Abstract
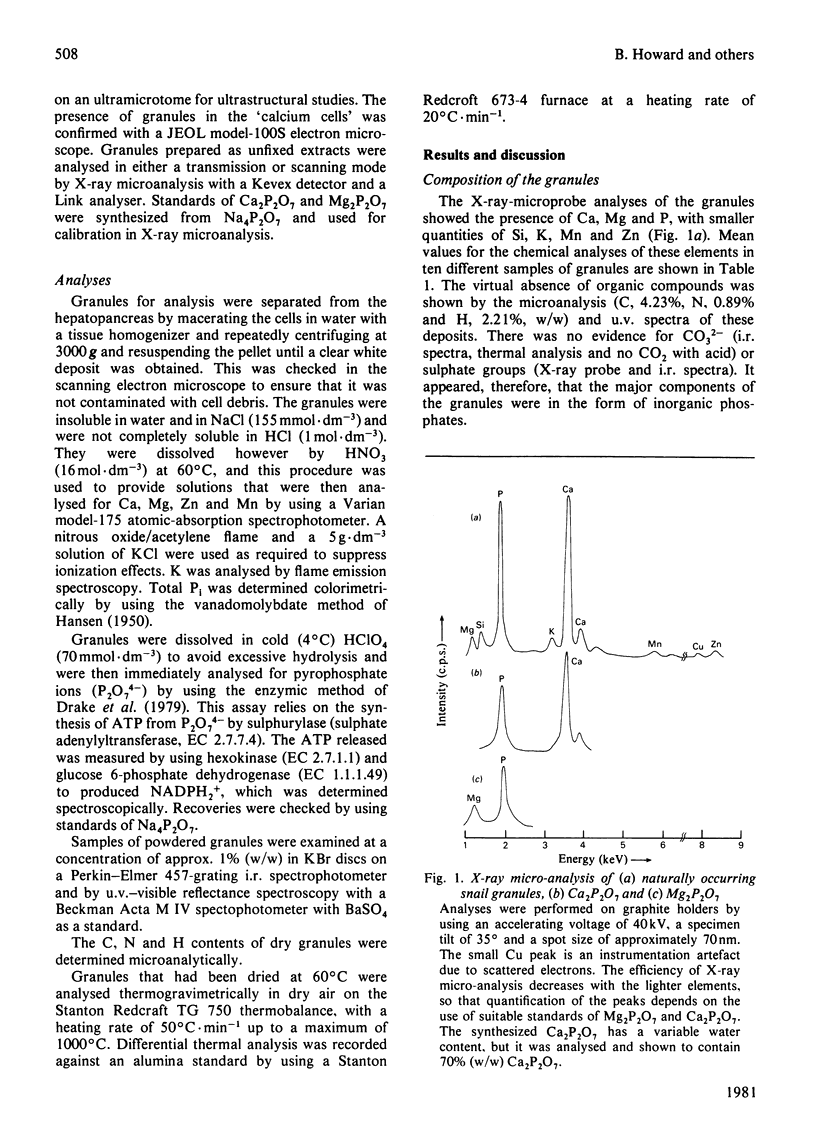
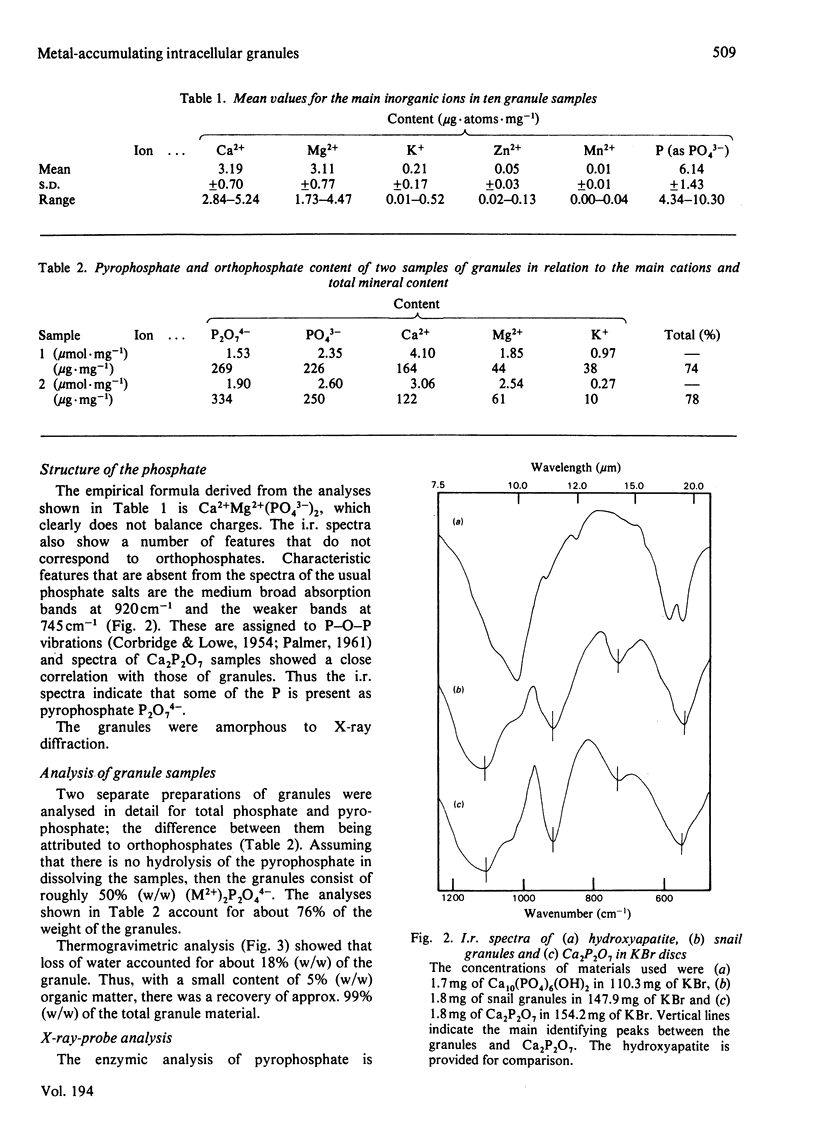
Certain cells in the hepatopancreas of the common garden snail (Helix aspersa) contain intracellular granules that are sites of metal-ion accumulation. These granules have been extracted and investigated by u.v. and i.r. spectroscopy, atomic-absorption spectroscopy, X-ray microanalysis, thermogravimetric analysis, enzymic assay and microanalysis. The deposits contain about 18% (w/w) water, 5% (w/w) organic matter and 76% (w/w) inorganic material of which the main components are Ca2+, Mg2+ and P2O7(4)-. The possible origin of these granules is discussed, as is their role in detoxifying heavy-metal ions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abolins-Krogis A. Electron microscope studies of the intracellular origin and formation of calcifying granules and calcium spherites in the hepatopancreas of the snail, Helix pomatia L. Z Zellforsch Mikrosk Anat. 1970;108(4):501–515. doi: 10.1007/BF00339656. [DOI] [PubMed] [Google Scholar]
- Becker G. L., Chen C. H., Greenawalt J. W., Lehninger A. L. Calcium phosphate granules in the hepatopancreas of the blue crab Callinectes sapidus. J Cell Biol. 1974 May;61(2):316–326. doi: 10.1083/jcb.61.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R. F. The storage of calcium and magnesium phosphates and of calcite in the digestive glands of the pulmonata (gastropoda). Comp Biochem Physiol A Comp Physiol. 1972 Nov 1;43(3):655–663. doi: 10.1016/0300-9629(72)90252-6. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Goss N. H., Wood H. G. A new, convenient method for the rapid analysis of inorganic pyrophosphate. Anal Biochem. 1979 Apr 1;94(1):117–120. doi: 10.1016/0003-2697(79)90800-5. [DOI] [PubMed] [Google Scholar]
- Flodgaard H., Fleron P. Thermodynamic parameters for the hydrolysis of inorganic pyrophosphate at pH 7.4 as a function of (Mg2+), (K+), and ionic strength determined from equilibrium studies of the reaction. J Biol Chem. 1974 Jun 10;249(11):3465–3474. [PubMed] [Google Scholar]
- Rosenberg H. The isolation and identification of "volutin" granules from Tetrahymena. Exp Cell Res. 1966 Feb;41(2):397–410. doi: 10.1016/s0014-4827(66)80147-7. [DOI] [PubMed] [Google Scholar]
- Russell R. G. Metabolism of inorganic pyrophosphate (PPi). Arthritis Rheum. 1976 May-Jun;19 (Suppl 3):465–478. doi: 10.1002/1529-0131(197605/06)19:3+<465::aid-art1780190722>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- VONBRAND T., SCOTT D. B., NYLEN M. U., PUGH M. H. VARIATIONS IN THE MINERALOGICAL COMPOSITION OF CESTODE CALCAREOUS CORPUSCLES. Exp Parasitol. 1965 Jun;16:382–391. doi: 10.1016/0014-4894(65)90061-5. [DOI] [PubMed] [Google Scholar]


