Abstract
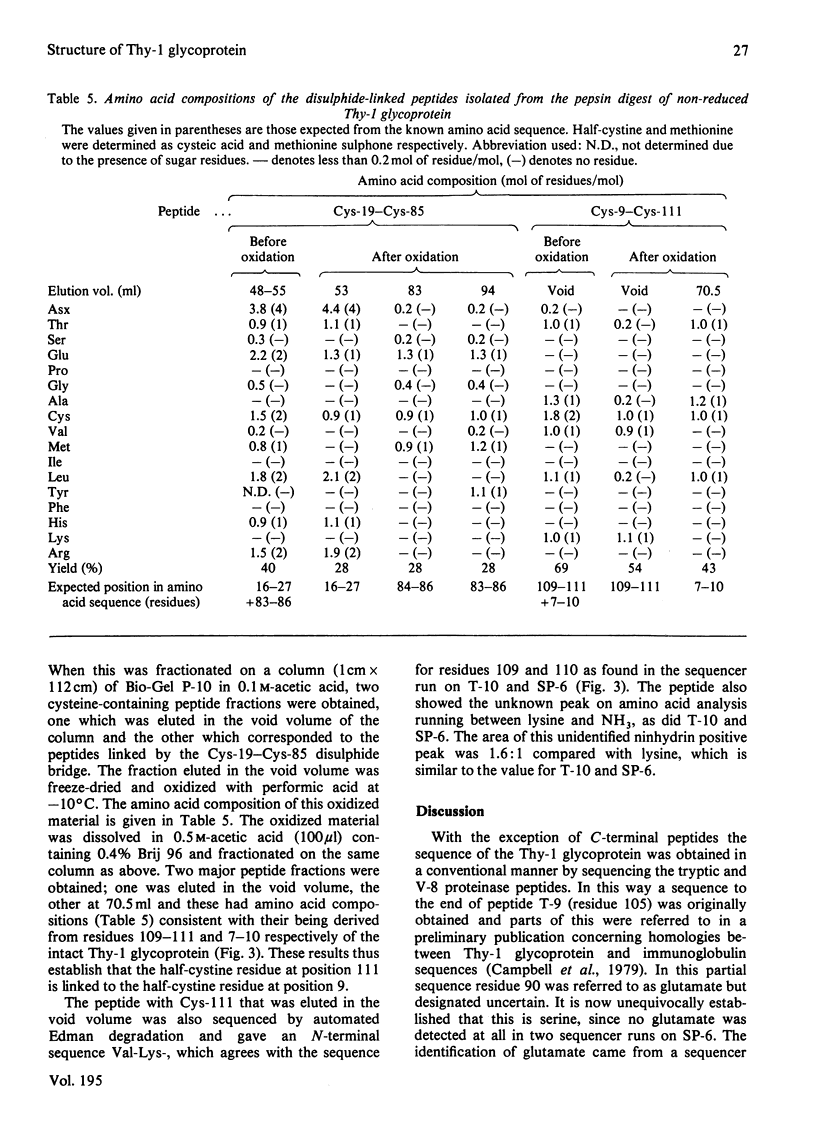
The full sequence of the Thy-1 membrane glycoprotein of rat brain is reported. The sequence was determined from tryptic and V-8 proteinase peptides and consisted of 111 amino acids. The amino terminus was blocked and consisted of a pyroglutamic acid residue. The molecule contained two disulphide bonds, namely Cys-9--Cys-111 and Cys-19--Cys-85. Three N-linked amino sugars were located at Asn-23, Asn-74 and Asn-98. In each case the sequence on the C-terminal side of the attachment point was Asn-Xaa-Thr as would be expected for N-linkage. The C-terminal peptides were unusual, in that they were either obtained in a highly aggregated form, or could only be purified after binding to Brij 96 micelles. Thus they appeared to have hydrophobic properties, yet did not contain any extended sequence of hydrophobic amino acids. Other unusual features of the C-terminal peptides were the presence of unidentified ninhydrin-positive material and of glucosamine and galactosamine. The C-terminal residue has not been directly identified but Cys-111 is the last conventional amino acid. It is suggested that the hydrophobic properties of the C-terminal peptides may be due to the linkage of lipid. The sequence of the Thy-1 glycoprotein showed homologies with immunoglobulin domains. This relationship is examined in detail in the paper following [Cohen et al. (1981) Biochem. J. 193, 000--000].
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton R. T., Morris R. J., Williams A. F. Estimation of the amount and tissue distribution of rat Thy-1.1 antigen. Eur J Immunol. 1974 Sep;4(9):598–602. doi: 10.1002/eji.1830040904. [DOI] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A. The quantitation of glucosamine and galactosamine in glycoproteins after hydrolysis in p-toluenesulphonic acid. FEBS Lett. 1975 Dec 1;60(1):76–80. doi: 10.1016/0014-5793(75)80422-4. [DOI] [PubMed] [Google Scholar]
- Arndt R., Stark R., Thiele H. G. Detection and molecular characterization of the thymus-brain antigen in human brain. Immunology. 1977 Jul;33(1):101–107. [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Hydén H. Localizatin of the Thy-1 antigen in rat brain and spinal cord by immunofluorescence. J Neurochem. 1978 Dec;31(6):1375–1391. doi: 10.1111/j.1471-4159.1978.tb06563.x. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F., Faulkes R. A. Chemical characterisation of the Thy-1 glycoproteins from the membranes of rat thymocytes and brain. Nature. 1976 Oct 14;263(5578):563–567. doi: 10.1038/263563a0. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F. Purification of the Thy-1 molecule from rat brain. Biochem J. 1975 Dec;151(3):699–706. doi: 10.1042/bj1510699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bause E., Hettkamp H. Primary structural requirements for N-glycosylation of peptides in rat liver. FEBS Lett. 1979 Dec 15;108(2):341–344. doi: 10.1016/0014-5793(79)80559-1. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Williams A. F., Bayley P. M., Reid K. B. Structural similarities between Thy-1 antigen from rat brain and immunoglobulin. Nature. 1979 Nov 15;282(5736):341–342. doi: 10.1038/282341a0. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Novotný J., Sternberg M. J., Campbell D. G., Williams A. F. Analysis of structural similarities between brain Thy-1 antigen and immunoglobulin domains. Evidence for an evolutionary relationship and a hypothesis for its functional significance. Biochem J. 1981 Apr 1;195(1):31–40. doi: 10.1042/bj1950031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas T. C. Occurrence of a theta-like antigen in rats. J Exp Med. 1972 Nov 1;136(5):1054–1062. doi: 10.1084/jem.136.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Brockes J. P., Mirsky R., Wendon L. M. Cell surface markers for distinguishing different types of rat dorsal root ganglion cells in culture. Cell. 1978 May;14(1):43–51. doi: 10.1016/0092-8674(78)90299-4. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Finch P. R., Wood D. D., Moscarello M. A. Isolation of a highly purified myelin protein. Biochemistry. 1971 Dec 7;10(25):4756–4763. doi: 10.1021/bi00801a024. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gordon L. K., Morris R. J. Demonstration of Thy-1 antigen on pluripotent hemopoietic stem cells in the rat. J Exp Med. 1978 Nov 1;148(5):1351–1366. doi: 10.1084/jem.148.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Mason D. W., Williams A. F. In rat bone marrow Thy-1 antigen is present on cells with membrane immunoglobulin and on precursors of peripheral B lymphocytes. Eur J Immunol. 1977 Nov;7(11):817–823. doi: 10.1002/eji.1830071114. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Kuchel P. W., Campbell D. G., Barclay A. N., Williams A. F. Molecular weights of the Thy-1 glycoproteins from rat thymus and brain in the presence and absence of deoxycholate. Biochem J. 1978 Feb 1;169(2):411–417. doi: 10.1042/bj1690411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Unger M., Dulbecco R. Thy-1: a differentiation marker of potential mammary myoepithelial cells in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6093–6097. doi: 10.1073/pnas.75.12.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J. F., Lennon V. A. Transitory expression of Thy-1 antigen in skeletal muscle development. Nature. 1977 Jul 14;268(5616):163–165. doi: 10.1038/268163a0. [DOI] [PubMed] [Google Scholar]
- Letarte-Muirhead M., Barclay A. N., Williams A. F. Purification of the Thy-1 molecule, a major cell-surface glycoprotein of rat thymocytes. Biochem J. 1975 Dec;151(3):685–697. doi: 10.1042/bj1510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte M., Meghji G. Purification and characterization of mouse brain Thy-1 glycoprotein antigens. J Immunol. 1978 Nov;121(5):1718–1725. [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain L. D., Tomana M., Acton R. T. Purification and characterization of mouse brain Thy-1.2 differentiation alloantigen. Brain Res. 1978 Dec 22;159(1):161–171. doi: 10.1016/0006-8993(78)90117-8. [DOI] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Owen M. J., Knott J. C., Crumpton M. J. Labeling of lymphocyte surface antigens by the lipophilic, photoactivatable reagent hexanoyldiiodo-N-(4-azido-2-nitrophenyl)tyramine. Biochemistry. 1980 Jun 24;19(13):3092–3099. doi: 10.1021/bi00554a040. [DOI] [PubMed] [Google Scholar]
- Ozols J., Gerard C. Primary structure of the membranous segment of cytochrome b5. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3725–3729. doi: 10.1073/pnas.74.9.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M. Thy-1 antigen on astrocytes in long-term cultures of rat central nervous system. Nature. 1979 Aug 23;280(5724):688–690. doi: 10.1038/280688a0. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Ritter M. A., Morris R. J. Thy-1 antigen: selective association in lymphoid organs with the vascular basement membrane involved in lymphocyte recirculation. Immunology. 1980 Jan;39(1):85–91. [PMC free article] [PubMed] [Google Scholar]
- Scheid M., Boyse E. A., Carswell E. A., Old L. J. Serologically demonstrable alloantigens of mouse epidermal cells. J Exp Med. 1972 Apr 1;135(4):938–955. doi: 10.1084/jem.135.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2481–2485. doi: 10.1073/pnas.73.7.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P. L. Theta alloantigen on mouse and rat fibroblasts. Nat New Biol. 1973 Nov 21;246(151):76–78. doi: 10.1038/newbio246076a0. [DOI] [PubMed] [Google Scholar]
- Stoffyn P., Folch-Pi J. On the type of linkage binding fatty acids present in brain white matter proteolipid apoprotein. Biochem Biophys Res Commun. 1971 Jul 2;44(1):157–161. doi: 10.1016/s0006-291x(71)80172-9. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Thierfelder S. Haemopoietic stem cells of rats but not of mice express Th-1.1 alloantigen. Nature. 1977 Oct 20;269(5630):691–693. doi: 10.1038/269691a0. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Weissman I. L., Bevan M. J. Mouse T-cell surface glycoprotein recognised by heterologous anti-thymocyte sera and its relationship to Thy-1 antigen. Nature. 1975 Aug 21;256(5519):652–654. doi: 10.1038/256652a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Letarte-Muirhead M., Morris R. J. Rat thy-1 antigens from thymus and brain: their tissue distribution, purification, and chemical composition. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):51–61. doi: 10.1101/sqb.1977.041.01.009. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Many cells in rat bone marrow have cell-surface Thy-1 antigen. Eur J Immunol. 1976 Jul;6(7):526–528. doi: 10.1002/eji.1830060716. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Zwerner R. K., Barstad P. A., Acton R. T. Isolation and characterizaiton of murine cell surface components. I. Purification of milligram quantities of Thy-1.1. J Exp Med. 1977 Oct 1;146(4):986–1000. doi: 10.1084/jem.146.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]


