Abstract
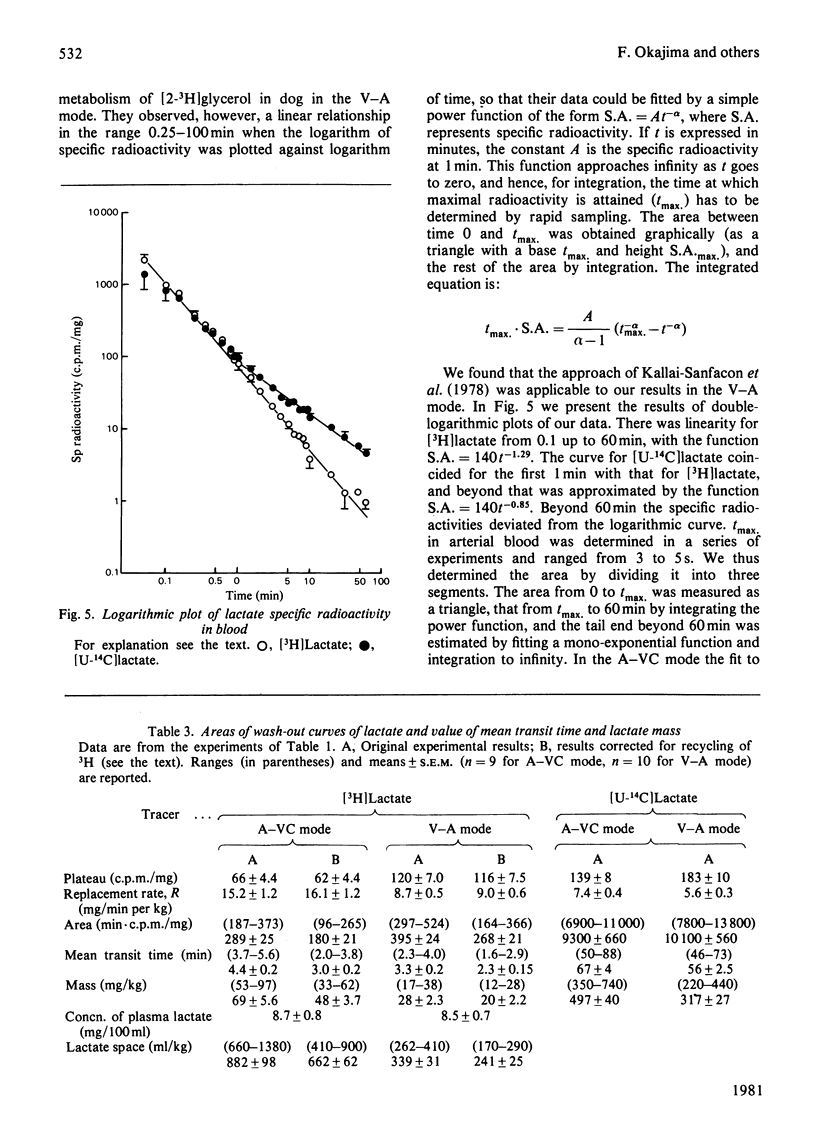
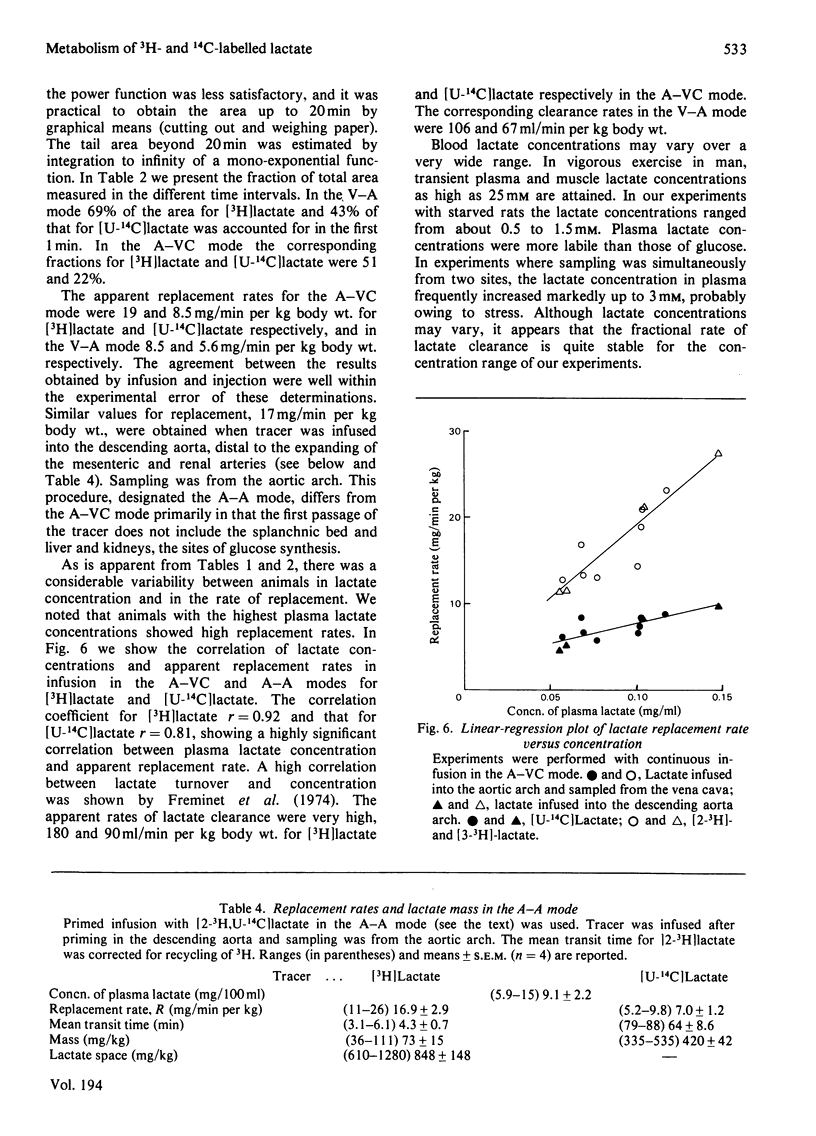
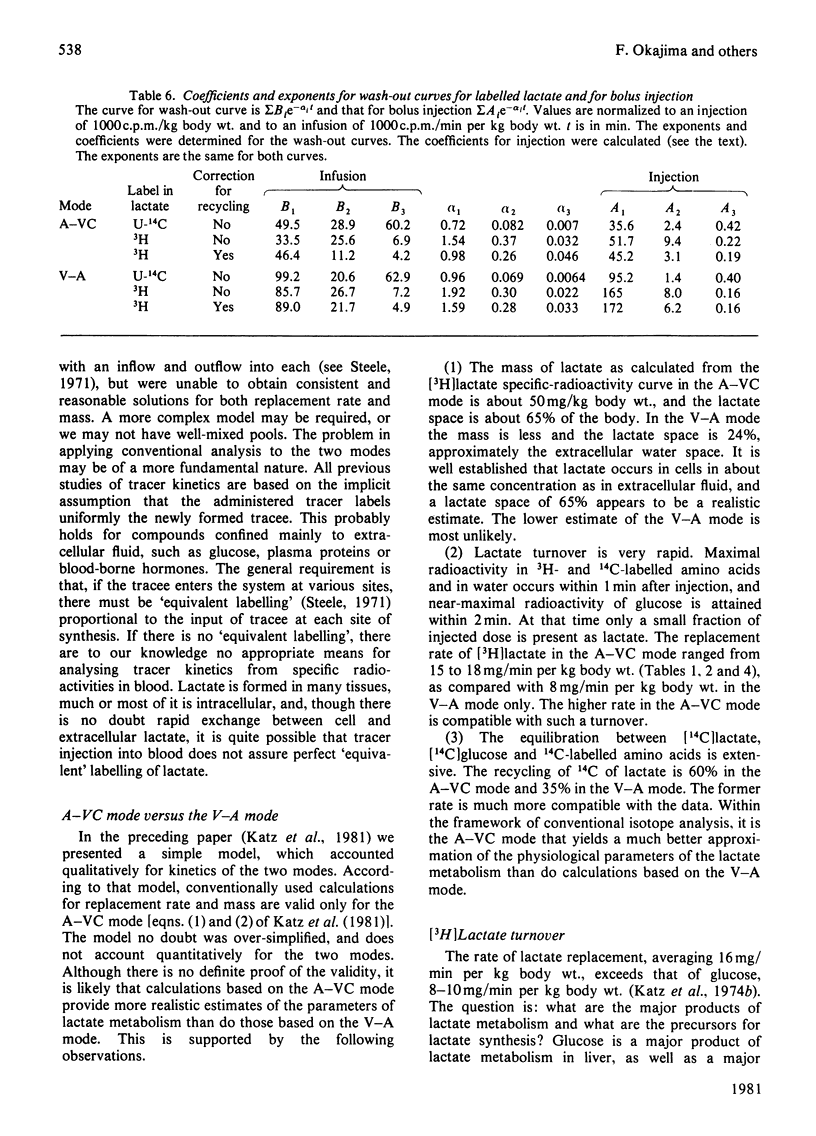
1. [2-3H,U-14C]- or [3-3H,U-14C]-Lactate was administered by infusion or bolus injection to overnight-starved rats. Tracer lactate was injected or infused through indwelling cannulas into the aorta and blood was sampled from the vena cava (A–VC mode), or it was administered into the vena cava and sampled from the aorta (V–A mode). Sampling was continued after infusion was terminated to obtain the wash-out curves for the tracer. The activities of lactate, glucose, amino acids and water were followed. 2. The kinetics of labelled lactate in the two modes differed markedly, but the kinetics of labelled glucose were much the same irrespective of mode. 3. The kinetics of 3H-labelled lactate differed markedly from those for [U-14C]lactate. Isotopic steady state was attained in less than 1h of infusion of [3H]lactate but required over 6h for [U-14C]lactate. 4. 3H from [2-3H]lactate labels glucose more extensive than does that from [3-3H]lactate. [3-3H]Lactate also labels plasma amino acids. The distribution of 3H in glucose was determined. 5. Maximal radioactivity in 3HOH in plasma is attained in less than 1min after injection. Near-maximal radioactivity in [14C]glucose and [3H]glucose is attained within 2–3min after injection. 6. The apparent replacement rates for lactate were calculated from the areas under the specific-radioactivity curves or plateau specific radioactivities after primed infusion. Results calculated from bolus injection and infusion agreed closely. The apparent replacement rate for [3H]lactate from the A–VC mode averaged about 16mg/min per kg body wt. and that in the V–A mode about 8.5mg/min per kg body wt. The apparent rates for [14C]lactate (`rate of irreversible disposal') were 8mg/min per kg body wt. for the A–VC mode and 5.5mg/min per kg body wt. for the V–A mode. Apparent recycling of lactate carbon was 55–60% according to the A–VC mode and 35% according to the V–A mode. 7. The specific radioactivities of [U-14C]glucose at isotopic steady state were 55% and 45% that of [U-14C]lactate in the A–VC and V–A modes respectively. We calculated, correcting for the dilution of 14C in gluconeogenesis via oxaloacetate, that over 70% of newly synthesized glucose was derived from circulating lactate. 8. Recycling of 3H between lactate and glucose was evaluated. It has no significant effect on the calculation of the replacement rate, but affects considerably the areas under the wash-out curves for both [2-3H]- and [3-3H]-lactate, and calculation of mean transit time and total lactate mass in the body. Corrected for recycling, in the A–VC mode the mean transit time is about 3min, the lactate mass about 50mg/kg body wt. and the lactate space about 65% of body space. The V–A mode yields a mass and lactate space about half those with the A–VC mode. 9. The area under the wash-out curve for [14C]lactate is some 20–30 times that for [3H]lactate, and apparent carbon mass is 400–500mg/kg body wt. and presumably includes the carbon of glucose, pyruvate and amino acids, which are exchanging rapidly with that of lactate.
Full text
PDF


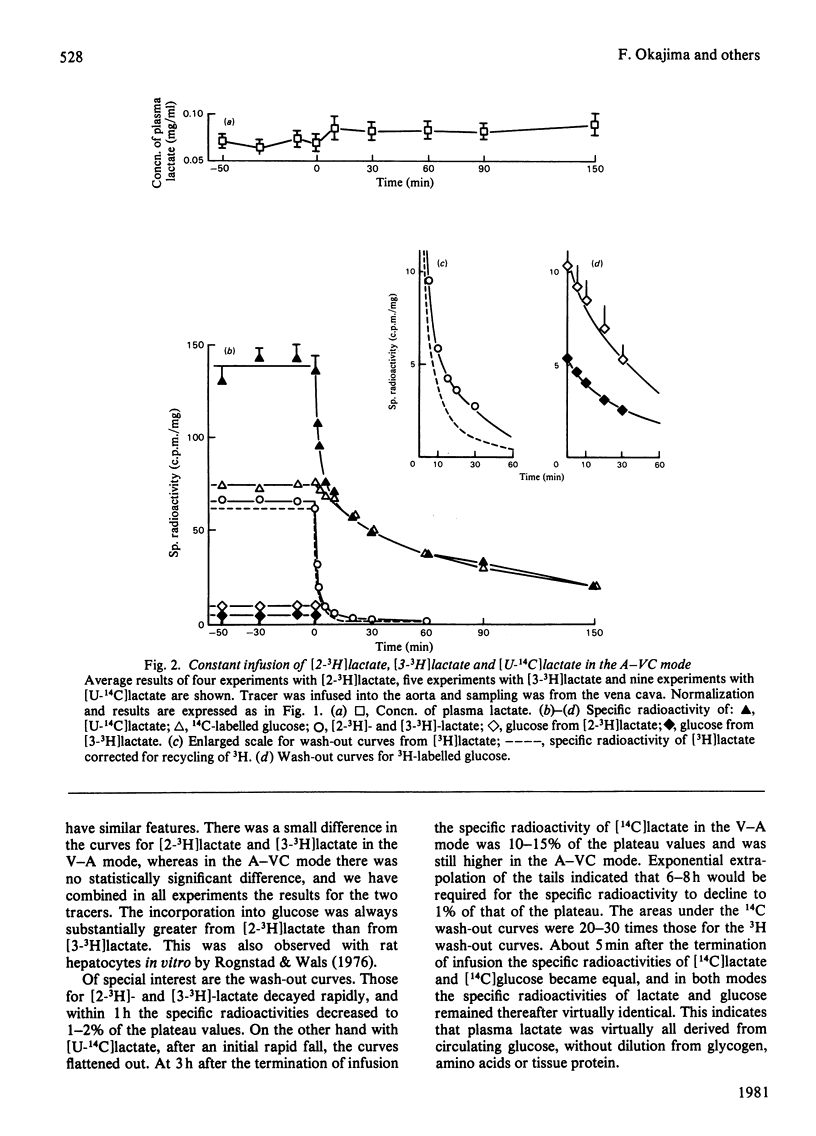
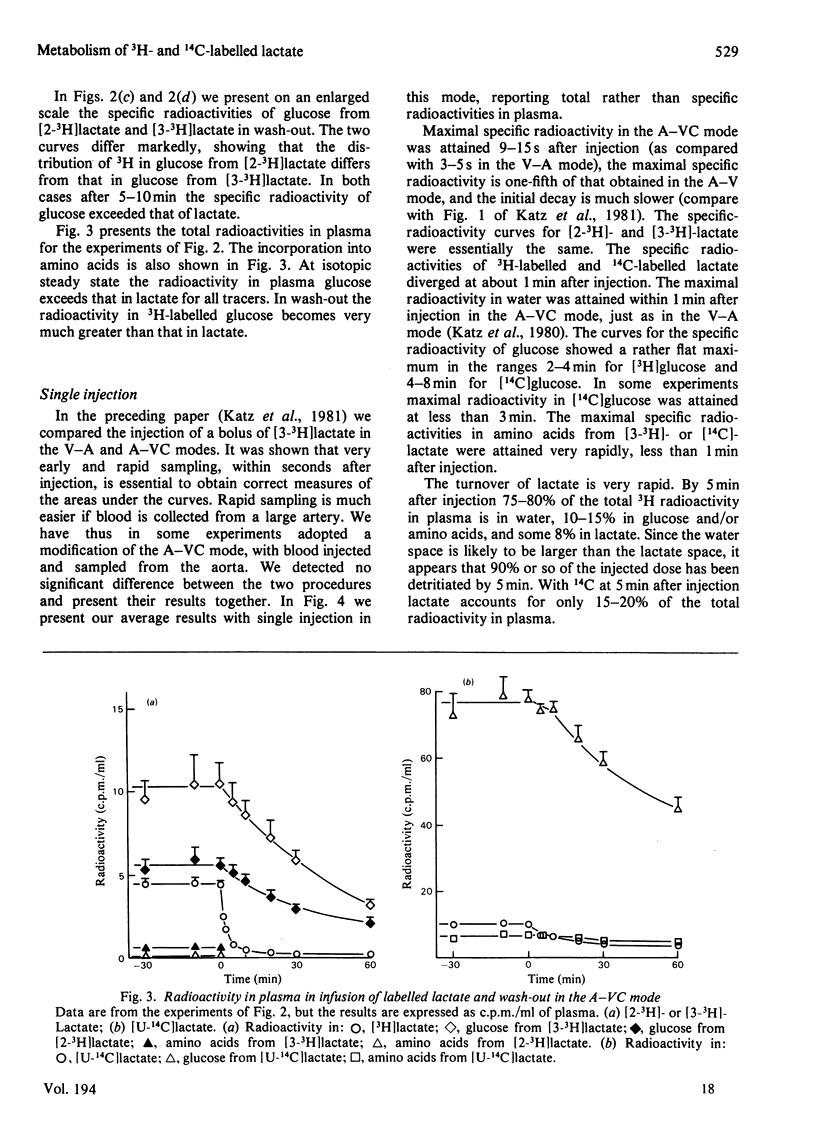
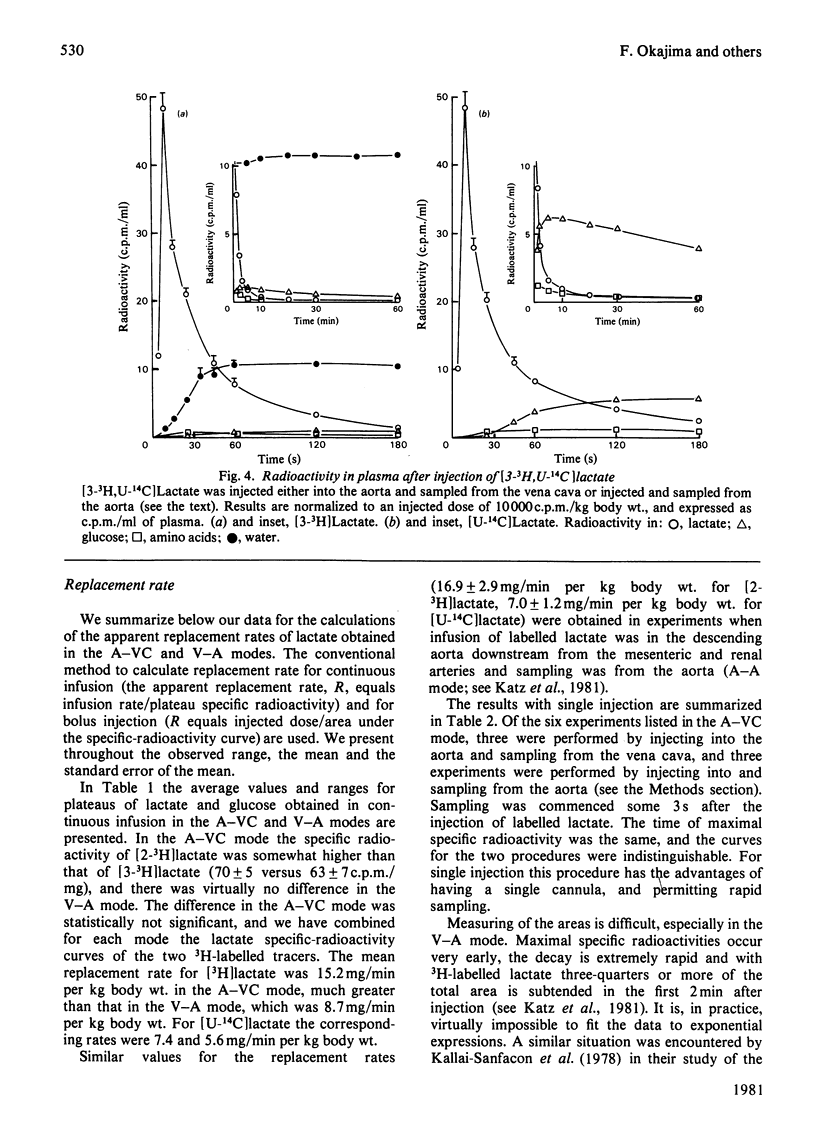
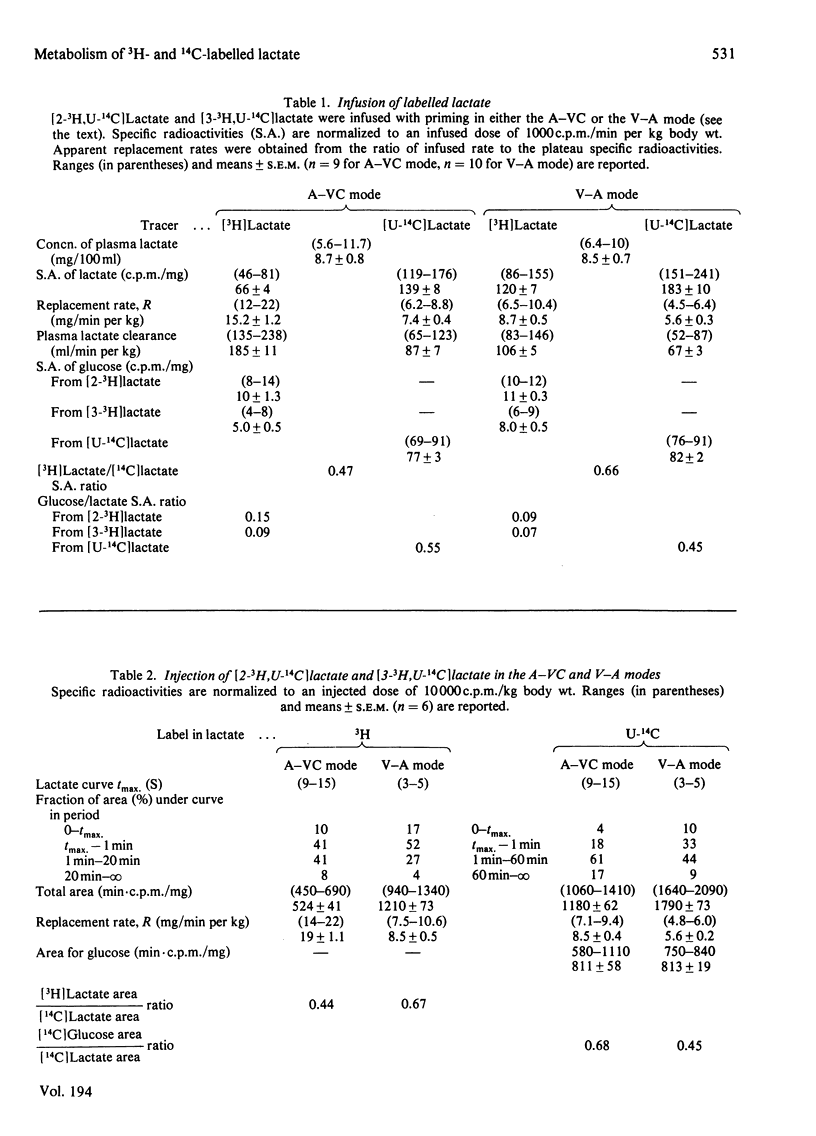






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM B. The simultaneous determination of C14 and H3 in the terminal groups of glucose. Anal Biochem. 1962 Jan;3:85–87. doi: 10.1016/0003-2697(62)90048-9. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Lipogenesis in rat hepatocytes. J Biol Chem. 1974 Apr 10;249(7):2028–2036. [PubMed] [Google Scholar]
- Depocas F., DeFreitas A. S. Method for estimating rates of formation and interconversion of glucose-glycerol and glucose-lactic acid in intact animals. Can J Physiol Pharmacol. 1970 Aug;48(8):557–560. doi: 10.1139/y70-084. [DOI] [PubMed] [Google Scholar]
- Dunn A., Chenoweth M., Schaeffer L. D. Incorporation of 3H and 14C into plasma lactate following administration of [6-3H-14C]glucose. Biochim Biophys Acta. 1968 Aug 6;165(1):170–171. doi: 10.1016/0304-4165(68)90202-x. [DOI] [PubMed] [Google Scholar]
- Forbath N., Hetenyi G., Jr Metabolic interrelations of glucose and lactate in unanesthetized normal and diabetic dogs. Can J Physiol Pharmacol. 1970 Feb;48(2):115–122. doi: 10.1139/y70-019. [DOI] [PubMed] [Google Scholar]
- Freminet A., Bursaux E., Poyart C. F. Effect of elevated lactataemia on the rates of lactate turnover and oxidation in rats. Pflugers Arch. 1974 Jan 16;346(1):75–86. doi: 10.1007/BF00592652. [DOI] [PubMed] [Google Scholar]
- Freminet A., Poyart C. Lactate-glucose interrelations, glucose recycling and the Cori cycle in normal fed rats. Pflugers Arch. 1975 Dec 19;361(1):25–31. doi: 10.1007/BF00587336. [DOI] [PubMed] [Google Scholar]
- HOBERMAN H. D. Coupling of oxidation of substrates to reductive biosyntheses. II. Location of deuterium in glycogen formed from DL-2-deuteriolactate. J Biol Chem. 1958 Nov;233(5):1045–1048. [PubMed] [Google Scholar]
- Kallai-Sanfaçon M. A., Norwich K. H., Steiner G. A new approach to the measurement of glycerol turnover. Can J Physiol Pharmacol. 1978 Dec;56(6):934–939. doi: 10.1139/y78-148. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A., Chenoweth M., Golden S. Determination of synthesis, recycling and body mass of glucose in rats and rabbits in vivo 3H-and 14C-labelled glucose. Biochem J. 1974 Jul;142(1):171–183. doi: 10.1042/bj1420171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Golden S., Dunn A., Chenoweth M. Estimation of glucose turnover in rats in vivo with tritium labeled glucoses. Hoppe Seylers Z Physiol Chem. 1976 Dec;357(10):1387–1394. doi: 10.1515/bchm2.1976.357.2.1387. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rostami H., Dunn A. Evaluation of glucose turnover, body mass and recycling with reversible and irreversible tracers. Biochem J. 1974 Jul;142(1):161–170. doi: 10.1042/bj1420161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka M., Ui M. Tracer kinetic analysis of Cori cycle activity in the rat: effect of feeding. Am J Physiol. 1977 Feb;232(2):E136–E144. doi: 10.1152/ajpendo.1977.232.2.E136. [DOI] [PubMed] [Google Scholar]
- OSHIMA T., TAMIYA N. Mechanism of transaminase action. Biochem J. 1961 Jan;78:116–119. doi: 10.1042/bj0780116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R. Gluconeogenesis in the kidney cortex. Flow of malate between compartments. Biochem J. 1970 Feb;116(3):493–502. doi: 10.1042/bj1160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Wals P. The metabolism of l-[3-3h]lactate by isolated hamster liver cells. Biochim Biophys Acta. 1976 Jun 23;437(1):16–21. doi: 10.1016/0304-4165(76)90343-3. [DOI] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L., Noce P., Utter M. F., Wood H. G., Willard J. M., Cooper T. G., Benziman M. Stereochemistry of the enzymatic carboxylation of phosphoenolpyruvate. J Biol Chem. 1969 Nov 25;244(22):6130–6133. [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. The effect of short term physiological elevations of plasma glucagon concentration on plasma triglyceride concentration in normal and diabetic man. Horm Metab Res. 1977 Jul;9(4):253–257. doi: 10.1055/s-0028-1093546. [DOI] [PubMed] [Google Scholar]


