Abstract
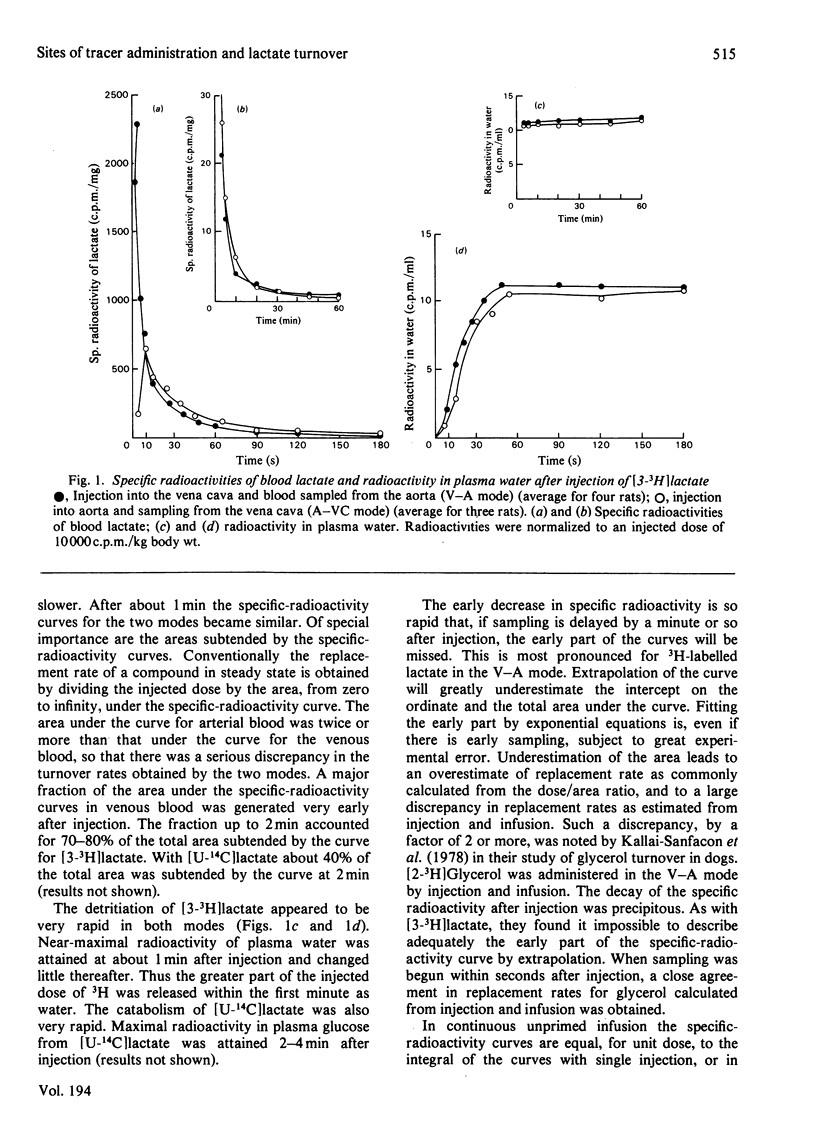
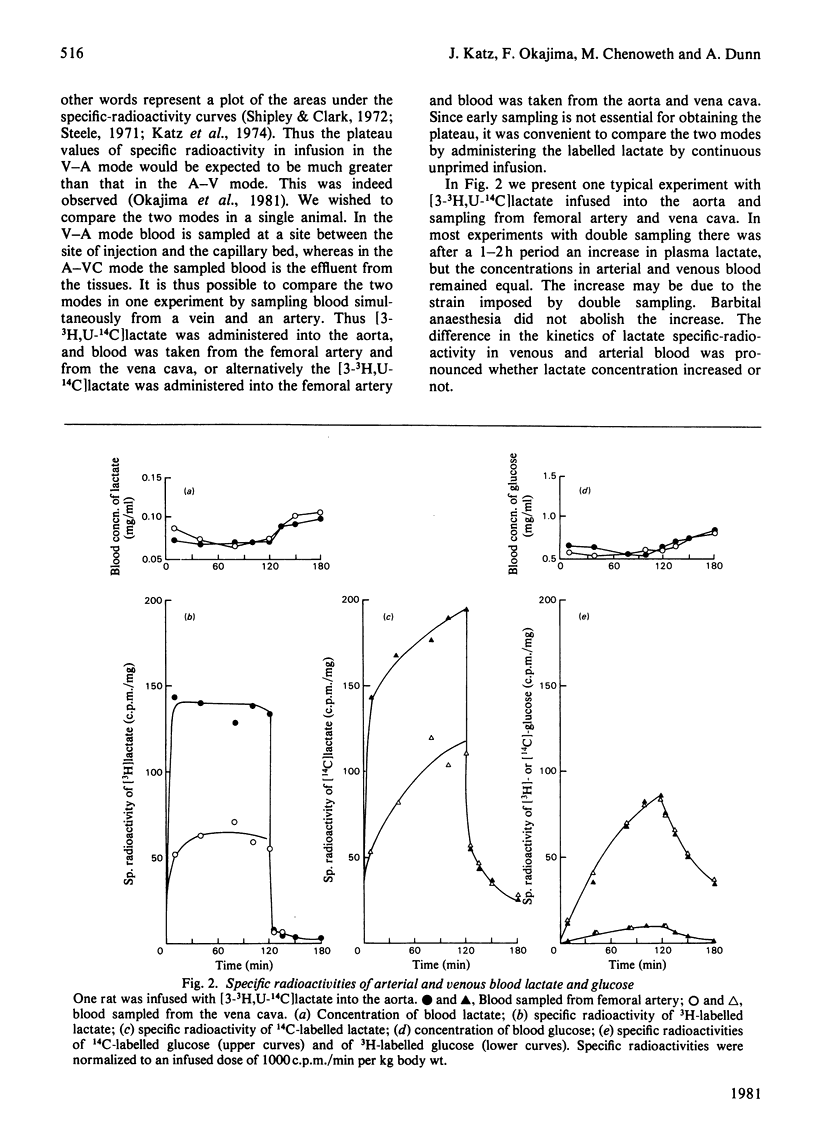
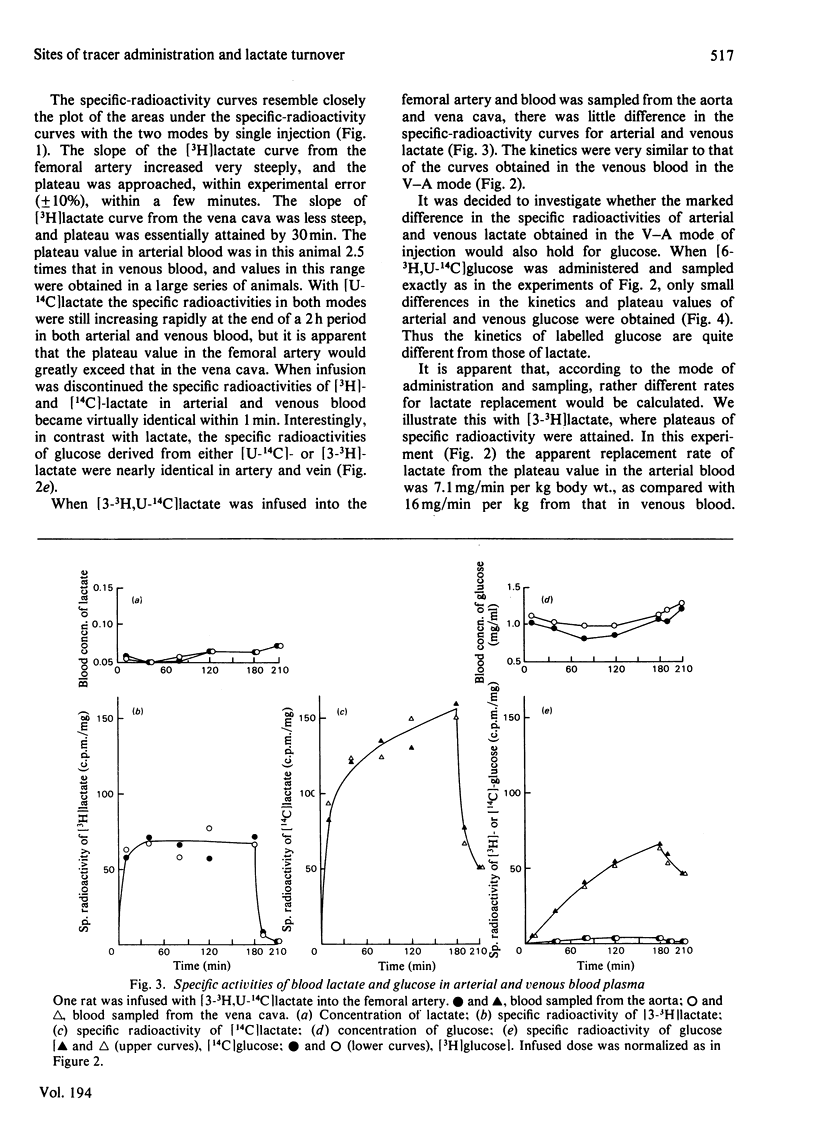
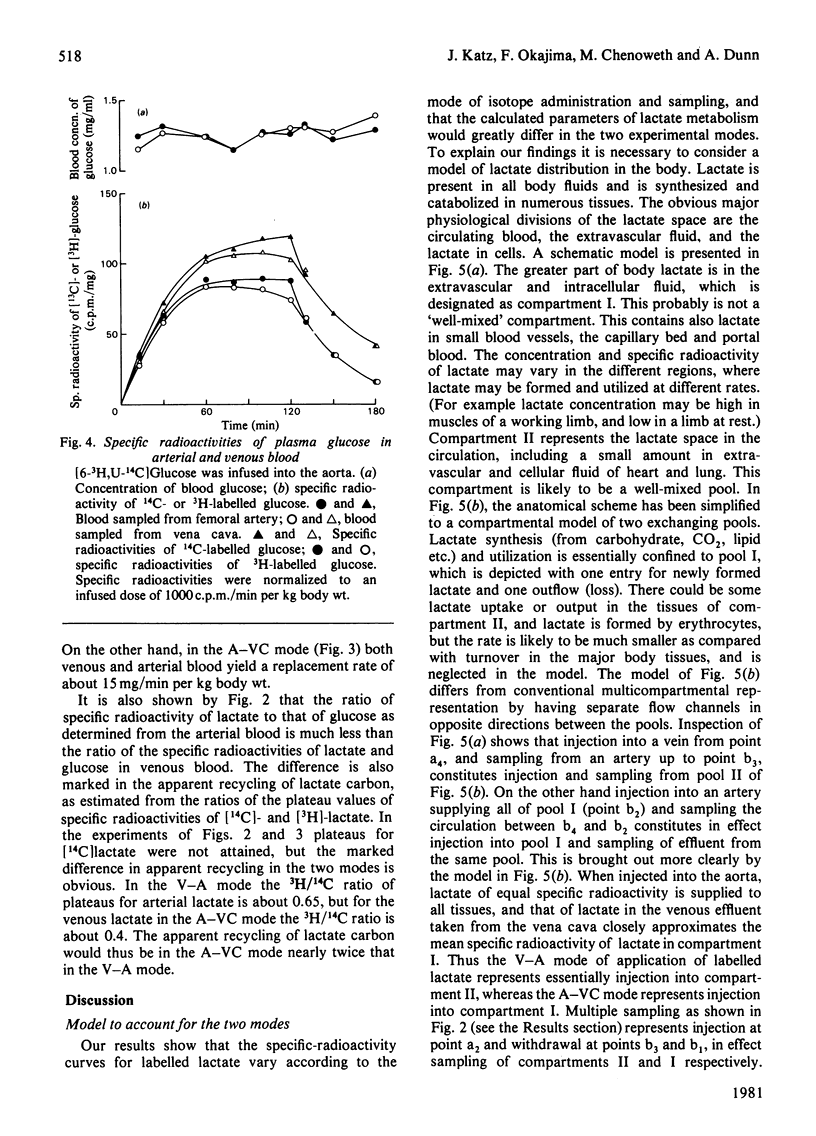
L-[3-3H,U-14C]Lactate was administered to starved rats either as a bolus or by continuous infusion. Tracer administration was performed two ways: injection into the vena cava and sampling from the aorta (V-A mode), or injection into the aorta and sampling from the vena cava (A-VC mode). The specific-radioactivity curves after infusion or injection differed markedly with the two procedures. However, the specific radioactivities of 14C-labelled glucose derived from [U-14C]lactate were similar in the two modes. The apparent turnover rates of lactate calculated from the 3H specific-radioactivity curves in the V-A mode were about half those obtained from the 3H specific-radioactivity curves in the A-VC mode. The apparent contribution of lactate carbon to glucose carbon calculated from specific-radioactivity curves of the A-VC mode was greater than that obtained from the V-A mode. The apparent recycling of lactate carbon calculated from the specific radioactivities for [U-14C]- and [3-3H]-lactate was greater in the A-VC mode than the V-A mode. [U-14C] Glucose was administered in the two modes, but in contrast with lactate the specific radioactivities were only slightly different. An analysis to account for these observations is presented. It is shown that the two modes represent sampling from different pools of lactate. The significance of sites of tracer administration and sampling for the interpretation of tracer kinetics of compounds present in intracellular and extracellular spaces, and with a high turnover rate, is discussed. We propose that for such compounds, including lactate, alanine and glycerol, the widely used V-A mode leads to a marked underestimate of replacement, mass and carbon recycling, and that the A-VC mode is the preferred method for the assessment of these parameters.
Full text
PDF

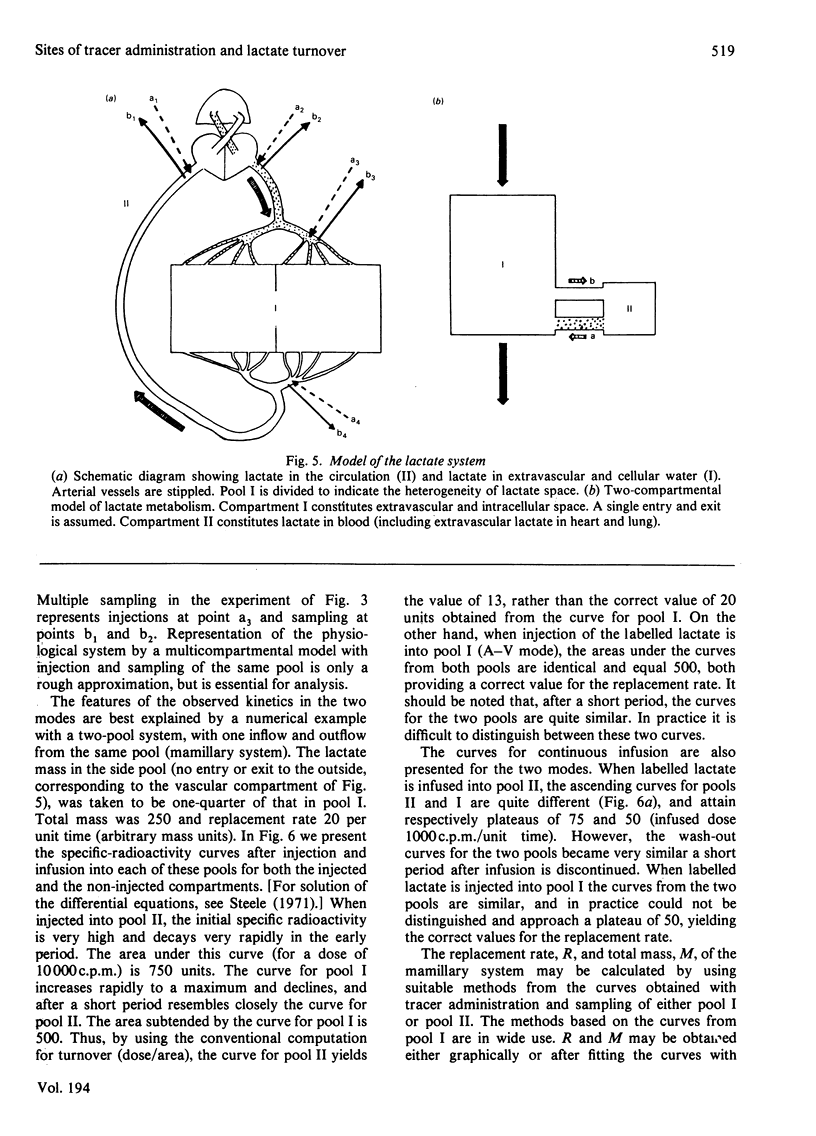
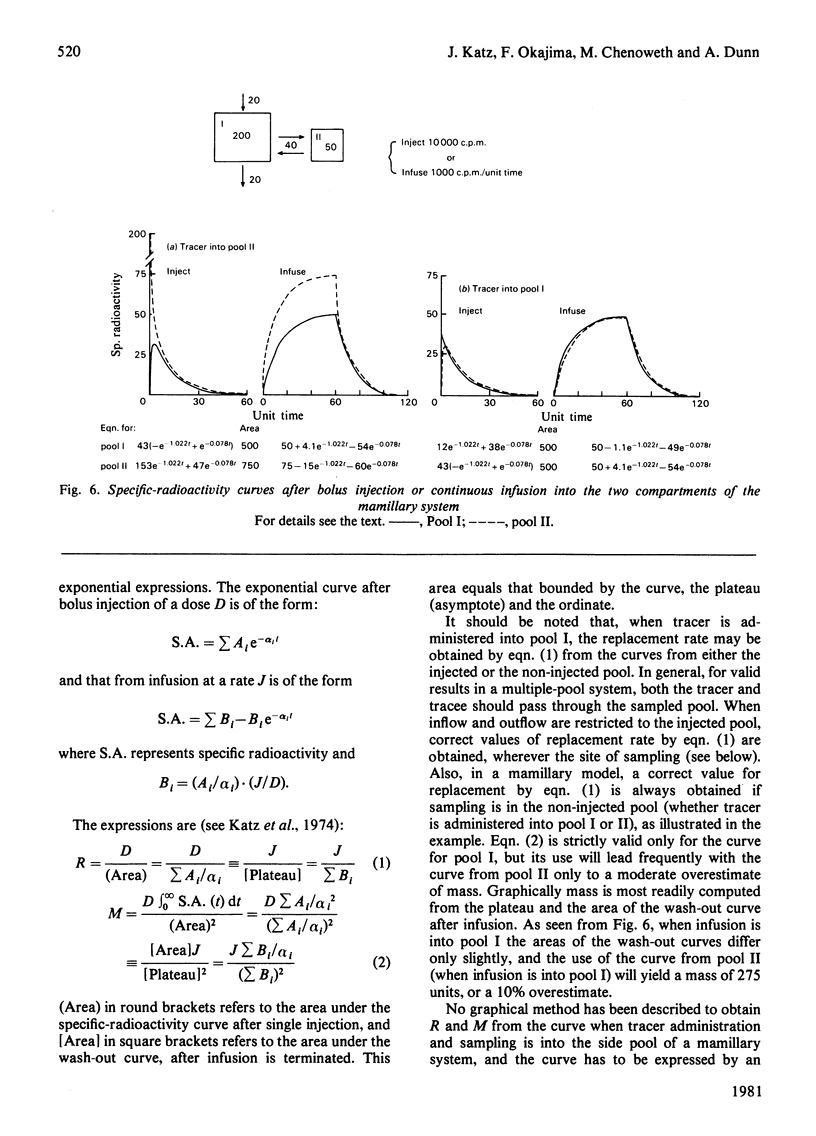



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- Depocas F., Minaire Y., Chatonnet J. Rates of formation and oxidation of lactic acid in dogs at rest and during moderate exercise. Can J Physiol Pharmacol. 1969 Jul;47(7):603–610. doi: 10.1139/y69-106. [DOI] [PubMed] [Google Scholar]
- Forbath N., Hetenyi G., Jr Metabolic interrelations of glucose and lactate in unanesthetized normal and diabetic dogs. Can J Physiol Pharmacol. 1970 Feb;48(2):115–122. doi: 10.1139/y70-019. [DOI] [PubMed] [Google Scholar]
- Freminet A., Poyart C. Lactate-glucose interrelations, glucose recycling and the Cori cycle in normal fed rats. Pflugers Arch. 1975 Dec 19;361(1):25–31. doi: 10.1007/BF00587336. [DOI] [PubMed] [Google Scholar]
- Kallai-Sanfaçon M. A., Norwich K. H., Steiner G. A new approach to the measurement of glycerol turnover. Can J Physiol Pharmacol. 1978 Dec;56(6):934–939. doi: 10.1139/y78-148. [DOI] [PubMed] [Google Scholar]
- Katz J., Rostami H., Dunn A. Evaluation of glucose turnover, body mass and recycling with reversible and irreversible tracers. Biochem J. 1974 Jul;142(1):161–170. doi: 10.1042/bj1420161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg R. A., Pennington L. F., Boshell B. R. Lactate turnover and gluconeogenesis in normal and obese humans. Effect of starvation. Diabetes. 1970 Jan;19(1):53–63. doi: 10.2337/diab.19.1.53. [DOI] [PubMed] [Google Scholar]
- Kusaka M., Ui M. Tracer kinetic analysis of Cori cycle activity in the rat: effect of feeding. Am J Physiol. 1977 Feb;232(2):E136–E144. doi: 10.1152/ajpendo.1977.232.2.E136. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Minaire Y., Pernod A., Jomain M. J., Mottaz M. Lactate turnover and oxidation in normal and adrenal-demedullated dogs during cold exposure. Can J Physiol Pharmacol. 1971 Dec;49(12):1063–1070. doi: 10.1139/y71-151. [DOI] [PubMed] [Google Scholar]
- ROSE I. A. Studies on the enolization of pyruvate by pyruvate kinase. J Biol Chem. 1960 Apr;235:1170–1177. [PubMed] [Google Scholar]
- Reilly P. E., Chandrasena L. G. Sheep lactate entry-rate measurements: error due to sampling jugular blood. Am J Physiol. 1977 Sep;233(3):E138–E140. doi: 10.1152/ajpendo.1977.233.3.E138. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Wals P. The metabolism of l-[3-3h]lactate by isolated hamster liver cells. Biochim Biophys Acta. 1976 Jun 23;437(1):16–21. doi: 10.1016/0304-4165(76)90343-3. [DOI] [PubMed] [Google Scholar]
- Searle G. L., Cavalieri R. R. Determination of lactate kinetics in the human analysis of data from single injection vs. continuous infusion methods. Proc Soc Exp Biol Med. 1972 Mar;139(3):1002–1006. doi: 10.3181/00379727-139-36284. [DOI] [PubMed] [Google Scholar]
- Wiener R., Spitzer J. J. Lactate metabolism following severe hemorrhage in the conscious dog. Am J Physiol. 1974 Jul;227(1):58–62. doi: 10.1152/ajplegacy.1974.227.1.58. [DOI] [PubMed] [Google Scholar]


