Abstract
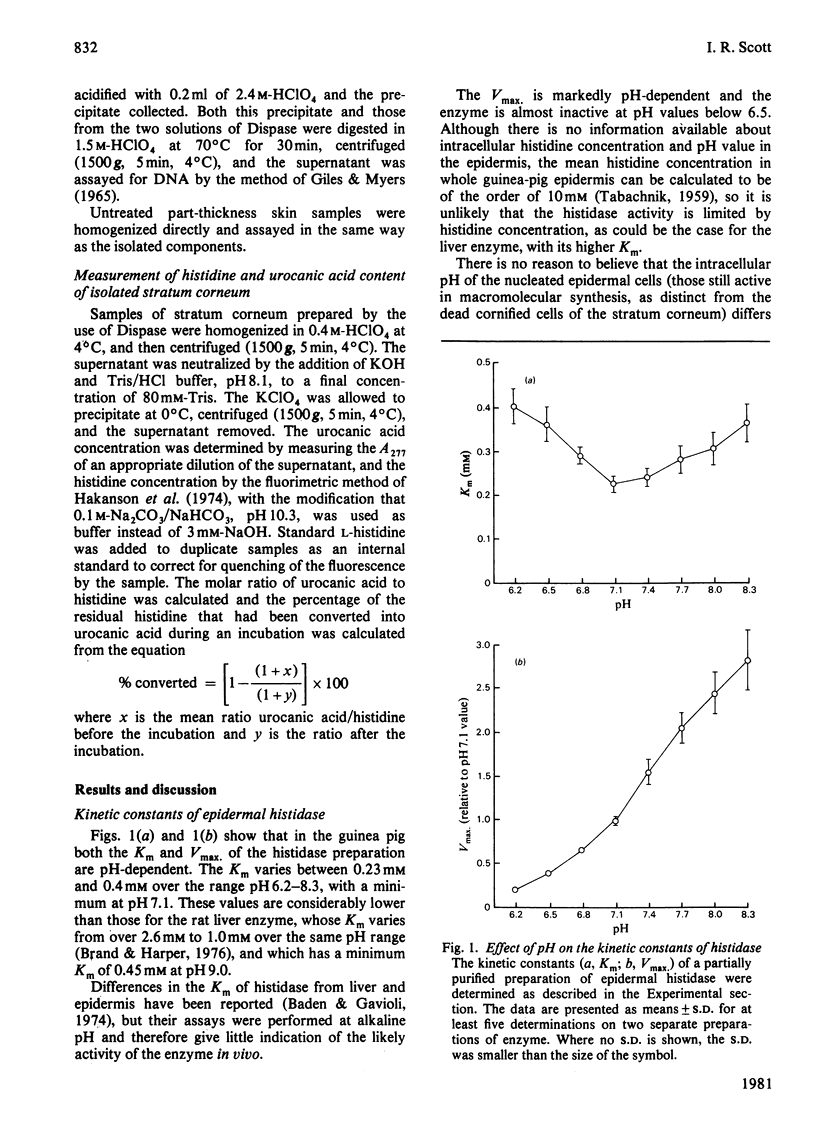
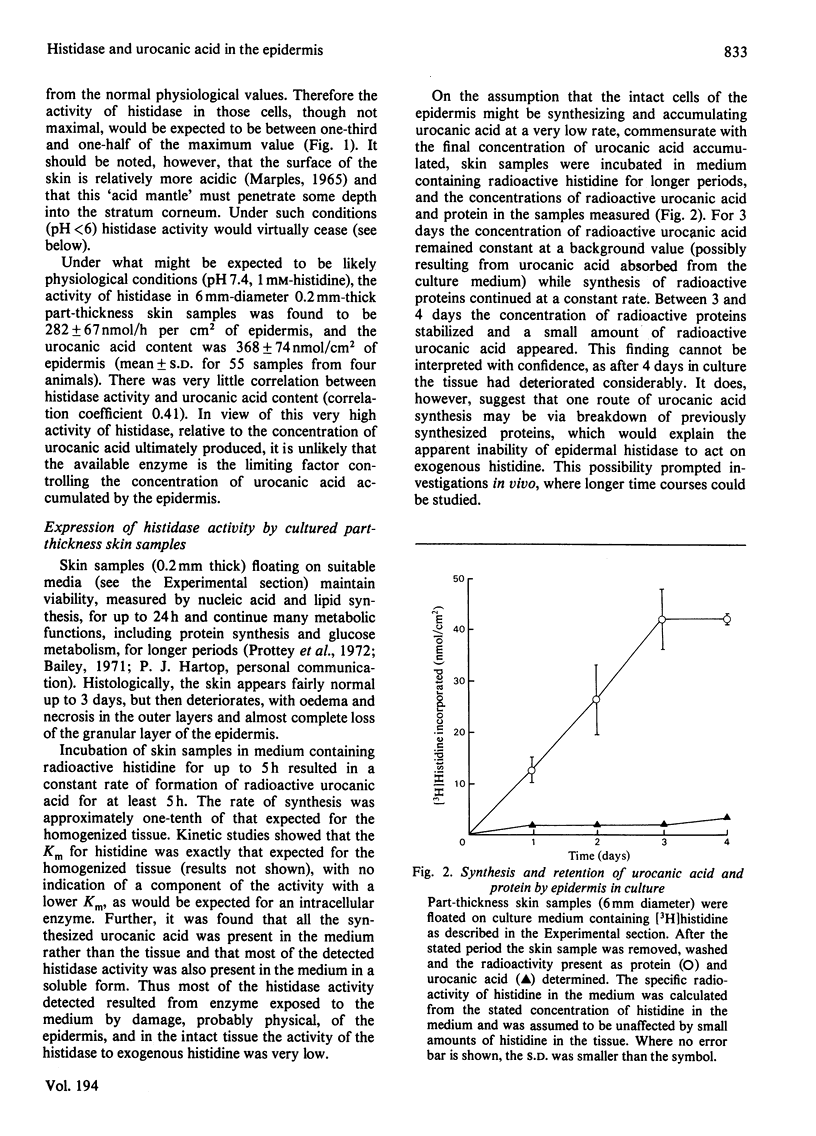
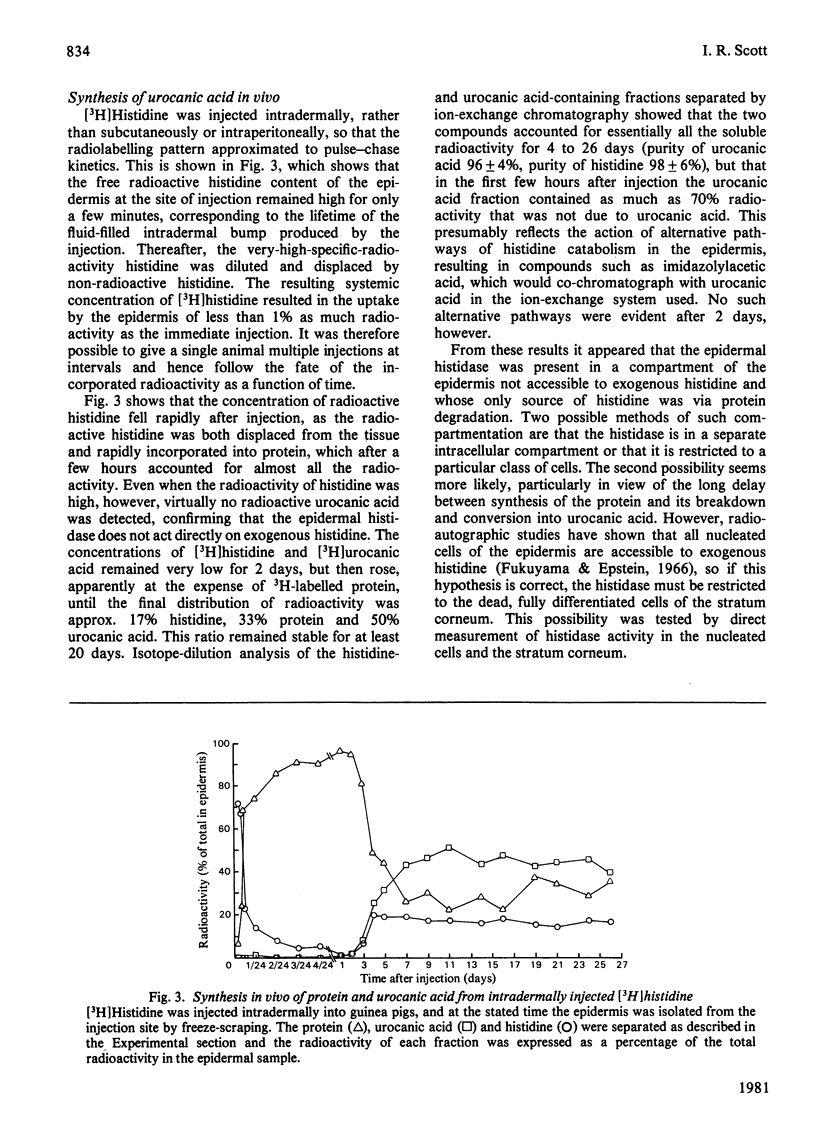
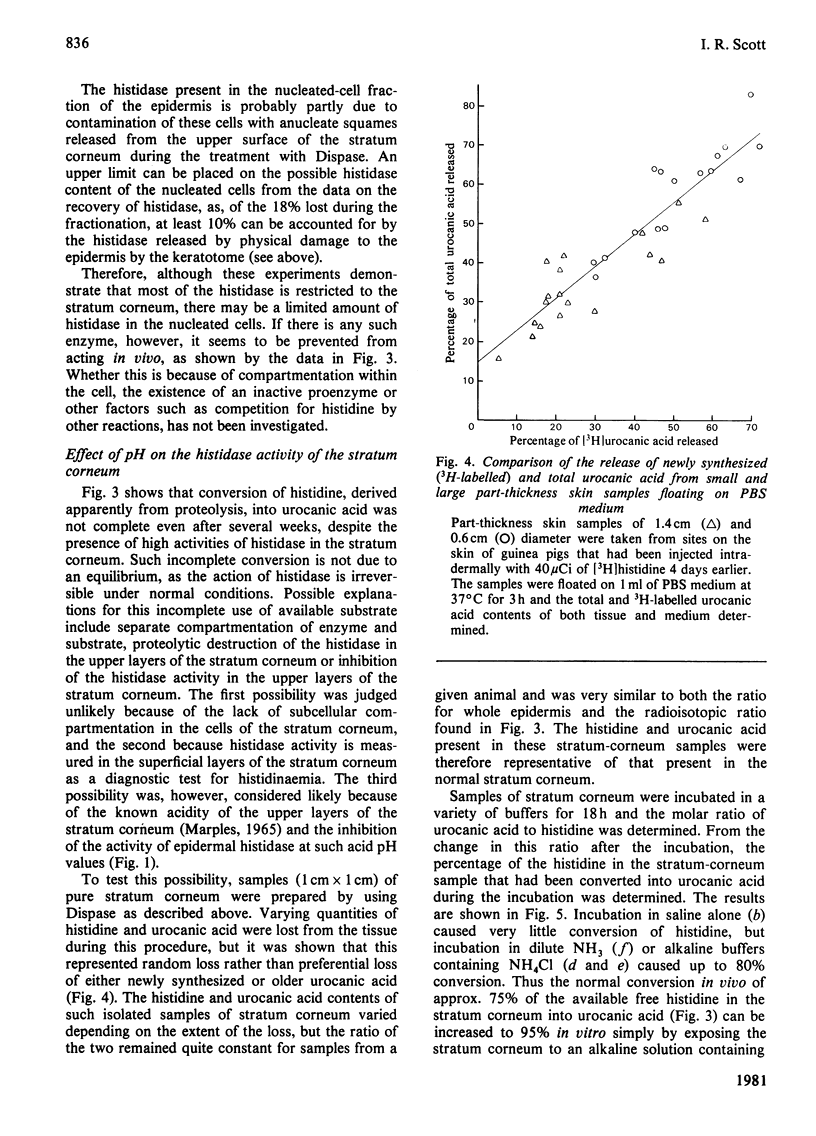
The synthesis of urocanic acid by histidine ammonia-lyase in guinea-pig epidermis was investigated in various ways. 1. In epidermal homogenates the enzyme obeys Michaelis-Menten kinetics and shows marked dependence of its activity of pH, such that below pH 6 it is inactive. 2. Part-thickness skin samples cultured with radioactive histidine do not accumulate detectable radioactive urocanic acid during 3 days in culture. 3. Very little histidine ammonia-lyase activity can be detected in the living cells of the epidermis. The enzyme is almost completely restricted to the dead cells of the stratum corneum. 4. Radioactive histidine injected into living animals does not result immediately in the accumulation of radioactive urocanic acid. By 3 days after the injection, however, both radioactive urocanic acid and histidine appear, apparently at the expense of radioactive proteins, 5. In isolated stratum corneum, the residual histidine can be converted into urocanic acid by the histidine ammonia-lyase in the tissue only if the natural acidity of the tissue is neutralized. It is concluded from these observations that the biosynthesis of urocanic acid occurs naturally only in the stratum corneum, which contains only dead cells. The amount of urocanic acid accumulated is limited by the availability of free histidine produced by proteolysis of residual protein in these cells and by the penetration into the stratum corneum of the 'acid mantle' of the skin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baden H. P., Gavioli L. Histidase activity in rat liver and epidermis. J Invest Dermatol. 1974 Dec;63(6):479–481. doi: 10.1111/1523-1747.ep12680423. [DOI] [PubMed] [Google Scholar]
- Baden H. P., Pathak M. A. The metabolism and function of urocanic acid in skin. J Invest Dermatol. 1967 Jan;48(1):11–17. [PubMed] [Google Scholar]
- Bailey P. J. The metabolism of glucose in skin maintained in tissue culture. Br J Dermatol. 1971 Sep;85(3):264–271. doi: 10.1111/j.1365-2133.1971.tb07229.x. [DOI] [PubMed] [Google Scholar]
- Ball R. D., Walker G. K., Bernstein I. A. Histidine-rich proteins as molecular markers of epidermal differentiation. J Biol Chem. 1978 Aug 25;253(16):5861–5868. [PubMed] [Google Scholar]
- Brand L. M., Harper A. E. Histidine ammonia-lyase from rat liver. Purification, properties, and inhibition by substrate analogues. Biochemistry. 1976 May 4;15(9):1814–1821. doi: 10.1021/bi00654a005. [DOI] [PubMed] [Google Scholar]
- EVERETT M. A., ANGLIN J. H., Jr, BEVER A. T. Ultraviolet induced biochemical alterations in skin. I. Urocanic acid. Arch Dermatol. 1961 Nov;84:717–719. doi: 10.1001/archderm.1961.01580170011002. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama K., Epstein W. L. Epidermal keratinization: localization of isotopically labeled amino acids. J Invest Dermatol. 1966 Dec;47(6):551–560. doi: 10.1038/jid.1966.184. [DOI] [PubMed] [Google Scholar]
- Hais I. M., Strych A. Increase in urocanate concentration in human epidermis following insolation. Experientia. 1968 Mar 15;24(3):231–232. doi: 10.1007/BF02152786. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Rönnberg A. L., Sjölund K. Improved fluorometric assay of histidine and peptides having NH2-terminal histidine using o-Phthalaldehyde. Anal Biochem. 1974 May;59(1):98–109. doi: 10.1016/0003-2697(74)90014-1. [DOI] [PubMed] [Google Scholar]
- Král J. A., Zenísek A., Strych A., Hais I. M. Tloustka pokozky a koncentrace kyseliny urokanové na posterolaterální (pigmentovanejsí) a anteromediální (méne pigmentované) stane paze. Cas Lek Cesk. 1968;107(10):302–307. [PubMed] [Google Scholar]
- Pratzel H., Fries P. Zur Veränderung der relativen Konzentrationen der freien Aminosäuren im Stratum corneum menschlicher Epidermis durch gezielte Umwelteinflüsse. I. Der Einfluss von UV-Strahlung. Arch Dermatol Res. 1977 Aug 22;259(2):157–160. doi: 10.1007/BF00557956. [DOI] [PubMed] [Google Scholar]
- Prottey C., Hartop P. J., Ferguson T. F. Lipid synthesis in rat skin. Br J Dermatol. 1972 Dec;87(6):586–607. doi: 10.1111/j.1365-2133.1972.tb07450.x. [DOI] [PubMed] [Google Scholar]
- Prottey C., Tovell P. W., Ferguson T. F. The morphology and longevity of cells derived from primary cultures of guinea-pig dorsal skin cells. Br J Dermatol. 1974 Dec;91(6):667–679. doi: 10.1111/j.1365-2133.1974.tb12453.x. [DOI] [PubMed] [Google Scholar]
- TABACHNICK J. Studies on the biochemistry of epidermis. I. The free amino acids, ammonia, urocanic acid and nucleic acid content of normal albino guinea pig epidermis. J Invest Dermatol. 1959 May;32(5):563–568. doi: 10.1038/jid.1959.94. [DOI] [PubMed] [Google Scholar]
- Vasantha L. Histidine urocanic acid and histidine alpha-deaminase in the stratum corneum in pellagrins. Indian J Med Res. 1970 Aug;58(8):1079–1084. [PubMed] [Google Scholar]
- Wadia A. S., Sule S. M., Mathur G. P. Epidermal urocanic acid & histidase of albino guineapig following total body UV-irradiation. Indian J Exp Biol. 1975 May;13(3):234–237. [PubMed] [Google Scholar]
- ZANNONI V. G., LA DU B. N. Determination of histidine alpha-deaminase in human stratum corneum and its absence in histidinaemia. Biochem J. 1963 Jul;88:160–162. doi: 10.1042/bj0880160. [DOI] [PMC free article] [PubMed] [Google Scholar]


