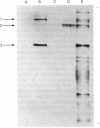
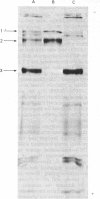
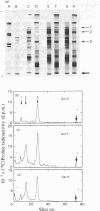
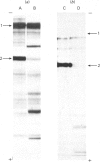
Abstract
1. Cell cultures propagated from foetal bovine ligamentum nuchae synthesized and secreted two glycoproteins, designated MFP I and MFP II, that are closely related to elastic-fibre microfibrils. Glycoproteins MFP I (apparent mol.wt. 150 000) and MFP II (apparent mol.wt. 300 000) were metabolically labelled, separated from other culture-medium components by immunoprecipitation with a specific anti-(microfibrillar protein) serum, and analysed by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and sodium dodecyl sulphate/gel-filtration chromatography. 2. Ligament cells also synthesized and secreted fibronectin, but salt-fractionation and immunoprecipitation studies with a specific anti-(cold-insoluble globulin) serum established that neither glycoprotein MFP I nor glycoprotein MFP II was related to fibronectin. 3. The secretion of glycoprotein MFP I, but not that of glycoprotein MFP II, was enhanced by the addition of ascorbate to the culture medium. 4. Ascorbate-supplemented ligament cells incorporated [3H]proline into glycoprotein MFP I, and 36% of the nondiffusible proline residues were hydroxylated, exclusively as 4-hydroxy[3H]proline. Less than 1% of the total proline residues in [3H]proline-labelled glycoprotein MFP II were hydroxylated. 5. Ascorbate-supplemented cells incorporated [14C]lysine into glycoprotein MFP I and 30% of the non-diffusible lysine residues were hydroxylated. 6. Newly secreted glycoprotein MFP I was digested by highly purified bacterial collagenase to yield polypeptide fragments of apparent mol.wts. 50 000 and 30 000. Glycoprotein MFP II was not digested by bacterial collagenase. 7. The results suggest that elastic-fibre microfibrils are composed of a novel collagenous glycoprotein MFP I in association, as yet undefined, with a non-collagenous glycoprotein MFP II.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham P. A., Hart M. L., Winge A. R., Carnes W. H. The biosynthesis of elastin by an aortic medial cell culture. Adv Exp Med Biol. 1977;79:397–412. doi: 10.1007/978-1-4684-9093-0_35. [DOI] [PubMed] [Google Scholar]
- Abraham P. A., Smith D. W., Carnes W. H. Synthesis of soluble elastin by aortic medial cells in culture. Biochem Biophys Res Commun. 1974 Jun 4;58(3):597–604. doi: 10.1016/s0006-291x(74)80461-4. [DOI] [PubMed] [Google Scholar]
- Alitalo K. Production of both interstitial and basement membrane procollagens by fibroblastic WI-38 cells from human embryonic lung. Biochem Biophys Res Commun. 1980 Apr 14;93(3):873–880. doi: 10.1016/0006-291x(80)91157-2. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Vaheri A., Krieg T., Timpl R. Biosynthesis of two subunits of type IV procollagen and of other basement membrane proteins by a human tumor cell line. Eur J Biochem. 1980 Aug;109(1):247–255. doi: 10.1111/j.1432-1033.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Anderson J. C. Glycoproteins of the connective tissue matrix. Int Rev Connect Tissue Res. 1976;7:251–322. doi: 10.1016/b978-0-12-363707-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Barnes M. J. Function of ascorbic acid in collagen metabolism. Ann N Y Acad Sci. 1975 Sep 30;258:264–277. doi: 10.1111/j.1749-6632.1975.tb29287.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bressan G. M., Prockop D. J. Synthesis of elastin in aortas from chick embryos. Conversion of newly secreted elastin to cross-linked elastin without apparent proteolysis of the molecule. Biochemistry. 1977 Apr 5;16(7):1406–1412. doi: 10.1021/bi00626a026. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Ross R. Synthesis of connective tissue macromolecules by smooth muscle. Int Rev Connect Tissue Res. 1979;8:119–157. doi: 10.1016/b978-0-12-363708-6.50010-2. [DOI] [PubMed] [Google Scholar]
- Cleary E. G., Sandberg L. B., Jackson D. S. The changes in chemical composition during development of the bovine nuchal ligament. J Cell Biol. 1967 Jun;33(3):469–479. doi: 10.1083/jcb.33.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Balian G., Holbrook K., Duksin D., Bornstein P. Amniotic fluid fibronectin. Characterization and synthesis by cells in culture. J Cell Biol. 1978 Sep;78(3):701–715. doi: 10.1083/jcb.78.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Bornstein P. Characterization of a type IV procollagen synthesized by human amniotic fluid cells in culture. J Biol Chem. 1979 May 25;254(10):4197–4204. [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Fierer J. A. Ultrastructural studies of developing pulmonary alveolar septal elastin. Adv Exp Med Biol. 1977;79:31–37. doi: 10.1007/978-1-4684-9093-0_4. [DOI] [PubMed] [Google Scholar]
- Finlay J. B., Steven F. S. The fibrous components of bovine ligamentum nuchae observed in the scanning electron microscope. J Microsc. 1973 Sep;99(1):57–63. doi: 10.1111/j.1365-2818.1973.tb03852.x. [DOI] [PubMed] [Google Scholar]
- Foster J. A., Mecham R. P., Rich C. B., Cronin M. F., Levine A., Imberman M., Salcedo L. L. Proelastin. Synthesis in cultured smooth muscle cells. J Biol Chem. 1978 Apr 25;253(8):2797–2803. [PubMed] [Google Scholar]
- Frederickson R. G., Morse D. E., Low F. N. High-voltage electron microscopy of extracellular fibrillogenesis. Am J Anat. 1977 Sep;150(1):1–33. doi: 10.1002/aja.1001500102. [DOI] [PubMed] [Google Scholar]
- GORHAM L. W., WAYMOUTH C. DIFFERENTIATION IN VITRO OF EMBRYONIC CARTILAGE AND BONE IN A CHEMICALLY-DEFINED MEDIUM. Proc Soc Exp Biol Med. 1965 May;119:287–290. doi: 10.3181/00379727-119-30160. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Jackson D. S. The biosynthesis of procollagen. Essays Biochem. 1976;12:77–113. [PubMed] [Google Scholar]
- Grant M. E., Kefalides N. A., Prockop D. J. The biosynthesis of basement membrane collagen in embryonic chick lens. I. Delay between the synthesis of polypeptide chains and the secretion of collagen by matrix-free cells. J Biol Chem. 1972 Jun 10;247(11):3539–3544. [PubMed] [Google Scholar]
- Grant M. E., Steven F. S., Jackson D. S., Sandberg L. B. Carbohydrate content of insoluble elastins prepared from adult bovine and calf ligamentum nuchae and tropoelastin isolated from copper-deficient porcine aorta. Biochem J. 1971 Jan;121(2):197–202. doi: 10.1042/bj1210197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Ross R., Hartman J. L. The fine structure of elastic fibers. J Cell Biol. 1966 Jul;30(1):59–71. doi: 10.1083/jcb.30.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood R., Merry A. H., Woolley D. E., Grant M. E., Jackson D. S. The disulphide-bonded nature of procollagen and the role of the extension peptides in the assembly of the molecule. Biochem J. 1977 Feb 1;161(2):405–418. doi: 10.1042/bj1610405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote G., Sear C. H., Grant M. E. Studies on the assembly of the rat lens capsule. Biosynthesis and partial characterization of the collagenous components. Biochem J. 1978 Oct 15;176(1):283–294. doi: 10.1042/bj1760283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote J. G., Bailey A. J., Grant M. E. Studies on the assembly of the rat lens capsule. Biosynthesis of a cross-linked collagenous component of high molecular weight. Biochem J. 1980 Aug 15;190(2):229–237. doi: 10.1042/bj1900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. J., Sear C. H., Grant M. E. An ultrastructural study of fibroblasts derived from bovine ligamentum nuchae and their capacity for elastogenesis in culture. J Pathol. 1980 May;131(1):35–53. doi: 10.1002/path.1711310104. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kewley M. A., Steven F. S., Williams G. Preparation of a specific antiserum towards the microfibrillar protein of elastic tissues. Immunology. 1977 Apr;32(4):483–489. [PMC free article] [PubMed] [Google Scholar]
- Kewley M. A., Williams G., Steven F. S. Studies of elastic tissue formation in the developing bovine ligamentum nuchae. J Pathol. 1978 Feb;124(2):95–101. doi: 10.1002/path.1711240205. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee-Own V., Anderson J. C. The preparation of a bacterial collagenase containing negligible non-specific protease activity. Prep Biochem. 1975;5(3):229–245. doi: 10.1080/00327487508061574. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Branson R. E., Hewgley P. B., Cunningham L. W. The synthesis of macromolecular 3-hydroxyproline by attaching and confluent cultures of human fibroblasts. Eur J Biochem. 1977 Jan;72(2):379–383. doi: 10.1111/j.1432-1033.1977.tb11262.x. [DOI] [PubMed] [Google Scholar]
- Levene C. I., Bates C. J. Ascorbic acid and collagen synthesis in cultured fibroblasts. Ann N Y Acad Sci. 1975 Sep 30;258:288–306. doi: 10.1111/j.1749-6632.1975.tb29289.x. [DOI] [PubMed] [Google Scholar]
- Macarak E. J., Kirby E., Kirk T., Kefalides N. A. Synthesis of cold-insoluble globulin by cultured calf endothelial cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2621–2625. doi: 10.1073/pnas.75.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir L. W., Bornstein P., Ross R. A presumptive subunit of elastic fiber microfibrils secreted by arterial smooth-muscle cells in culture. Eur J Biochem. 1976 Apr 15;64(1):105–114. doi: 10.1111/j.1432-1033.1976.tb10278.x. [DOI] [PubMed] [Google Scholar]
- Olsen B. R., Hoffmann H., Prockop D. J. Interchain disulfide bonds at the COOH-terminal end of procollagen synthesized by matrix-free cells from chick embryonic tendon and cartilage. Arch Biochem Biophys. 1976 Jul;175(1):341–350. doi: 10.1016/0003-9861(76)90516-6. [DOI] [PubMed] [Google Scholar]
- Richmond V. Lung parenchymal elastin isolated by non-degradative means. Biochim Biophys Acta. 1974 Jun 7;351(2):173–177. doi: 10.1016/0005-2795(74)90178-0. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Cywinski A. Biosynthesis and secretion of tropoelastin by chick aorta cells. Biochem Biophys Res Commun. 1976 Apr 5;69(3):613–620. doi: 10.1016/0006-291x(76)90920-7. [DOI] [PubMed] [Google Scholar]
- Ross R., Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969 Feb;40(2):366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Fialkow R. J., Altman L. K. The morphogenesis of elastic fibers. Adv Exp Med Biol. 1977;79:7–17. doi: 10.1007/978-1-4684-9093-0_2. [DOI] [PubMed] [Google Scholar]
- Ross R. The elastic fiber. J Histochem Cytochem. 1973 Mar;21(3):199–208. doi: 10.1177/21.3.199. [DOI] [PubMed] [Google Scholar]
- Schwartz C. E., Hellerqvist C. G., Cunningham L. W. Attaching human fibroblasts secrete a type I procollagen rich in 3-hydroxyproline. Biochem Biophys Res Commun. 1979 Sep 12;90(1):240–246. doi: 10.1016/0006-291x(79)91616-4. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Davies P. J., Gevers W. Elastin biosynthesis by smooth muscle cells cultured under scorbutic conditions. Biochem Biophys Res Commun. 1979 Dec 14;91(3):739–746. doi: 10.1016/0006-291x(79)91942-9. [DOI] [PubMed] [Google Scholar]
- Scott D. M., Harwood R., Grant M. E., Jackson D. S. Characterisation of the major collagen species present in porcine aortae and the synthesis of their precursors by smooth muscle cells in culture. Connect Tissue Res. 1977;5(1):7–13. doi: 10.3109/03008207709152606. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Anderson J. C., Jackson D. S. The incorporation of L-[1-3-H]= fucose into non-collagenous glycoproteins secreted by human fibroblasts in culture. Biochem Soc Trans. 1975;3(1):138–140. doi: 10.1042/bst0030138. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Jackson D. S. Biosynthesis and release of glycoproteins by human skin fibroblasts in culture. Biochem J. 1977 Oct 15;168(1):91–103. doi: 10.1042/bj1680091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sear C. H., Kewley M. A., Grant M. E., Steven F. S., Jackson D. S. Biosynthesis of a structural glycoprotein component of elastic tissues by cultured human skin fibroblasts. Biochem Soc Trans. 1977;5(2):430–431. doi: 10.1042/bst0050430. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Kewley M. A., Jones C. J., Grant M. E., Jackson D. S. The identification of glycoproteins associated with elastic-tissue microfibrils. Biochem J. 1978 Mar 15;170(3):715–718. doi: 10.1042/bj1700715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Field J. M., Armitt C. Characterisation of the microfibrillar component of bovine ligamentum nuchae. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1146–1152. doi: 10.1016/s0006-291x(75)80505-5. [DOI] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Field J. M. The ultrastructure and mechanics of elastic ligaments. Adv Exp Med Biol. 1977;79:97–103. doi: 10.1007/978-1-4684-9093-0_9. [DOI] [PubMed] [Google Scholar]
- Uitto J. Biosynthesis of type II collagen. Removal of amino-and carboxy-terminal extensions from procollagen synthesized by chick embryo cartilage cells. Biochemistry. 1977 Jul 26;16(15):3421–3429. doi: 10.1021/bi00634a020. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Wirtschafter Z. T., Cleary E. G., Jackson D. S., Sandberg L. B. Histological changes during the development of the bovine nuchal ligament. J Cell Biol. 1967 Jun;33(3):481–488. doi: 10.1083/jcb.33.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- de Clerck Y. A., Jones P. A. The effect of ascorbic acid on the nature and production of collagen and elastin by rat smooth-muscle cells. Biochem J. 1980 Jan 15;186(1):217–225. doi: 10.1042/bj1860217. [DOI] [PMC free article] [PubMed] [Google Scholar]