Abstract
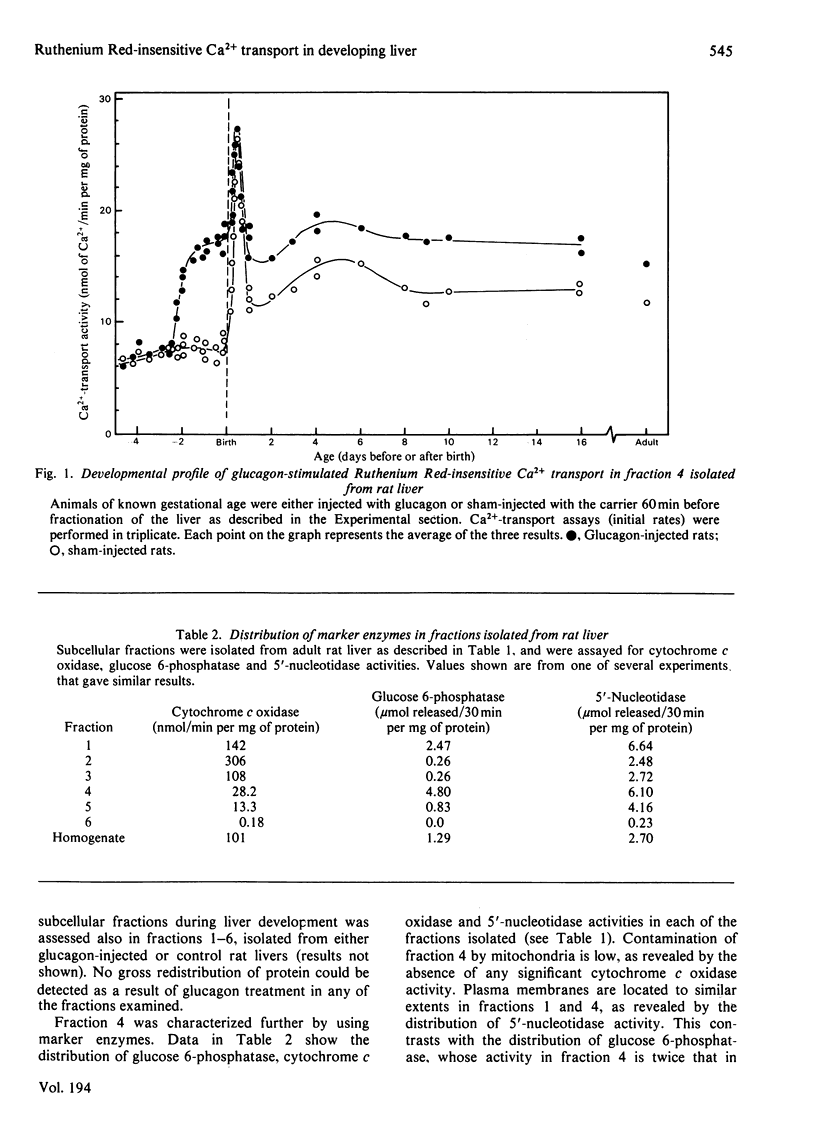
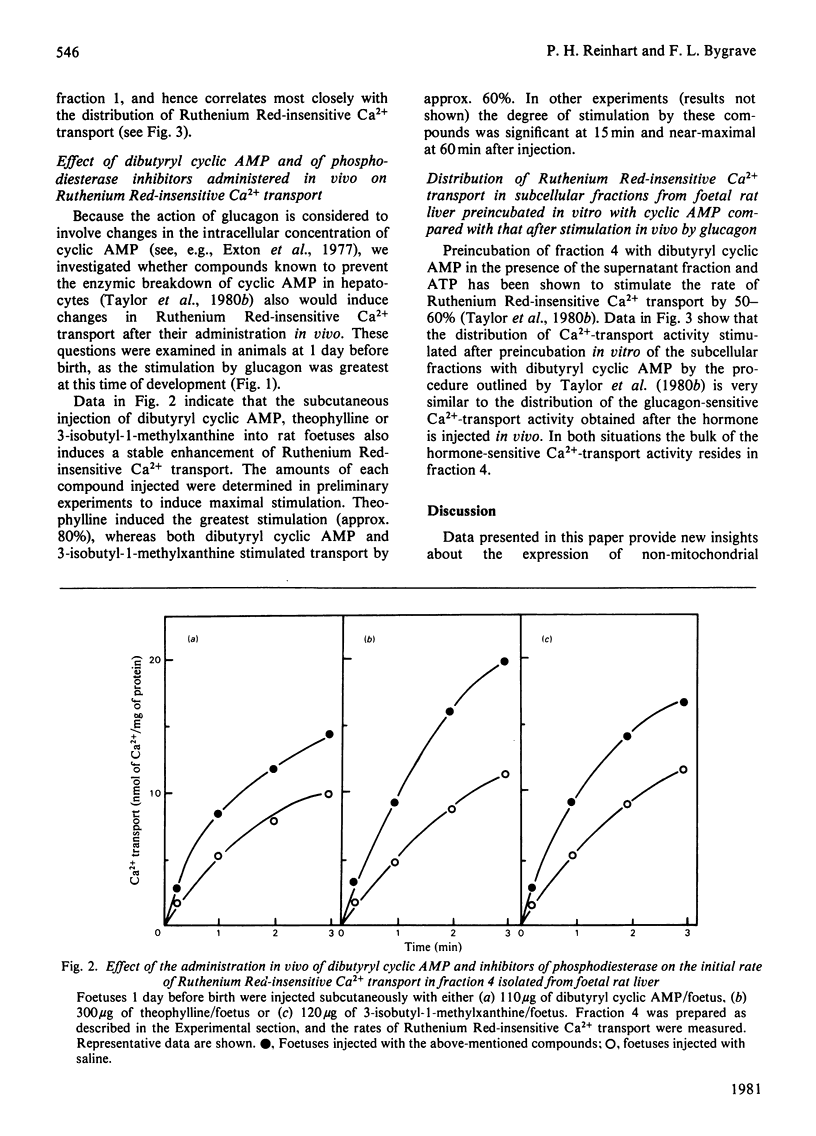
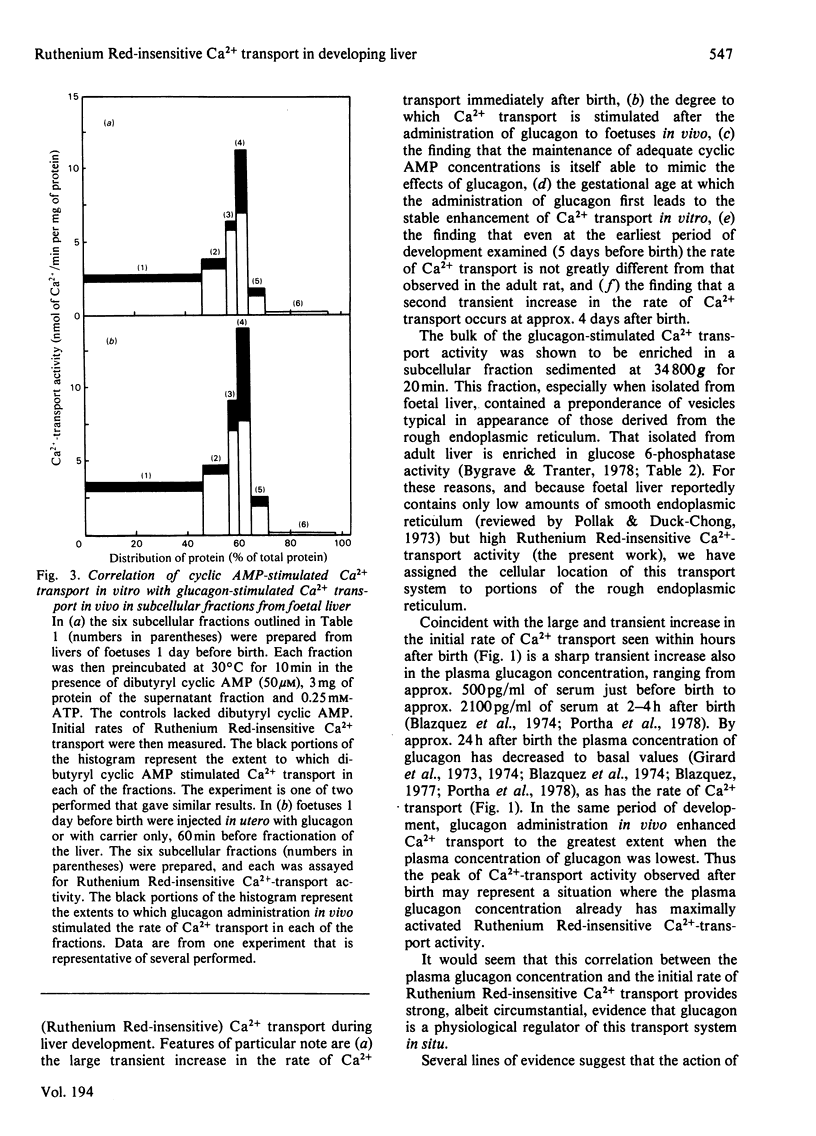
The maturation of glucagon-stimulated Ruthenium Red-insensitive Ca2+-transport activity was determined in livers of rats ranging in age from 5 days preterm to 10 weeks of adult life. Previous indications are that this activity is confined to vesicles derived mainly from the endoplasmic reticulum. Perinatal-rat liver contains near-adult values of Ruthenium Red-insensitive Ca2+-transport activity, and exhibits large transient increases in the rate of this activity at two stages of development, immediately after birth, and at 2-5 days after birth. The administration of glucagon to foetal rats, at developmental stages after 19.5 days of gestation (2.5 days before birth), results in a large stable increase (greater than 100%) of Ca2+-transport activity in a subsequently isolated 'heavy' microsomal fraction. That this fraction was enriched in vesicles derived from the rough endoplasmic reticulum was indicated by both an electron-microscopic examination and a marker-enzyme analysis of the subcellular fractions. The administration of glucagon into newborn animals only hours old does not enhance further the initial rate of Ca2+-transport activity, and from day 1 to 10 weeks after birth the administration of the hormone results in the moderate enhancement of Ca2+ transport. Experiments with cyclic AMP and inhibitors of phosphodiesterase activity suggest that cyclic AMP plays a key role in the enhancement by glucagon of Ruthenium Red-insensitive Ca2+ transport, and arguments are presented that this transport system has an important metabolic role in the redistribution of intracellular Ca2+ in liver tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andia-Waltenbaugh A. M., Lam A., Hummel L., Friedmann N. Characterization of the hormone-sensitive Ca2+ uptake activity of the hepatic endoplasmic reticulum. Biochim Biophys Acta. 1980 Jun 19;630(2):165–175. doi: 10.1016/0304-4165(80)90418-3. [DOI] [PubMed] [Google Scholar]
- Ash G. R., Bygrave F. L. Ruthenium red as a probe in assessing the potential of mitochondria to control intracellular calcium in liver. FEBS Lett. 1977 Jun 15;78(2):166–168. doi: 10.1016/0014-5793(77)80297-4. [DOI] [PubMed] [Google Scholar]
- Bashan N., Gross Y., Moses S., Gutman A. Rat liver glycogen metabolism in the perinatal period. Biochim Biophys Acta. 1979 Oct 4;587(2):145–154. doi: 10.1016/0304-4165(79)90349-0. [DOI] [PubMed] [Google Scholar]
- Bhalla R. C., Webb R. C., Singh D., Brock T. Role of cyclic AMP in rat aortic microsomal phosphorylation and calcium uptake. Am J Physiol. 1978 May;234(5):H508–H514. doi: 10.1152/ajpheart.1978.234.5.H508. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. The role of mitochondrial calcium release in alpha-adrenergic activation of phosphorylase in perfused rat liver. J Biol Chem. 1979 Aug 10;254(15):6945–6950. [PubMed] [Google Scholar]
- Blázquez E., Sugase T., Blázquez M., Foá P. P. Neonatal changes in the concentration of rat liver cyclic AMP and of serum glucose, free fatty acids, insulin, pancreatic, and total glucagon in man and in the rat. J Lab Clin Med. 1974 Jun;83(6):957–967. [PubMed] [Google Scholar]
- Butcher F. R., Scott D. F., Potter V. R. Effect of glucagon on the level of adenosine 3',5'-monophosphate in rat liver: dependence on the route of administration. Endocrinology. 1971 Jul;89(1):130–134. doi: 10.1210/endo-89-1-130. [DOI] [PubMed] [Google Scholar]
- Bygrave F. L., Heaney T. P., Ramachandran C. Submitochondrial location of ruthenium red-sensitive calcium-ion transport and evidence for its enrichment in a specific population of rat liver mitochondria. Biochem J. 1978 Sep 15;174(3):1011–1019. doi: 10.1042/bj1741011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L., Tranter C. J. The subcellular location, maturation and response to increased plasma glucagon of ruthenium red-insensitive calcium-ion transport in rat liver. Biochem J. 1978 Sep 15;174(3):1021–1030. doi: 10.1042/bj1741021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen T., Morland J., Osnes J. B., Oye I. Development of cyclic AMP metabolism in rat liver. A correlative study of tissue levels of cyclic AMP, accumulation of cyclic AMP in slices, adenylate cyclase activity and cyclic nucleotide phosphodiesterase activity. Biochim Biophys Acta. 1973 Jul 28;313(2):338–349. doi: 10.1016/0304-4165(73)90033-0. [DOI] [PubMed] [Google Scholar]
- De Wulf H., Keppens S. Proceedings: Is calcium the second messenger in liver for cyclic AMP-independent glycogenolytic hormones? Arch Int Physiol Biochim. 1976 Feb;84(1):159–160. [PubMed] [Google Scholar]
- Di Marco P. N., Oliver I. T. Adenosine 3':5'-monophosphate in perinatal rat liver. Ontogeny and response to hormones. Eur J Biochem. 1978 Jun 15;87(2):235–241. doi: 10.1111/j.1432-1033.1978.tb12371.x. [DOI] [PubMed] [Google Scholar]
- Girard J. R., Kervran A., Soufflet E., Assan R. Factors affecting the secretion of insulin and glucagon by the rat fetus. Diabetes. 1974 Apr;23(4):310–317. doi: 10.2337/diab.23.4.310. [DOI] [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. The premature deposition or lysis of glycogen in livers of fetal rats injected with hydrocortisone or glucagon. Dev Biol. 1970 Mar;21(3):452–461. doi: 10.1016/0012-1606(70)90135-1. [DOI] [PubMed] [Google Scholar]
- Hales C. N., Campbell A. K., Luzio J. P., Siddle K. Calcium as a mediator of hormone action [proceedings]. Biochem Soc Trans. 1977;5(4):866–872. doi: 10.1042/bst0050866a. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Effects of glucagon and N6O2'-dibutyryladenosine 3':5'-cyclic monophosphate on calcium transport in isolated rat liver mitochondria. Biochem J. 1978 Oct 15;176(1):295–304. doi: 10.1042/bj1760295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Peuch C. J., Haiech J., Demaille J. G. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium--calmodulin-dependent phosphorylations. Biochemistry. 1979 Nov 13;18(23):5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- Lyons R. T., Pitot H. C. The regulation of ornithine aminotransferase synthesis by glucagon in the rat. Arch Biochem Biophys. 1976 May;174(1):262–272. doi: 10.1016/0003-9861(76)90345-3. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Nishikori K., Takenaka T., Maeno H. Stimulation of microsomal calcium uptake and protein phosphorylation by adenosine cyclic 3', 5'-monophosphate in rat uterus. Mol Pharmacol. 1977 Jul;13(4):671–678. [PubMed] [Google Scholar]
- Pollak J. K., Duck-Chong C. G. Changes in rat liver mitochondria and endoplasmic reticulum during development and differentiation. Enzyme. 1973;15(1):139–160. [PubMed] [Google Scholar]
- Portha B., Picon L., Rosselin G. Postmaturity in the rat: high levels of glucagon in the plasma of the foetus and neonate. J Endocrinol. 1978 Apr;77(1):153–154. doi: 10.1677/joe.0.0770153. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K. Effect of sodium butyrate on mammalian cells in culture: a review. In Vitro. 1976 Feb;12(2):125–132. doi: 10.1007/BF02796360. [DOI] [PubMed] [Google Scholar]
- Prpić V., Bygrave F. L. On the inter-relationship between glucagon action, the oxidation-reduction state of pyridine nucleotides, and calcium retention by rat liver mitochondria. J Biol Chem. 1980 Jul 10;255(13):6193–6199. [PubMed] [Google Scholar]
- Prpić V., Spencer T. L., Bygrave F. L. Stable enhancement of calcium retention in mitochondria isolated from rat liver after the administration of glucagon to the intact animal. Biochem J. 1978 Dec 15;176(3):705–714. doi: 10.1042/bj1760705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylatt D. B., Embi N., Cohen P. The role of calmodulin in regulation of glycogen metabolism. Biochem Soc Trans. 1979 Aug;7(4):622–624. doi: 10.1042/bst0070622. [DOI] [PubMed] [Google Scholar]
- Sands H., Mascali J. Effects of cyclic AMP and of protein kinase on the calcium uptake by various tracheal smooth muscle organelles. Arch Int Pharmacodyn Ther. 1978 Dec;236(2):180–191. [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Lattke H. K., Wieland O. H. Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. Biochem J. 1977 Aug 15;166(2):225–235. doi: 10.1042/bj1660225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Ohmori F., Yamada M., Abe H. Mechanism of the stimulation of Ca2+-dependent ATPase of cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. Role of the 22,000-dalton protein. J Biol Chem. 1979 Jan 25;254(2):319–326. [PubMed] [Google Scholar]
- Taylor W. M., Bygrave F. L., Blackmore P. F., Exton J. H. Stable enhancement of ruthenium red-insensitive calcium transport in an endoplasmic reticulum-rich fraction following the exposure of isolated rat liver cells to glucagon. FEBS Lett. 1979 Aug 1;104(1):31–34. doi: 10.1016/0014-5793(79)81079-0. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Prpić V., Exton J. H., Bygrave F. L. Stable changes to calcium fluxes in mitochondria isolated from rat livers perfused with alpha-adrenergic agonists and with glucagon. Biochem J. 1980 May 15;188(2):443–450. doi: 10.1042/bj1880443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. M., Reinhart P., Hunt N. H., Bygrave F. L. Role of 3',5'-cyclic AMP in glucagon-induced stimulation of ruthenium red-insensitive calcium transport in an endoplasmic reticulum-rich fraction of rat liver. FEBS Lett. 1980 Mar 24;112(1):92–96. doi: 10.1016/0014-5793(80)80136-0. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Clippinger M. S., Sivaramakrishnan S., McCullough T. E. Cyclic adenosine monophosphate dependent and independent phosphorylation of sarcolemma membrane proteins in perfused rat heart. Biochemistry. 1979 Mar 6;18(5):871–877. doi: 10.1021/bi00572a021. [DOI] [PubMed] [Google Scholar]
- Waltenbaugh A. M., Friedmann N. Hormone sensitive calcium uptake by liver microsomes. Biochem Biophys Res Commun. 1978 May 30;82(2):603–608. doi: 10.1016/0006-291x(78)90917-8. [DOI] [PubMed] [Google Scholar]