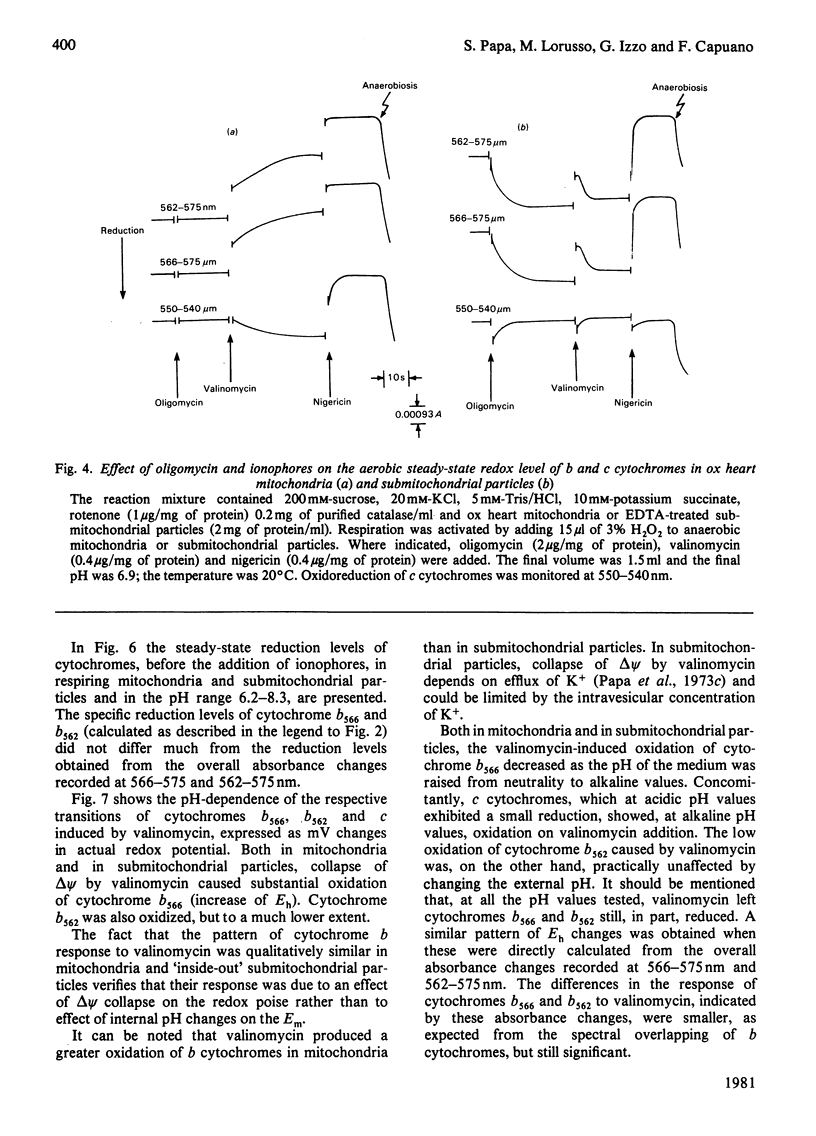
1. A study is presented of the effects of pH, transmembrane pH gradient and electrical potential on oxidoreductions of b and c cytochromes in ox heart mitochondria and 'inside-out' submitochondrial particles. 2. Kinetic analysis shows that, in mitochondria at neutral pH, there is a restraint on the aerobic oxidation of cytochrome b566 with respect to cytochrome b562. Valinomycin plus K+ accelerates cytochrome b566 oxidation and retards net oxidation of cytochrome b562. At alkaline pH the rate of cytochrome b566 oxidation approaches that of cytochrome b562 and the effects of valinomycin on b cytochromes are impaired. 3. At slightly acidic pH, oxygenation of antimycin-supplemented mitochondria causes rapid reduction of cytochrome b566 and small delayed reduction of cytochrome b562. Valinomycin or a pH increase in the medium promote reduction of cytochrome b562 and decrease net reduction of cytochrome b566. 4. Addition of valinomycin to mitochondria and submitochondrial particles in the respiring steady state causes, at pH values around neutrality, preferential oxidation of cytochrome b566 with respect to cytochrome b562. The differential effect of valinomycin on oxidation of cytochromes b566 and b562 is enhanced by substitution of 1H2O of the medium with 2H2O and tends to disappear as the pH of the medium is raised to alkaline values. 5. Nigericin addition in the aerobic steady state causes, both in mitochondria and submitochondrial particles, preferential oxidation of cytochrome b562 with respect to cytochrome b566. This is accompanied by c cytochrome oxidation in mitochondria but c cytochrome reduction in submitochondrial particles. 6. In mitochondria as well as in submitochondrial particles, the aerobic transmembrane potential (delta psi) does not change by raising the pH of the external medium from neutrality to alkalinity. The transmembrane pH gradient (delta pH) on the other hand, decrease slightly. 7. The results presented provide evidence that the delta psi component of the aerobic delta microH+ (the sum of the proton chemical and electrical activities) exerts a pH-dependent constraint on forward electron flow from cytochrome b566 to cytochrome b562. This effect is explained as a consequence of anisotropic location of cytochromes b566 and b562 in the membrane and the pH-dependence of the redox function of these cytochromes. Transmembrane delta pH, on the other hand, exerts control on electron flow from cytochrome b562 to c cytochromes.
Full text
PDF

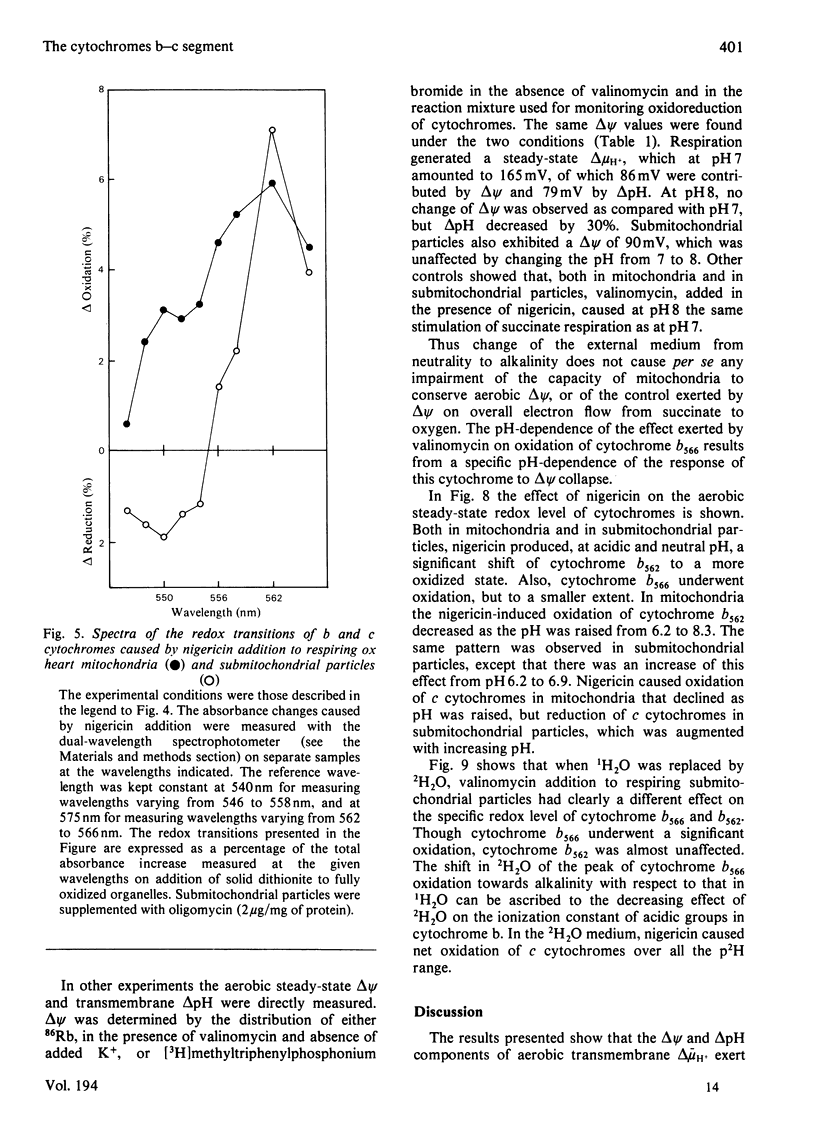
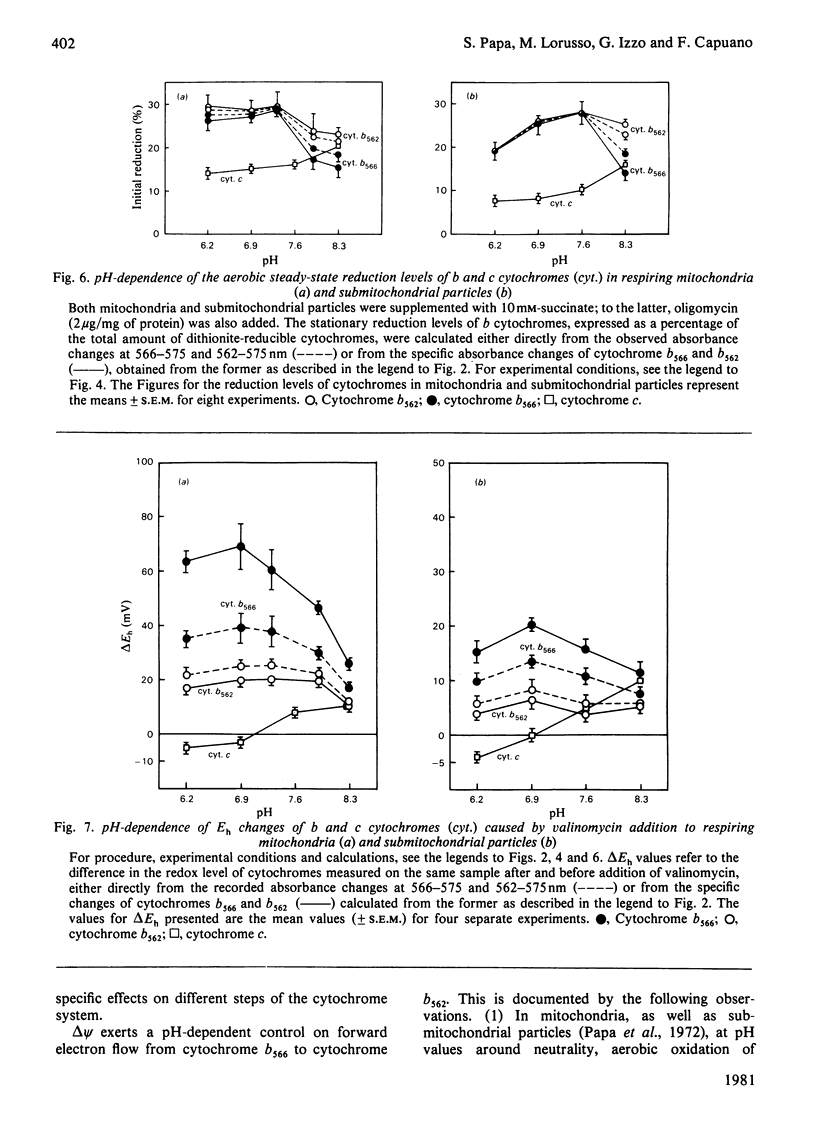
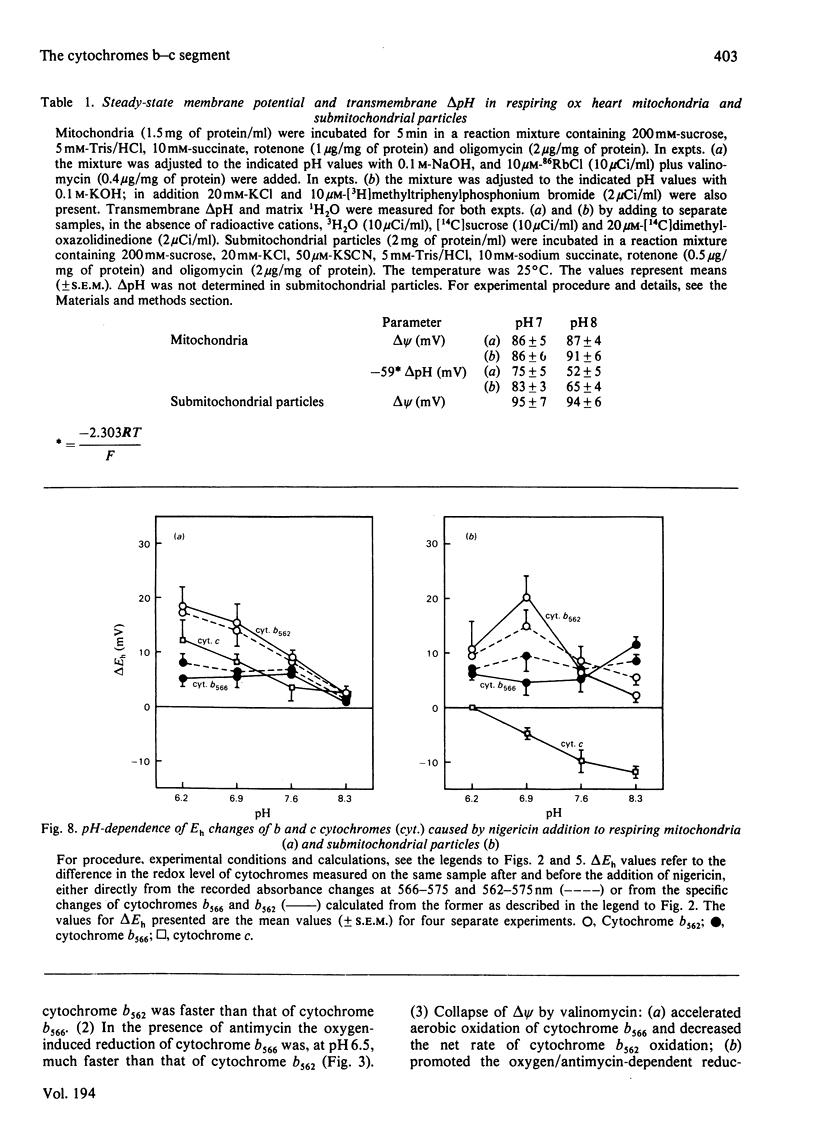
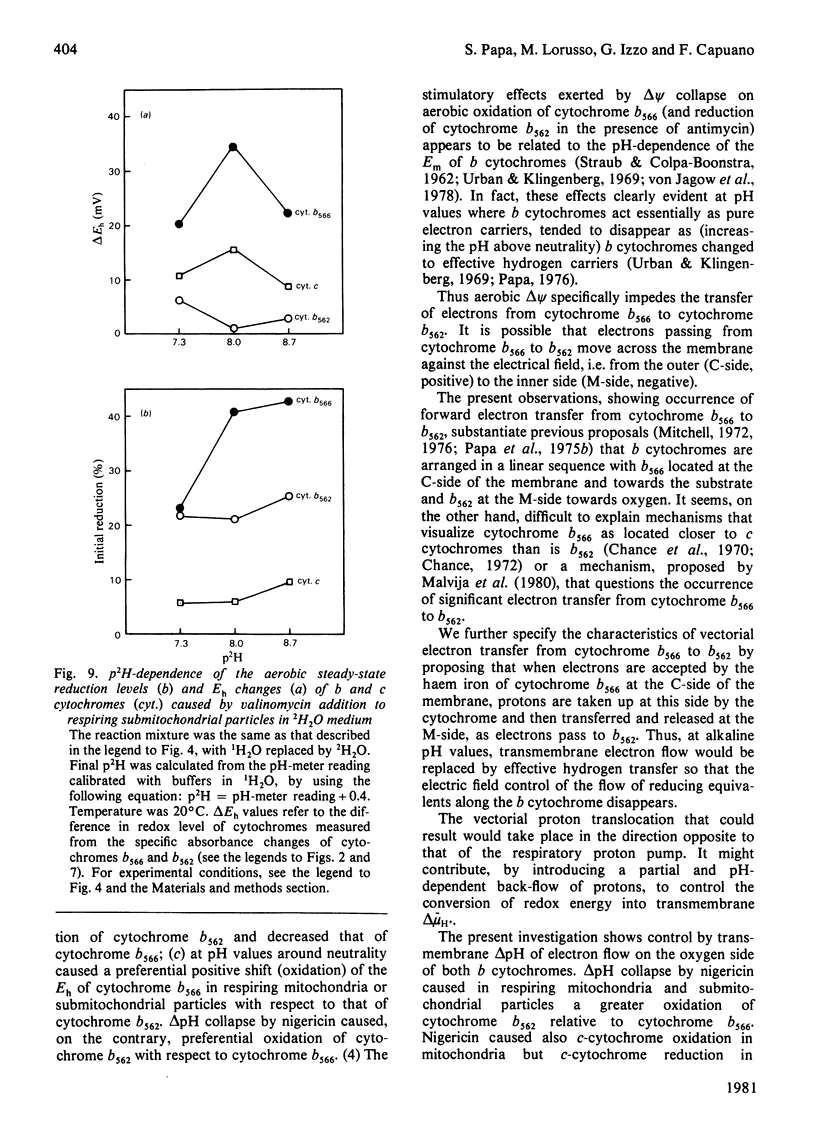


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum H., Rieske J. S., Silman H. I., Lipton S. H. On the mechanism of electron transfer in complex iii of the electron transfer chain. Proc Natl Acad Sci U S A. 1967 Mar;57(3):798–805. doi: 10.1073/pnas.57.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden J. A., Opperdoes F. R., Slater E. C. The effect of ATP on the apparent mid-point potentials of cytochrome b and cytochrome c in beef-heart mitochondria. Biochim Biophys Acta. 1972 Feb 28;256(2):594–599. doi: 10.1016/0005-2728(72)90088-6. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B. The nature of electron transfer and energy coupling reactions. FEBS Lett. 1972 Jun 1;23(1):3–20. doi: 10.1016/0014-5793(72)80272-2. [DOI] [PubMed] [Google Scholar]
- Chance B., Wilson D. F., Dutton P. L., Erecińska M. Energy-coupling mechanisms in mitochondria: kinetic, spectroscopic, and thermodynamic properties of an energy-transducing form of cytochrome b. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1175–1182. doi: 10.1073/pnas.66.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Erecinska M., Sato N., Mukai Y., Pring M., Wilson D. F. Reactions of b-cytochromes with ATP and antimycin A in pigeon heart mitochondria. Biochim Biophys Acta. 1972 Apr 20;267(1):15–24. doi: 10.1016/0005-2728(72)90134-x. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F. Redox potentiometry in mitochondrial and photosynthetic bioenergetics. Biochim Biophys Acta. 1974 Oct 31;346(2):165–212. doi: 10.1016/0304-4173(74)90008-1. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Chance B., Wilson D. F., Dutton P. L. Aerobic reduction of cytochrome b 566 in pigeon-heart mitochondria (succinate-cytochrome C1 reductase-stopped-flow kinetics). Proc Natl Acad Sci U S A. 1972 Jan;69(1):50–54. doi: 10.1073/pnas.69.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F., Miyata Y. Mitochondrial cytochrome b-c complex: its oxidation-reduction components and their stoichiometry. Arch Biochem Biophys. 1976 Nov;177(1):133–143. doi: 10.1016/0003-9861(76)90423-9. [DOI] [PubMed] [Google Scholar]
- Kell D. B., John P., Sorgato M. C., Ferguson S. J. Continuous monitoring of the electrical potential across energy-transducing membranes using ion-selective electrodes. Application to submitochondrial particles and chromatophores. FEBS Lett. 1978 Feb 15;86(2):294–298. doi: 10.1016/0014-5793(78)80583-3. [DOI] [PubMed] [Google Scholar]
- Lawford H. G., Garland P. B. Proton translocation coupled to quinol oxidation in ox heart mitochondria. Biochem J. 1973 Nov;136(3):711–720. doi: 10.1042/bj1360711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Ernster L. Studies of the energy-transfer system of submitochondrial particles. 2. Effects of oligomycin and aurovertin. Eur J Biochem. 1968 Feb;3(4):391–400. doi: 10.1111/j.1432-1033.1967.tb19542.x. [DOI] [PubMed] [Google Scholar]
- Leung K. H., Hinkle P. C. Reconstitution of ion transport and respiratory control in vesicles formed from reduced coenzyme Q-cytochrome c reductase and phospholipids. J Biol Chem. 1975 Nov 10;250(21):8467–8471. [PubMed] [Google Scholar]
- Malviya A. N., Nicholls P., Elliott W. B. Observations on the oxidoreduction of the two cytochromes b in cytochrome c-deficient mitochondria and submitochondrial particles. Biochim Biophys Acta. 1980 Jan 4;589(1):137–149. doi: 10.1016/0005-2728(80)90137-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976 Oct 21;62(2):327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Izzo G. Redox Bohr-effects in the cytochrome system of mitochondria. FEBS Lett. 1979 Sep 15;105(2):213–216. doi: 10.1016/0014-5793(79)80614-6. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Lorusso M. Mechanism of respiration-driven proton translocation in the inner mitochondrial membrane. Analysis of proton translocation associated to oxido-reductions of the oxygen-terminal respiratory carriers. Biochim Biophys Acta. 1974 Aug 23;357(2):181–192. doi: 10.1016/0005-2728(74)90059-0. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Lorusso M., Simone S. Proton translocation and energy transduction in mitochondria. Biochimie. 1973;55(6):703–716. doi: 10.1016/s0300-9084(73)80024-0. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Rossi Bernardi L., Tager J. M. Effect of oligomygin on proton translocation in submitochondrial particles. Biochim Biophys Acta. 1970 Jan 13;197(1):100–103. doi: 10.1016/0005-2728(70)90016-2. [DOI] [PubMed] [Google Scholar]
- Papa S., Guerrieri F., Simone S., Lorusso M., Larosa D. Mechanism of respiration-driven proton translocation in the inner mitochondrial membrane. Biochim Biophys Acta. 1973 Jan 18;292(1):20–38. doi: 10.1016/0005-2728(73)90247-8. [DOI] [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Papa S., Scarpa A., Lee C. P., Chance B. Effect of ion conductance changes in the mitochondrial membrane on the kinetics of respiratory carriers. Biochemistry. 1972 Aug 1;11(16):3091–3098. doi: 10.1021/bi00766a024. [DOI] [PubMed] [Google Scholar]
- Papa S., Storey B. T., Lorusso M., Lee C. P., Chance B. Energy-linked swelling of EDTA submitochondrial particles. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1395–1402. doi: 10.1016/0006-291x(73)90656-6. [DOI] [PubMed] [Google Scholar]
- Prince R. G., Dutton P. L. Further studies on the Rieske iron-sulfur center in mitochondrial and photosynthetic systems: a pK on the oxidized form. FEBS Lett. 1976 May 15;65(1):117–119. doi: 10.1016/0014-5793(76)80634-5. [DOI] [PubMed] [Google Scholar]
- Rieske J. S. Composition, structure, and function of complex III of the respiratory chain. Biochim Biophys Acta. 1976 Sep 27;456(2):195–247. doi: 10.1016/0304-4173(76)90012-4. [DOI] [PubMed] [Google Scholar]
- STRAUB J. P., COLPA-BOONSTRA J. P. The effect of pH on the oxidation-reduction potential of cytochrome b in heart-muscle preparations. Biochim Biophys Acta. 1962 Jul 16;60:650–652. doi: 10.1016/0006-3002(62)90887-9. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Urban P. F., Klingenberg M. On the redox potentials of ubiquinone and cytochrome b in the respiratory chain. Eur J Biochem. 1969 Jul;9(4):519–525. doi: 10.1111/j.1432-1033.1969.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Weiss H. Subunit composition and biogenesis of mitochondrial cytochrome b. Biochim Biophys Acta. 1976 Nov 30;456(3-4):291–313. doi: 10.1016/0304-4173(76)90002-1. [DOI] [PubMed] [Google Scholar]
- Wikström M. K., Lambowitz A. M. The interaction of redox mediators with the 'second phosphorylation site'; significance for the cytochrome bT hypothesis. FEBS Lett. 1974 Mar 15;40(1):149–153. doi: 10.1016/0014-5793(74)80915-4. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M. Thermodynamic relationships among cytochrome b k, cytochrome b t, and ubiquinone in mitochondria. Arch Biochem Biophys. 1975 Mar;167(1):116–128. doi: 10.1016/0003-9861(75)90447-6. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Lindsay J. G., Brocklehurst E. S. Heme-heme interaction in cytochrome oxidase. Biochim Biophys Acta. 1972 Feb 28;256(2):277–286. doi: 10.1016/0005-2728(72)90058-8. [DOI] [PubMed] [Google Scholar]
- Wyman J. Regulation in macromolecules as illustrated by haemoglobin. Q Rev Biophys. 1968 May;1(1):35–80. doi: 10.1017/s0033583500000457. [DOI] [PubMed] [Google Scholar]


