Abstract
Objective
The objective of this study was to investigate the long-term outcomes between laparoscopic anatomical liver resection (LAR) and laparoscopic non-anatomical liver resection (LNAR) in patients with hepatocellular carcinoma (HCC).
Methods
In this single-center retrospective cohort study, 1773 patients, from January 2009 to December 2017, were assessed for inclusion. After exclusions, 661 patients were included: 304 patients received LAR and 357 patients received LNAR. Propensity score matching (PSM) with 1:1 ratio was used to eliminate the selection bias between LAR and LNAR groups. The Kaplan–Meier and Cox models were used for survival analysis.
Results
After PSM, 250 patients were in LAR or LNAR group, respectively. The overall survival (OS) had no significant difference between LAR and LNAR by Kaplan–Meier analysis. While, LAR had better disease-free survival (DFS) compared with LNAR (Log-rank P=0.035). The cumulative 5-year DFS rates were 48% for LAR, and 38% for LNAR. By Cox analysis, LAR was an independent risk factor of DFS (HR=1.308, P=0.030). In subgroup analysis for tumor size ≤ 5 cm, 207 patients were in LAR or LNAR subgroup after PSM. LAR had better DFS compared with LNAR (Log-rank P=0.033). LAR was an independent risk factor of DFS (HR=1.333, P=0.036). The cumulative 5-year DFS rates were 50% for LAR, and 39% for LNAR. In another subgroup analysis for tumor size > 5 cm, 43 patients were in LAR or LNAR subgroup after PSM. The DFS had no significant difference between LAR and LNAR (Log-rank P=0.912).
Conclusion
LAR is preferred for HCC patients with tumor size ≤5cm compared with LNAR because of the better DFS. For patients with tumor size >5cm, LAR and LNAR might be alternative procedures with comparable long-term outcomes.
Keywords: Hepatocellular carcinoma, laparoscopic anatomical liver resection, laparoscopic non-anatomical liver resection, outcome, tumor size
Introduction
Hepatocellular carcinoma (HCC), accounting for 75–85% of primary liver cancer, which is the sixth common malignancy and the third-leading cause of tumor-related death, has always been a global health challenge.1 Surgery, including liver resection (LR) and liver transplantation, is the mainstay of HCC treatment, and LR represents the first option in candidates with adequate liver function.2,3
The most appropriate technique of LR for HCC remains a matter of debate. There are two types of LR, anatomical liver resection (AR) and non-anatomical liver resection (NAR). AR is defined as the complete removal of the tumor-bearing portal territories, while NAR is defined as the removal of the tumor with a rim of non-neoplastic liver parenchyma.4 Up to now, the clinical reports of long-term outcomes of AR and NAR for patients with HCC are still under debate. Several studies reported that AR had better long-term outcomes compared with NAR.5–7 On the other hand, some studies showed that AR had comparable long-term outcomes with NAR, including overall survival (OS) and disease-free survival (DFS).8–10 Therefore, it need to be investigated in more studies.
With the development of surgical technique and equipment, laparoscopic liver resection (LLR), including laparoscopic anatomical liver resection (LAR) and laparoscopic non-anatomical liver resection (LNAR), has been widely accepted and recommended because of the better short-term outcomes compared with open liver resection (OLR).11,12 The recent reports of long-term outcomes of LAR and LNAR for patients with HCC were also under debate.13–16 In this retrospective cohort study, we aimed to compare the long-term outcomes of LAR with those of LNAR for HCC. Besides, the outcomes of LAR and LNAR with different tumor size were also studied in subgroups analyses.
Materials and Methods
Study Design and Patients
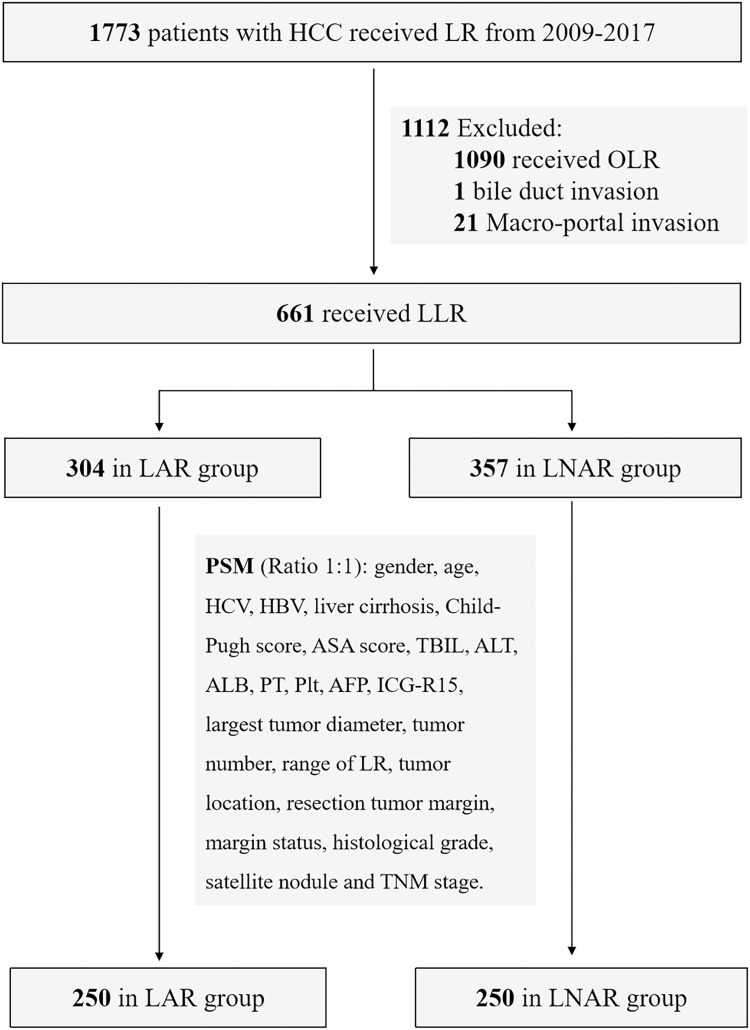
In this single-center retrospective cohort study, 1773 patients with HCC who received LR from our center were evaluated for inclusion from January 2009 to December 2017. The inclusion criteria include: (1) received LLR; (2) pathological diagnosis of HCC; (3) Child-Pugh A or B; (4) without macro-portal invasion and bile duct invasion and (5) no extrahepatic metastasis. The exclusion criteria were as follows: (1) recurrent HCC; (2) LR combined with abdominal organ resection other than gallbladder; and (3) patients who underwent prior TACE or RFA. Thus, a total of 661 hCC patients were included in this study: 304 patients received LAR and 357 patients received LNAR (Figure 1). Preoperative evaluations included routine blood test (white cell, red cell, platelet), liver function, coagulation examinations, serum alpha-fetoprotein (AFP), indocyanine green retention test at 15 minutes (ICG-R15), and triphasic enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI). Routine blood test and hepatic function test was performed after surgery. Abdominal ultrasonography was routinely conducted for patients before discharge. Laparoscopic minor liver rection (LMLR) (<3 segments) or Laparoscopic major hepatectomy (LMH) (≥3 contiguous segments) were defined according to the previous study.17 The severity of postoperative complications was graded by the Clavien-Dindo classification, and the severe complications were defined as Clavien-Dindo grade III and above. All the HCC patients with HBV infection received anti-viral therapy. This study was approved by the Ethics Committee of Southwest Hospital of Army Medical University.
Figure 1.
Diagram of patient selection in the present study.
Abbreviations: HCC, Hepatocellular carcinoma; LR, Liver resection; OLR, Open liver resection; LLR, Laparoscopic liver resection; LAR, Laparoscopic anatomical liver resection; LNAR, Laparoscopic non-anatomical liver resection; PSM, Propensity score matching; HBV, Hepatitis B virus; HCV, Hepatitis C virus; ASA, American Society of Anesthesiologists; TBIL, Total bilirubin; ALT, Alanine transaminase; PT, Prothrombin time; AFP, Alpha-fetoprotein; Plt, Platelet; ICG-R15, Indocyanine green retention test at 15 minutes.
Surgical Procedure
LAR was defined as the systematic removal of the tumor-bearing portal territories under laparoscopy. Briefly, the Glissonian pedicles of the resected segments were ligated. Intraoperative ultrasonography was performed to confirm the position of tumors. The liver parenchyma was dissected along the ischemic line and hepatic veins by using the ultrasonic dissector. The hepatic veins were exposed on the cut surface of the preserved liver parenchyma.
LNAR was defined as the removal of the tumor plus a rim of nonneoplastic liver parenchyma under laparoscopy. Intraoperative ultrasonography was performed to confirm the positions of tumors and the dissection line. The liver parenchyma was dissected by using the ultrasonic dissector. A surgical margin more than 10 mm from the tumor was secured, unless the tumor was attached to the hepatic vein or Glissonian pedicle.
Follow-Up
The follow-up was performed every 1 month within 3 months after operation, and then every 3 months within 2 years and 3 or 6 months afterwards. Routine investigations at each follow-up included routine blood tests, liver function, AFP and abdominal ultrasonography. CT or MRI was performed if necessary. OS was defined as the time from the surgery date to death from any cause or the last follow-up. DFS was defined as the time from the surgery date to tumor recurrence. All patients were followed up with the same protocol.
Propensity Score Matching (PSM) and Statistical Analysis
PSM with 1:1 ratio was used to eliminate the selection bias between LAR and LNAR groups based on the nearest neighbor matching method without replacement. The propensity score covariates in this study included gender, age, HCV, HBV, liver cirrhosis, Child-Pugh score, American Society of Anesthesiologists score (ASA score), preoperative blood tests (TBIL, ALT, ALB, PT, platelet count and AFP), ICG-R15, largest tumor diameter, tumor number, range of LR, tumor location, resection tumor margin, margin status, histological grade, satellite nodule and TNM stage. After PSM, the covariables between the two groups were compared to validate the effect of PSM. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using a t test or Mann–Whitney test. A two-tailed P value <0.05 was considered significant. The Kaplan–Meier and Cox analyses were applied for OS and DFS. P values <0.05 were considered significant. All statistical analyses were performed using SPSS software version 25.0 (IBM SPSS Inc. Chicago, IL).
Results
The Baseline Characteristics Between LAR and LNAR Groups Had No Differences After PSM
A total of 661 hCC patients were included in this study, 304 were in the LAR group and 357 were in the LNAR group. The baseline characteristics were in Table 1. The characteristics, in terms of tumor diameter, tumor number, tumor location, TNM stage, ICG-R15 and the preoperative serum levels of ALT and Platelet count, were significantly different between LAR and LNAR groups. Thus, PSM with 1:1 ratio was used to eliminate the selection bias. After PSM, there were 250 patients in each group. All the baseline characteristics had no significant differences between LAR and LNAR groups after PSM (Table 1).
Table 1.
Baseline Patient Characteristics Between Laparoscopic Anatomical Liver Resection (LAR) and Laparoscopic Non-Anatomical Liver Resection (LNAR) Groups Before and After Propensity Score Matching (PSM)
| Characteristics | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| LAR (N=304) | LNAR (N=357) | P | LAR (N=250) | LNAR (N=250) | P | |
| Age | 52.70±11.15 | 51.08±11.37 | 0.067 | 52.09±11.07 | 52.28±11.39 | 0.855 |
| Gender | 0.082 | 0.797 | ||||
| Male | 252 (82.9%) | 313 (87.7%) | 214 (85.6%) | 216 (86.4%) | ||
| Female | 52 (17.1%) | 44 (12.3%) | 36 (14.4%) | 34 (13.6%) | ||
| HBV | 259 (85.2%) | 309 (86.6%) | 0.617 | 218 (87.2%) | 212 (84.8%) | 0.439 |
| HCV | 6 (2.0%) | 2 (0.6%) | 0.098 | 0 (0%) | 2 (0.8%) | 0.156 |
| Liver cirrhosis | 196 (64.5%) | 238 (66.7%) | 0.554 | 167 (66.8%) | 162 (64.8%) | 0.637 |
| Child-Pugh score | 1.000 | 1.000 | ||||
| A | 304 (100%) | 357 (100%) | 250 (100%) | 250 (100%) | ||
| B | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| ASA score | 0.650 | 0.471 | ||||
| I | 168 (55.3%) | 191 (53.5%) | 143 (57.2%) | 135 (54.0%) | ||
| II | 136 (44.7%) | 166 (46.5%) | 107 (42.8%) | 115 (46.0%) | ||
| TBIL (µmol/L) | 15.70±6.02 | 16.16±6.14 | 0.331 | 15.52±6.12 | 15.99±6.16 | 0.389 |
| ALT (IU/L) | 38.21±23.89 | 43.52±33.93 | 0.022* | 39.56±24.91 | 38.21±26.33 | 0.556 |
| ALB | 42.15±4.06 | 42.68±3.97 | 0.093 | 42.45±3.96 | 42.43±3.98 | 0.952 |
| PT (INR) | 1.01±0.06 | 1.02±0.08 | 0.119 | 1.01±0.06 | 1.01±0.07 | 0.756 |
| Plt (×103/μL) | 150.27±69.52 | 130.11±54.46 | <0.001* | 138.68±55.73 | 140.20±58.47 | 0.767 |
| ICG-R15 (%) | 4.68±2.94 | 5.24±3.06 | 0.017* | 4.75±2.94 | 4.68±2.73 | 0.777 |
| AFP (≥400 ng/mL) | 78 (25.7%) | 99 (27.7%) | 0.549 | 66 (26.4%) | 63 (25.2%) | 0.759 |
| Largest tumor diameter | 0.001* | 0.647 | ||||
| ≤ 5cm | 225 (74.0%) | 303 (84.9%) | 201 (80.4%) | 205 (82.0%) | ||
| >5cm | 79 (26.0%) | 54 (15.1%) | 49 (19.6%) | 45 (18.0%) | ||
| Tumor number | 0.016* | 0.112 | ||||
| 1 | 292 (96.1%) | 324 (90.8%) | 241 (96.4%) | 236 (94.4%) | ||
| 2–3 | 10 (3.3%) | 31 (8.7%) | 7 (2.8%) | 14 (5.6%) | ||
| ≥4 | 2 (0.7%) | 2 (0.6%) | 2 (0.8%) | 0 (0%) | ||
| Range of LR | 0.078 | 0.455 | ||||
| Minor | 245 (80.6%) | 306 (85.7%) | 215 (86.0%) | 209 (83.6%) | ||
| Major | 59 (19.4%) | 51 (14.4%) | 35 (14.0%) | 41 (16.4%) | ||
| Tumor Location | 0.024* | 0.845 | ||||
| Central segments (1, 4a, 7, 8) | 80 (26.3%) | 123 (34.5%) | 73 (29.2%) | 75 (30.0%) | ||
| Peripheral segments (2, 3, 4b, 5, 6) | 224 (73.7%) | 234 (65.5%) | 177 (70.8%) | 175 (70.0%) | ||
| Resection tumor margin | 0.306 | 0.432 | ||||
| ≥1cm | 290 (95.4%) | 346 (96.9%) | 241 (96.4%) | 244 (97.6%) | ||
| <1cm | 14 (4.6%) | 11 (3.1%) | 9 (3.6%) | 6 (2.4%) | ||
| Margin status | 0.356 | 1.000 | ||||
| Negative | 304 (100%) | 356 (99.7%) | 250 (100%) | 250 (100%) | ||
| Positive | 0 (0%) | 1 (0.3%) | 0 (0%) | 0 (0%) | ||
| Histological grade | 0.514 | 0.788 | ||||
| Low | 41 (13.5%) | 39 (10.9%) | 33 (13.2%) | 30 (12.0%) | ||
| Moderate | 238 (78.3%) | 292 (81.8%) | 197 (78.8%) | 203 (81.2%) | ||
| High | 25 (8.2%) | 26 (7.3%) | 20 (8.0%) | 17 (6.8%) | ||
| Satellite nodule | 0.064 | 1.000 | ||||
| Positive | 0 (0%) | 4 (1.1%) | 0 (0%) | 0 (0%) | ||
| Negative | 304 (100%) | 353 (98.9%) | 250 (100%) | 250 (100%) | ||
| TNM stage | 0.041* | 0.476 | ||||
| I–II | 301 (99.0%) | 345 (96.6%) | 247 (98.8%) | 245 (98.0%) | ||
| III–IV | 3 (1.0%) | 12 (3.4%) | 3 (1.2%) | 5 (2.0%) | ||
Note: *, P < 0.05, statistical significance.
Abbreviations: HBV, Hepatitis B virus; HCV, Hepatitis C virus; ASA, American Society of Anesthesiologists; TBIL, Total bilirubin; ALT, Alanine transaminase; PT, Prothrombin time; Plt, Platelet; AFP, Alpha-fetoprotein; ICG-R15, Indocyanine green retention test at 15 minutes.
The Patients in LAR Group Had Similar Short-Term Outcomes Compared with Them in LNAR Group
Short-term outcomes of LAR and LNAR groups were shown in Table 2. The operative time, intraoperative blood loss and transfusion had no significant difference between two groups. There was no perioperative death in two groups. The postoperative complications mainly included ascites, hydrothorax, infection, bile leak, hemorrhage, liver failure and respiratory failure. There was no significant difference, in terms of overall and severe complications, between LAR and LNAR groups. Besides, the postoperative hospital stay was also similar between two groups.
Table 2.
Operative Details and Postoperative Outcomes Between Laparoscopic Anatomical Liver Resection (LAR) and Laparoscopic Non-Anatomical Liver Resection (LNAR) Groups After Propensity Score Matching
| LAR (N=250) | LNAR (N=250) | P | |
|---|---|---|---|
| Operative time (min) | 216.69±96.04 | 210.53±84.09 | 0.446 |
| Blood loss (mL) | 292.44±188.69 | 300.16±243.60 | 0.692 |
| Blood transfusion | 18 (7.2%) | 22 (8.8%) | 0.510 |
| Perioperative mortality | 0 (0%) | 0 (0%) | 1.000 |
| Overall complications | 59 (23.6%) | 59 (23.6%) | 1.000 |
| Ascites | 24 (9.6%) | 24 (9.6%) | |
| Hydrothorax | 18 (7.2%) | 16 (6.4%) | |
| Infection | 11 (4.4%) | 10 (4.0%) | |
| Hemorrhage | 10 (4.0%) | 11 (4.4%) | |
| Bile leak | 2 (0.8%) | 2 (0.8%) | |
| Liver failure | 0 (0%) | 1 (0.4%) | |
| Respiratory failure | 1 (0.4%) | 0 (0%) | |
| Severe complications (Clavien–Dindo III–IV) | 13 (5.2%) | 9 (3.6%) | 0.640 |
| Postoperative hospital stay (D) | 10.87±4.58 | 10.24±3.73 | 0.093 |
The Patients in LAR Group Had Better DFS but Comparable OS with Them in LNAR Group
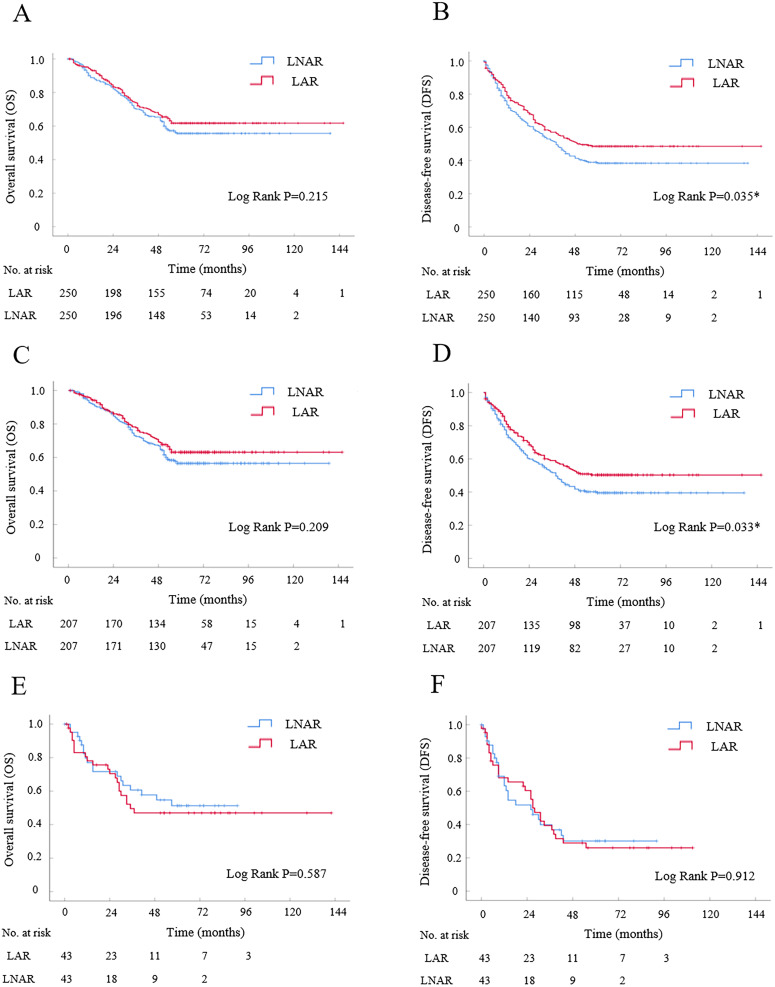
In the Kaplan–Meier analysis, the OS had no significant difference between LAR and LNAR groups (Log-rank P=0.215, Figure 2A). The cumulative 1-, 3-, and 5-year OS rates were 85%, 68%, and 62% for patients in LAR group, and were 83%, 65%, and 55% for patients in LNAR group, respectively. Consistently, in the Cox univariate and multivariate analysis, LAR was not a risk factor for OS (HR, 1.196; 95% CI, 0.900–1.588; P=0.217; Table 3). In the Kaplan-Meier analysis, LAR had better DFS compared with LNAR (Log-rank P=0.035, Figure 2B). The cumulative 1-, 3-, and 5-year DFS rates were 69%, 51%, and 48% for patients in LAR group, and were 61%, 43%, and 38% for patients in LNAR group, respectively. In the Cox model, univariate analysis showed that LAR was significantly associated with better DFS compared with LNAR (HR, 1.295; 95% CI, 1.016–1.650; P=0.037; Table 3), and moreover, multivariate analysis indicated that LAR was an independent risk factor of DFS (HR, 1.308; 95% CI, 1.026–1.667; P=0.030; Table 3). Together, the results showed that the patients in LAR group had better DFS compared with them in LNAR group.
Figure 2.
The survival curve of laparoscopic anatomical liver resection (LAR) versus laparoscopic non-anatomical liver resection (LNAR) for HCC patients after propensity score matching (PSM) by Kaplan-Meier analysis. (A and B) Overall survival and disease-free survival analyses for HCC patients after PSM enrolled in present study (N=250 for each group). (C and D) Overall survival and disease-free survival analyses for patients with tumor size less than or equal to 5 cm after PSM by subgroup analyses (N=207 for each group). (E and F) Overall survival and disease-free survival analyses for patients with tumor size larger than 5 cm after PSM by subgroup analyses (N=43 for each group).
Table 3.
Univariate and Multivariate Analysis with Disease-Free Survival and Overall Survival Between Laparoscopic Anatomical Liver Resection (LAR) and Laparoscopic Non-Anatomical Liver Resection (LNAR) After Propensity Score Matching
| Variable | Disease-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis, HR (95% CI) | P value | Multivariate Analysis, HR (95% CI) | P value | Univariate Analysis, HR (95% CI) | P value | Multivariate Analysis, HR (95% CI) | P value | |
| Resection type (LNAR/LAR) | 1.295 (1.016–1.650) | 0.037* | 1.308 (1.026–1.667) | 0.030* | 1.196 (0.900–1.588) | 0.217 | / | / |
| Largest tumor diameter (≤ 5cm/>5cm) | 0.689 (0.514–0.925) | 0.013* | 0.725 (0.536–0.980) | 0.037* | 0.659 (0.468–0.928) | 0.017* | 0.747 (0.525–1.064) | 0.106 |
| Range of LR (Minor/Major) | 0.634 (0.461–0.872) | 0.005* | 0.684 (0.493–0.949) | 0.023* | 0.498 (0.350–0.708) | 0.000* | 0.533 (0.371–0.766) | 0.001* |
| Tumor Location (Peripheral/Central) | 0.893 (0.684–1.167) | 0.409 | / | / | 0.780 (0.565–1.076) | 0.130 | / | / |
| Resection tumor margin (≥1cm/<1cm) | 1.452 (0.747–2.824) | 0.271 | / | / | 1.733 (0.854–3.519) | 0.128 | / | / |
Note: *, P < 0.05, statistical significance.
Subgroup Analysis: LAR Had Better DFS but Comparable OS with LNAR for Patients with Tumor Size Less Than or Equal to 5 cm
In subgroup analysis for HCC patients with tumor size less than or equal to 5 cm, there were 207 patients in LAR or LNAR subgroups after PSM (Supplementary Table S1). In the Kaplan–Meier analysis, the OS had no significant difference between LAR and LNAR subgroups (Log-rank P=0.209, Figure 2C). The cumulative 1-, 3-, and 5-year OS rates were 87%, 71%, and 63% for patients in LAR subgroup, and were 86%, 67%, and 56% for patients in LNAR subgroup, respectively. While, LAR had better DFS compared with LNAR (Log-rank P=0.033, Figure 2D). The cumulative 1-, 3-, and 5-year DFS rates were 70%, 53%, and 50% for patients in LAR subgroup, and were 60%, 43%, and 39% for patients in LNAR subgroup, respectively. In the Cox model, the univariate and multivariate analyses showed that LAR was significantly associated with better DFS but not OS compared with LNAR (HR, 1.333; 95% CI, 1.020–1.742; P=0.036; HR, 1.222; 95% CI, 0.892–1.675; P=0.212; Table 4). Together, the data indicated that LAR had better DFS but comparable OS with LNAR for patients with tumor size less than or equal to 5 cm.
Table 4.
Univariate and Multivariate Analysis with Disease-Free Survival and Overall Survival Between Laparoscopic Anatomical Liver Resection (LAR) and Laparoscopic Non-Anatomical Liver Resection (LNAR) Subgroups for Patients with Tumor Size Less Than or Equal to 5 cm After Propensity Score Matching
| Variable | Disease-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis, HR (95% CI) | P value | Multivariate Analysis, HR (95% CI) | P value | Univariate Analysis, HR (95% CI) | P value | Multivariate Analysis, HR (95% CI) | P value | |
| Resection type (LNAR/LAR) | 1.333 (1.020–1.742) | 0.036* | / | / | 1.222 (0.892–1.675) | 0.212 | / | / |
| Range of LR (Minor/Major) | 0.799 (0.523–1.222) | 0.301 | / | / | 0.671 (0.415–1.084) | 0.103 | / | / |
| Tumor Location (Peripheral/Central) | 0.976 (0.727–1.311) | 0.873 | / | / | 0.781 (0.543–1.122) | 0.181 | / | / |
| Resection tumor margin (≥1cm/<1cm) | 1.049 (0.390–2.822) | 0.924 | / | / | 1.517 (0.562–4.095) | 0.411 | / | / |
Note: *, P < 0.05, statistical significance.
Subgroup Analysis: LAR Had Comparable DFS and OS with LNAR for Patients with Tumor Size Larger Than 5 cm
In subgroup analysis for patients with tumor size larger than 5 cm, there were 43 patients in LAR or LNAR subgroups after PSM (Supplementary Table S2). In the Kaplan–Meier analysis, both the OS and DFS had no significant difference between LAR and LNAR subgroups (Log-rank P=0.587; Log-rank P=0.912; Figure 2E and F). The cumulative 1-, 3-, and 5-year OS rates were 61%, 47%, and 47% for patients in LAR subgroup, and were 62%, 47%, and 47% for patients in LNAR subgroup, respectively. The cumulative 1-, 3-, and 5-year DFS rates were 61%, 29%, and 26% for patients in LAR subgroup, and were 52%, 31%, and 31% for patients in LNAR subgroup, respectively. The Cox analysis also showed that LAR was not significantly associated with DFS and OS compared with LNAR (Table 5). The data indicated that LAR had comparable DFS and OS with LNAR for patients with tumor size larger than 5 cm.
Table 5.
Univariate and Multivariate Analysis with Disease-Free Survival and Overall Survival Between Laparoscopic Anatomical Liver Resection (LAR) and Laparoscopic Non-Anatomical Liver Resection (LNAR) Subgroups in Patients with Tumor Size Larger Than 5 cm After Propensity Score Matching
| Variable | Disease-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis, HR (95% CI) | P value | Multivariate Analysis, HR (95% CI) | P value | Univariate Analysis, HR (95% CI) | P value | Multivariate Analysis, HR (95% CI) | P value | |
| Resection type (LNAR/LAR) | 0.971 (0.586–1.659) | 0.914 | / | / | 0.841 (0.448–1.579) | 0.590 | / | / |
| Range of LR (Minor/Major) | 0.518 (0.301–0.891) | 0.018* | / | / | 0.378 (0.200–0.716) | 0.003* | / | / |
| Tumor Location (Peripheral/Central) | 1.167 (0.587–2.323) | 0.660 | / | / | 1.297 (0.572–2.941) | 0.533 | / | / |
| Resection tumor margin (≥1cm/<1cm) | / | / | / | / | / | / | / | / |
Note: *, P < 0.05, statistical significance.
Discussion
The long-term outcomes between LAR and LNAR for HCC is one of the research focuses currently. A recent previous study showed that LAH versus LNAH for HCC patients with tumor size less than or equal to 10cm was associated with increased outcomes.14 In the present propensity-matched retrospective cohort study, we provided a new clinical evidence for the long-term outcomes of LAR versus LNAR for the HCC patients with different tumor size. Our results showed that LAR had better DFS but similar OS compared with LNAR for patients with tumor size less than or equal to 5 cm. While, for patients with tumor size larger than 5 cm, LAR and LNAR had comparable long-term outcomes.
AR was initially proposed by Makuuchi in 1985.4 As the high propensity of HCC to spread through the portal veins, AR had a theoretical benefit in terms of reducing tumor recurrence. However, the debate about the real-world long-term outcomes of AR and NAR for HCC has been going on for a long time, and the results of several recent meta-analyses were also contradictory.6,7,10,18 One recognized reason for these contradictory results was the study selection bias. In the present study, we conducted a large number study, established the patient inclusion and exclusion criteria, and used the PSM to balanced the bias in baseline characteristics. Based on the above measures which might largely decrease the study selection bias, we found that LAR was preferred for the HCC patients with tumor size less than or equal to 5 cm compared with LNAR because of the better DFS.
As the fact that the long-term outcome of HCC was largely affected by the complex tumor characteristics and that different patients had different tumor characteristics, LAR might be not preferred for all the HCC patients with different tumor characteristics. This was the reason why we conducted subgroup analysis based on an important tumor characteristic, the tumor size. Our subgroup results showed that LAR and LNAR had comparable OS and DFS for patients with tumor size larger than 5 cm, indicating that LAR and LNAR were alternative procedures for HCC patients with tumor size larger than 5 cm. The consistent result was got in open surgery.19 Apart from the tumor size, microvascular invasion (MVI) was considered as another important tumor characteristic. It was reported that AR had better DFS compared with NAR for HCC with MVI, while for HCC without MVI, AR and NAR had comparable long-term outcomes.7
LLR has been widely accepted and recommended because of the better short-term outcomes compared with open liver resection (OLR). However, the clinical evidences for the effect of LAR were relatively limited compared with open AR (OAR). Thus, more clinical studies in terms of LLR were needed. An previous study showed that LAR versus LNAR for HCC patients with tumor size less than 10 cm was associated with increased DFS and comparable OS.14 Our present study provided a new further evidence about the long-term outcomes of LAR versus LNAR for HCC patients with different tumor size. Based on the advantages of the laparoscopic procedure, we believed that LAR deserves more attention. With the development of surgical technique and equipment, some measures might facilitate the performance of LAR.20–22 First, some imaging evaluations, such as the three-dimensional liver modeling or augmented reality technology, could provide a precise and standardized approach for LAR.23,24 Second, the Glissonean approach might be suitable for LAR compared with the hilar approach.25,26 Third, 3D laparoscopy, which offers the surgeon binocular vision and depth perception, might be an advantage for LAR performance.27 At last, the parenchymal transection of LAR could be performed with more precision by using the indocyanine green fluorescence negative or positive staining.28,29
Although our study included a large number of patients, it had limitations because that it was a retrospective study. Hence, the well-designed prospective studies are needed to confirm the role of LAR and LNAR in HCC patients with different tumor size.
In conclusion, our result showed that LAR had better DFS but comparable OS with LNAR for patients with tumor size less than or equal to 5 cm. While, LAR and LNAR had comparable OS and DFS for patients with tumor size larger than 5 cm. The data indicated that LAR was preferred for the HCC patients with tumor size less than or equal to 5 cm compared with LNAR. While, LAR and LNAR were alternative procedures for HCC patients with tumor size larger than 5 cm.
Funding Statement
There is no funding to report.
Abbreviations
HCC, hepatocellular carcinoma; LAR, laparoscopic anatomical liver resection; LNAR, laparoscopic non-anatomical liver resection; PSM, Propensity score matching; OS, overall survival; DFS, disease-free survival; LR, liver resection; AR, anatomical liver resection; NAR, non-anatomical liver resection; LLR, laparoscopic liver resection; OLR, open liver resection; AFP, serum alpha-fetoprotein; ICG-R15, indocyanine green retention test at 15 minutes; LMLR, laparoscopic minor liver rection; LMH, laparoscopic major hepatectomy.
Data Sharing Statement
The original anonymous dataset is available on request from the corresponding author at ljianwei1015@yeah.net or tianfeng@tmmu.edu.cn.
Compliance with Ethics Guidelines
The study was reviewed and approved by Ethics Committee of Southwest Hospital of Army Medical University.
Informed Consent Statement
All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Disclosure
All the authors have no conflict of interest related to the manuscript.
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 4.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161(4):346–350. [PubMed] [Google Scholar]
- 5.Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64(3):594–600. doi: 10.1016/j.jhep.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 6.Jiao S, Li G, Zhang D, Xu Y, Liu J. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surg. 2020;80:243–255. doi: 10.1016/j.ijsu.2020.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Shin SW, Kim TS, Ahn KS, Kim YH, Kang KJ. Effect of anatomical liver resection for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Surg. 2023;109:2784–2793. doi: 10.1097/JS9.0000000000000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marubashi S, Gotoh K, Akita H, et al. Anatomical versus non-anatomical resection for hepatocellular carcinoma. Br J Surg. 2015;102:776–784. doi: 10.1002/bjs.9815 [DOI] [PubMed] [Google Scholar]
- 9.Li SQ, Huang T, Shen SL, et al. Anatomical versus non-anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria. Br J Surg. 2017;104:118–127. doi: 10.1002/bjs.10311 [DOI] [PubMed] [Google Scholar]
- 10.Famularo S, Ceresoli M, Giani A, et al. Is it just a matter of surgical extension to achieve the cure of hepatocarcinoma? A meta-analysis of propensity-matched and randomized studies for anatomic versus parenchyma-sparing liver resection. J Gastrointest Surg. 2021;25:94–103. doi: 10.1007/s11605-019-04494-5 [DOI] [PubMed] [Google Scholar]
- 11.Cheung TT, Han HS, She WH, et al. The Asia pacific consensus statement on laparoscopic liver resection for hepatocellular carcinoma: a report from the 7th Asia-Pacific primary liver cancer expert meeting held in Hong Kong. Liver Cancer. 2018;7:28–39. doi: 10.1159/000481834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. 2018;268:11–18. doi: 10.1097/SLA.0000000000002524 [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Liu F, Hao X, et al. Laparoscopically anatomical versus non-anatomical liver resection for large hepatocellular carcinoma. HPB. 2020;22(1):136–143. doi: 10.1016/j.hpb.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Liao K, Yang K, Cao L, et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: a randomised controlled trial. Int J Surg. 2022;102:106652. doi: 10.1016/j.ijsu.2022.106652 [DOI] [PubMed] [Google Scholar]
- 15.Lee B, Cho JY, Han HS, et al. Laparoscopic anatomical versus non-anatomical liver resection for hepatocellular carcinoma in the posterosuperior segments: a propensity score matched analysis. Hepatobiliary Surg Nutr. 2023;12:824–834. doi: 10.21037/hbsn-21-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badawy AR, Han HS, El-Mahdy TM, Soliman HEM, Abo-Ryia MH, Elkhadrawy OH. Laparoscopic anatomic vs. nonanatomic liver resection for large hepatocellular carcinoma (≥5 cm) in the right lobe. HPB. 2024;26:576–585. doi: 10.1016/j.hpb.2024.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg. 2016;263:761–777. doi: 10.1097/SLA.0000000000001413 [DOI] [PubMed] [Google Scholar]
- 18.Dai XM, Xiang ZQ, Wang Q, Li HJ, Zhu Z. Oncological outcomes of anatomic versus non-anatomic resections for small hepatocellular carcinoma: systematic review and meta-analysis of propensity-score matched studies. World J Surg Oncol. 2022;20(1):299. doi: 10.1186/s12957-022-02770-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon JH, Lee JW, Lee JW, Lee YJ. Effects of anatomical or non-anatomical resection of hepatocellular carcinoma on survival outcome. J Clin Med. 2022;11(5):1369. doi: 10.3390/jcm11051369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotohda N, Cherqui D, Geller DA, et al. Expert consensus guidelines: how to safely perform minimally invasive anatomic liver resection. J Hepatobiliary Pancreat Sci. 2022;29:16–32. doi: 10.1002/jhbp.1079 [DOI] [PubMed] [Google Scholar]
- 21.Wakabayashi G, Cherqui D, Geller DA, et al. The Tokyo 2020 terminology of liver anatomy and resections: updates of the Brisbane 2000 system. J Hepatobiliary Pancreat Sci. 2022;29:6–15. doi: 10.1002/jhbp.1091 [DOI] [PubMed] [Google Scholar]
- 22.Morimoto M, Monden K, Wakabayashi T, et al. Minimally invasive anatomic liver resection: results of a survey of world experts. J Hepatobiliary Pancreat Sci. 2022;29(1):33–40. doi: 10.1002/jhbp.1094 [DOI] [PubMed] [Google Scholar]
- 23.Benkabbou A, Azami A, Majbar MA. Open source-based 3-dimensional liver modeling serving accessibility of liver surgery virtual planning. JAMA Surg. 2023;158:974–975. doi: 10.1001/jamasurg.2023.0272 [DOI] [PubMed] [Google Scholar]
- 24.Zeng X, Tao H, Dong Y, et al. Impact of three-dimensional reconstruction visualization technology on short-term and long-term outcomes after hepatectomy in patients with hepatocellular carcinoma: a propensity-score-matched and inverse probability of treatment-weighted multicenter study. Int J Surg. 2024;110(3):1663–1676. doi: 10.1097/JS9.0000000000001047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujiyama Y, Wakabayashi T, Mishima K, Al-Omari MA, Colella M, Wakabayashi G. Latest findings on minimally invasive anatomical liver resection. Cancers. 2023;15:2218. doi: 10.3390/cancers15082218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morimoto M, Matsuo Y, Nonoyama K, et al. Glissonean pedicle isolation focusing on the Laennec’s capsule for minimally invasive anatomical liver resection. J Pers Med. 2023;13:1154. doi: 10.3390/jpm13071154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Au KP, Chan MY, Chu KW, et al. Impact of three-dimensional (3D) visualization on laparoscopic hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2022;29:6731–6744. doi: 10.1245/s10434-022-11716-9 [DOI] [PubMed] [Google Scholar]
- 28.Aoki T, Koizumi T, Mansour DA, et al. Ultrasound-guided preoperative positive percutaneous indocyanine green fluorescence staining for laparoscopic anatomical liver resection. J Am Coll Surg. 2020;230:e7–e12. doi: 10.1016/j.jamcollsurg.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 29.Jianxi W, Xiongfeng Z, Zehao Z, et al. Indocyanine green fluorescence-guided laparoscopic hepatectomy versus conventional laparoscopic hepatectomy for hepatocellular carcinoma: a single-center propensity score matching study. Front Oncol. 2022;12:930065. doi: 10.3389/fonc.2022.930065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original anonymous dataset is available on request from the corresponding author at ljianwei1015@yeah.net or tianfeng@tmmu.edu.cn.