Abstract
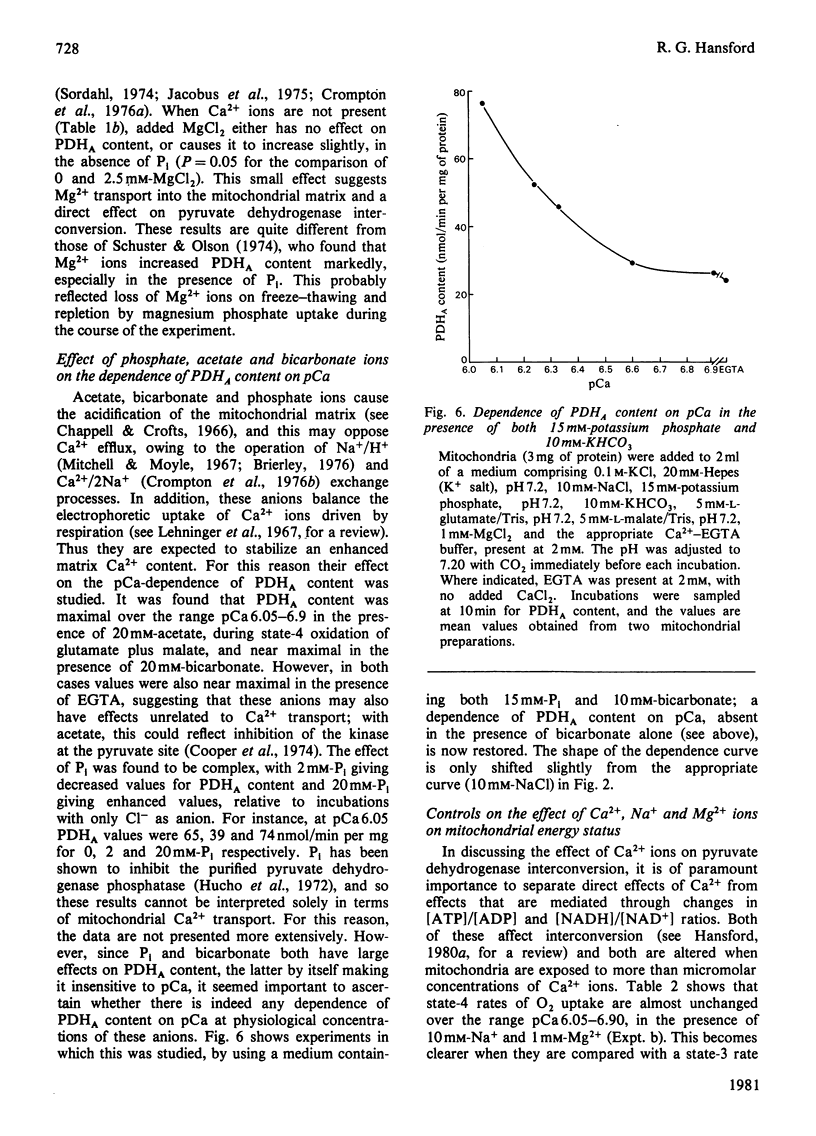
1. The mitochondrial content of active (dephospho) pyruvate dehydrogenase (PDHA) was found to be severalfold higher at an extramitochondrial Ca2+ concentration of 2 microM (pCa6) than at pCa7. The nature of the respiratory substrate did not affect this finding. 2. This Ca2+-dependence was shown in state-4 and 50%-state-3 conditions [see Chance & Williams (1956) Adv. Enzymol. 17, 65-134], but was absent in the presence of excess ADP (state 3). 3. Na+ and Mg2+ ions shifted the pCa value required for a maximal PDHA content to lower values. This was attributed to a stimulation of mitochondrial Ca2+ egress and an inhibition of uptake, respectively. Na+ ions diminished pyruvate dehydrogenase phosphate phosphatase activity in mitochondria which had been extensively depleted of Ca2+ ions by incubation with EGTA, raising the possibility of a direct inhibitory effect of Na+ ions, unrelated to Ca2+ movements. 4. Mg2+ ions lowered the mitochondrial PDHA content at pCa 6.24 and 6.48, but had only minimal effects in the presence of EGTA. 5. The effects of P1 and bicarbonate ions on PDHA content were also studied, as possible effectors of mitochondrial Ca2+ transport. Bicarbonate ions abolished the response to Ca2+ ions, by generating maximal values of PDHA content, but such a response was still observed when physiological concentrations of both P1 and bicarbonate were used. 6. The pCa of the medium in the range 6.33 to over 7 affected PDHA content, with only very minor changes in state-4 rates of O2 uptake and no change in [ATP]/[ADP] ratio or in mitochondrial [NADH]/[NAD+] ratio, provided that Mg2+ ions were present. Thus the effect of Ca2+ ions on PDHA content is unlikely to be mediated by changes in [ATP]/[ADP] and [NADH]/[NAD+] ratio and is more likely to be direct. Equally, changes in the [acetyl-CoA]/[CoA] ratio in response to Ca2+ ions when the substrate was pyruvate were the converse of those required to mediate changes in interconversion, and are probably secondary to changes in PDHA content.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Azzone G. F. Ion transport in liver mitochondria. II. Metabolism-linked ion extrusion. Biochim Biophys Acta. 1967 Jul 3;135(3):444–453. doi: 10.1016/0005-2736(67)90033-8. [DOI] [PubMed] [Google Scholar]
- Bremer J. Pyruvate dehydrogenase, substrate specificity and product inhibition. Eur J Biochem. 1969 Apr;8(4):535–540. doi: 10.1111/j.1432-1033.1969.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Brierley G. P. The uptake and extrusion of monovalent cations by isolated heart mitochondria. Mol Cell Biochem. 1976 Jan 31;10(1):41–63. doi: 10.1007/BF01731680. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- Bygrave F. L. Mitochondria and the control of intracellular calcium. Biol Rev Camb Philos Soc. 1978 Feb;53(1):43–79. doi: 10.1111/j.1469-185x.1978.tb00992.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Carafoli E. The calcium cycle of mitochondria. FEBS Lett. 1979 Aug 1;104(1):1–5. doi: 10.1016/0014-5793(79)81073-x. [DOI] [PubMed] [Google Scholar]
- Chiang P. K., Sacktor B. Control of pyruvate dehydrogenase activity in intact cardiac mitochondria. Regulation of the inactivation and activation of the dehydrogenase. J Biol Chem. 1975 May 10;250(9):3399–3408. [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Crompton M., Heid I. The cycling of calcium, sodium, and protons across the inner membrane of cardiac mitochondria. Eur J Biochem. 1978 Nov 15;91(2):599–608. doi: 10.1111/j.1432-1033.1978.tb12713.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Bridges B. J., Cooper R. H., Kerbey A. L., Pask H. T., Severson D. L., Stansbie D., Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975 Oct 31;9(1):27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Veech R. L., Wilson D. F. Thermodynamic relationships between the oxidation-reduction reactions and the ATP synthesis in suspensions of isolated pigeon heart mitochondria. Arch Biochem Biophys. 1974 Feb;160(2):412–421. doi: 10.1016/0003-9861(74)90415-9. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact tissue preparations by alpha-Cyano-4-hydroxycinnamate and related compounds. Biochem J. 1975 Apr;148(1):97–106. doi: 10.1042/bj1480097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Chappell J. B. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967 Jun 23;27(6):686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978 Nov;191(1):65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Lipid oxidation by heart mitochondria from young adult and senescent rats. Biochem J. 1978 Feb 15;170(2):285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Studies on inactivation of pyruvate dehydrogenase by palmitoylcarnitine oxidation in isolated rat heart mitochondria. J Biol Chem. 1977 Mar 10;252(5):1552–1560. [PubMed] [Google Scholar]
- Hansford R. G. Studies on the effects of coenzyme A-SH: acetyl coenzyme A, nicotinamide adenine dinucleotide: reduced nicotinamide adenine dinucleotide, and adenosine diphosphate: adenosine triphosphate ratios on the interconversion of active and inactive pyruvate dehydrogenase in isolated rat heart mitochondria. J Biol Chem. 1976 Sep 25;251(18):5483–5489. [PubMed] [Google Scholar]
- Hucho F., Randall D. D., Roche T. E., Burgett M. W., Pelley J. W., Reed L. J. -Keto acid dehydrogenase complexes. XVII. Kinetic and regulatory properties of pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase from bovine kidney and heart. Arch Biochem Biophys. 1972 Jul;151(1):328–340. doi: 10.1016/0003-9861(72)90504-8. [DOI] [PubMed] [Google Scholar]
- Hucho F. Regulation of the mammalian pyruvate dehydrogenase multienzyme complex by Mg2+ and the adenine nucleotide pool. Eur J Biochem. 1974 Aug 1;46(3):499–505. doi: 10.1111/j.1432-1033.1974.tb03643.x. [DOI] [PubMed] [Google Scholar]
- Illingworth J. A., Ford W. C., Kobayashi K., Williamson J. R. Regulation of myocardial energy metabolism. Recent Adv Stud Cardiac Struct Metab. 1975;8:271–290. [PubMed] [Google Scholar]
- Jacobus W. E., Tiozzo R., Lugli G., Lehninger A. L., Carafoli E. Aspects of energy-linked calcium accumulation by rat heart mitochondria. J Biol Chem. 1975 Oct 10;250(19):7863–7870. [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- Kohn M. C., Achs M. J., Garfinkel D. Computer simulation of metabolism in pyruvate-perfused rat heart. III. Pyruvate dehydrogenase. Am J Physiol. 1979 Sep;237(3):R167–R173. doi: 10.1152/ajpregu.1979.237.3.R167. [DOI] [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem J. 1980 Jul 15;190(1):95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Denton R. M., England P. J., Randle P. J. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972 Jun;128(1):147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978 Nov 15;176(2):463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki K., Erecińska M., Wilson D. F. Energy relationships between cytosolic metabolism and mitochondrial respiration in rat heart. Am J Physiol. 1978 Mar;234(3):C73–C81. doi: 10.1152/ajpcell.1978.234.3.C73. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Denton R. M., Pask H. T., Severson D. L. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;(39):75–88. [PubMed] [Google Scholar]
- Schuster S. M., Olson M. S. The regulation of pyruvate dehydrogenase in isolated beef heart mitochondria. The role of calcium, magnesium, and permeant anions. J Biol Chem. 1974 Nov 25;249(22):7159–7165. [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Bridges B. J., Randle P. J. Exchangeable and total calcium pools in mitochondria of rat epididymal fat-pads and isolated fat-cells. Role in the regulation of pyruvate dehydrogenase activity. Biochem J. 1976 Jan 15;154(1):209–223. doi: 10.1042/bj1540209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson D. L., Denton R. M., Pask H. T., Randle P. J. Calcium and magnesium ions as effectors of adipose-tissue pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1974 May;140(2):225–237. doi: 10.1042/bj1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordahl L. A. Effects of magnesium, Ruthenium red and the antibiotic ionophore A-23187 on initial rates of calcium uptake and release by heart mitochondria. Arch Biochem Biophys. 1975 Mar;167(1):104–115. doi: 10.1016/0003-9861(75)90446-4. [DOI] [PubMed] [Google Scholar]
- Tsai C. S., Burgett M. W., Reed L. J. Alpha-keto acid dehydrogenase complexes. XX. A kinetic study of the pyruvate dehydrogenase complex from bovine kidney. J Biol Chem. 1973 Dec 25;248(24):8348–8352. [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]
- Walajtys E. I., Gottesman D. P., Williamson J. R. Regulation of pyruvate dehydrogenase in rat liver mitochondria by phosphorylation-dephosphorylation. J Biol Chem. 1974 Mar 25;249(6):1857–1865. [PubMed] [Google Scholar]
- Wehrle J. P., Jurkowitz M., Scott K. M., Brierley G. P. Mg2+ and the permeability of heart mitochondria to monovalent cations. Arch Biochem Biophys. 1976 May;174(1):313–323. doi: 10.1016/0003-9861(76)90350-7. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Murphy E. Calcium homeostasis and compartmentation in liver. Adv Exp Med Biol. 1980;132:671–688. doi: 10.1007/978-1-4757-1419-7_70. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978 Jun 13;17(12):2364–2370. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]


