Abstract
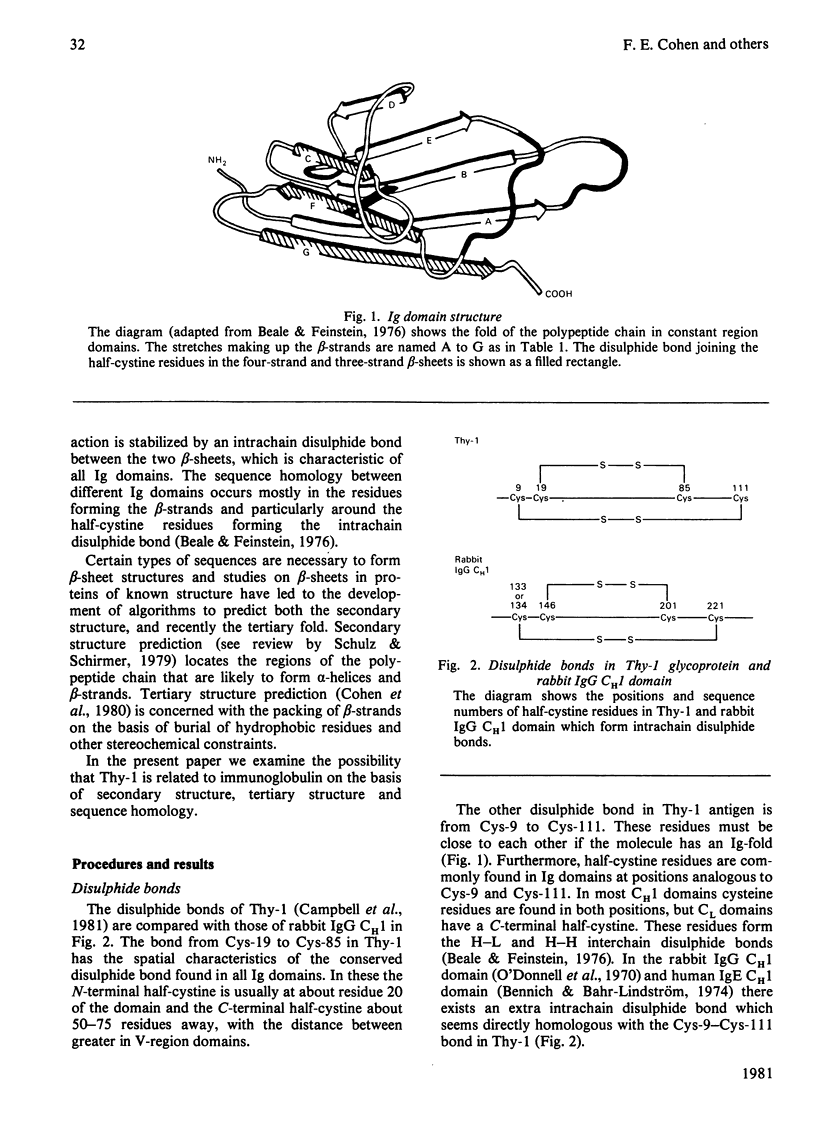
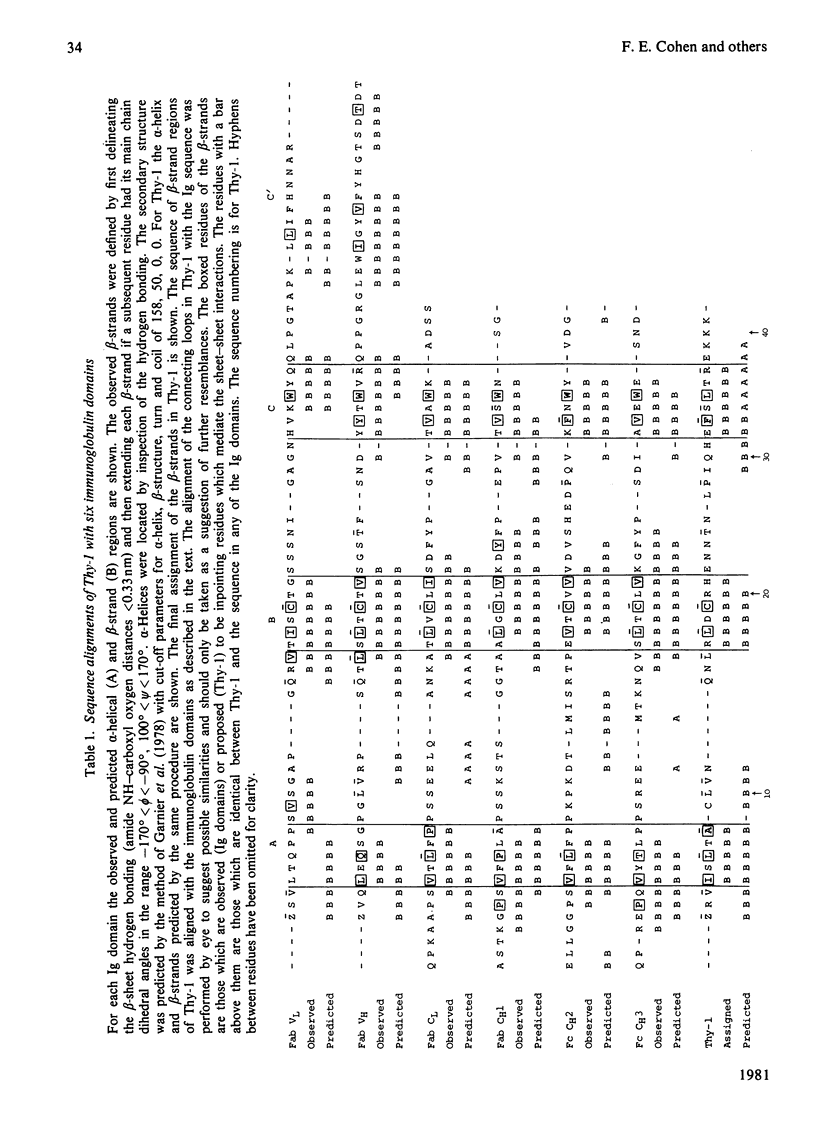
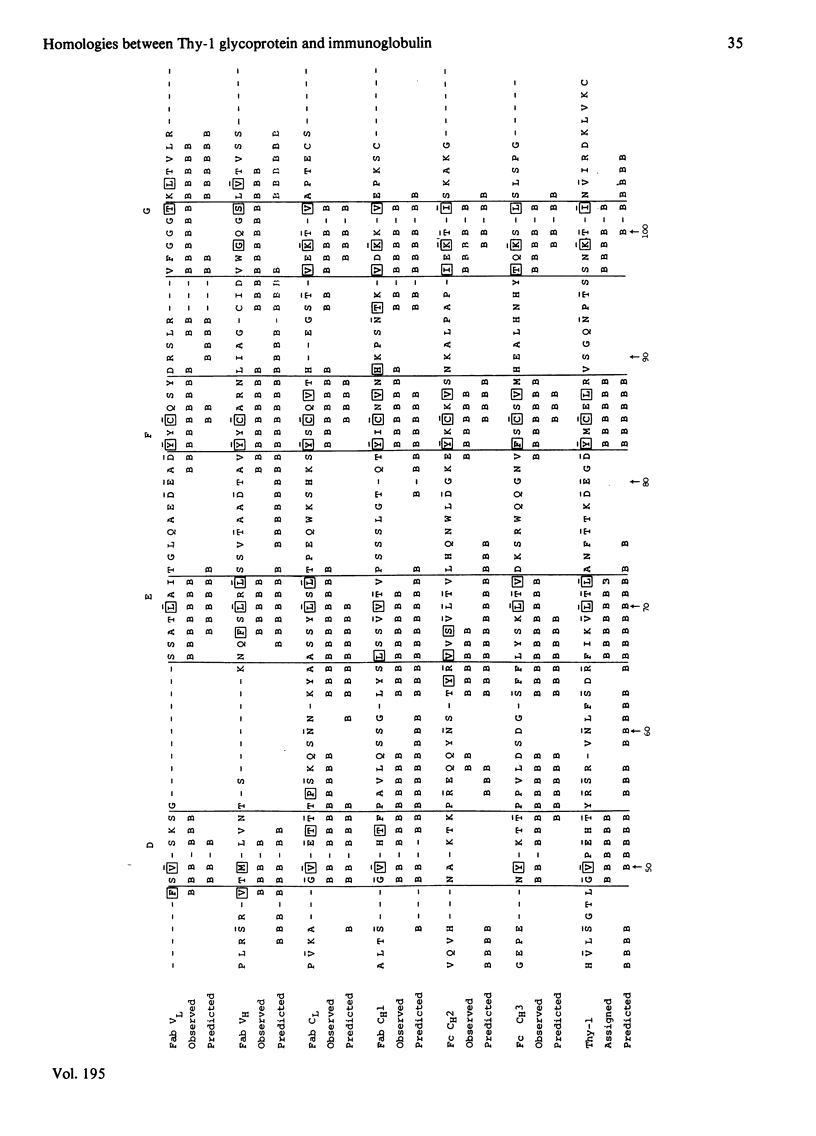
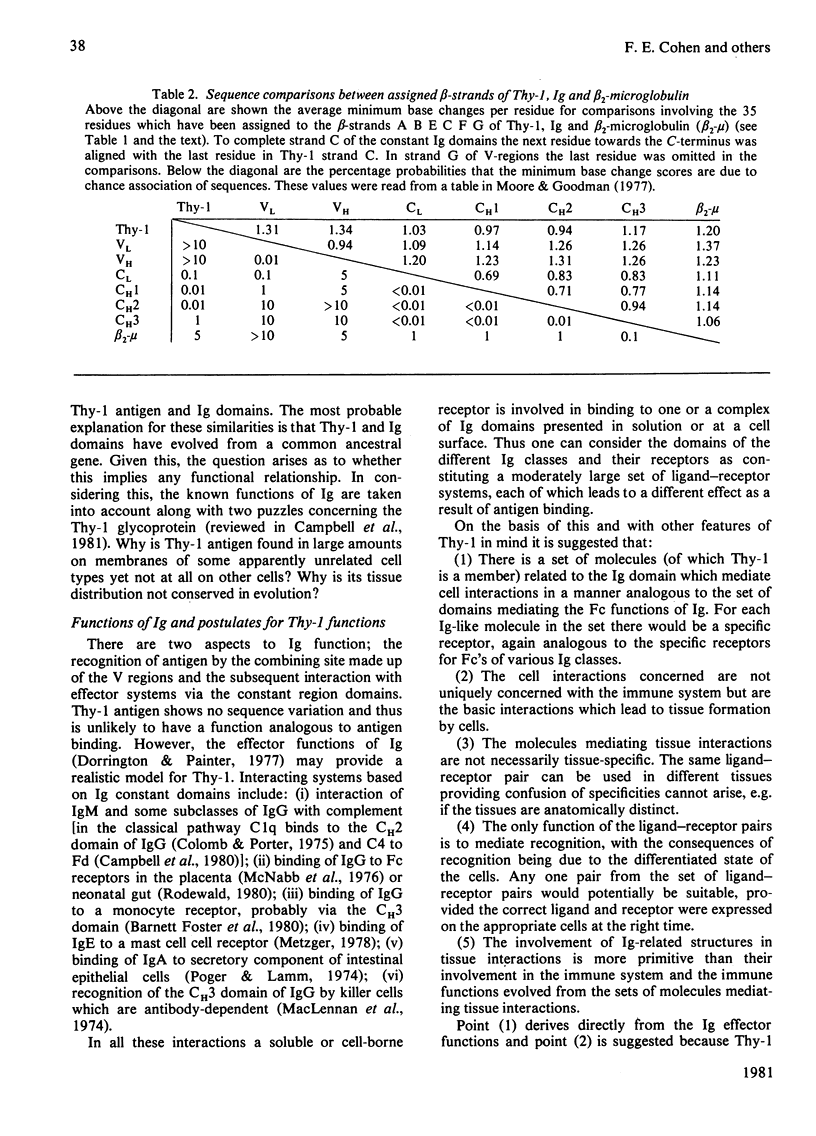
The Thy-1 membrane glycoprotein from rat brain is shown to have structural and sequence homologies with immunoglobulin (Ig) domains on the basis of the following evidence. 1. The two disulphide bonds of Thy-1 are both consistent with the Ig-fold. 2. The molecule contains extensive beta-structure as shown by the c.d. spectrum. 3. Secondary structure prediction locates beta-strands along the sequence in a manner consistent with the Ig-fold. 4. On the basis of rules derived from known beta-sheet structures, a three-dimensional structure with the Ig-fold is predicted as favourable for Thy-1. 5. Sequences in the proposed beta-strands of Thy-1 and known beta-strands of Ig domains show significant sequence homology. This homology is statistically more significant than for the comparison of proposed beta-strand sequences of beta 2-microglobulin with Ig domains. An hypothesis is presented for the possible functional significance of an evolutionary relationship between Thy-1 and Ig. It is suggested that both Thy-1 and Ig evolved from primitive molecules, with an Ig fold, which mediated cell--cell interactions. The present-day role of Thy-1 may be similar to that of the primitive domain.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Barnett Foster D. E., Dorrington K. J., Painter R. H. Structure and function of immunoglobulin domains. VIII. An analysis of the structural requirements in human IgG1 for binding to the Fc receptor of human monocytes. J Immunol. 1980 May;124(5):2186–2190. [PubMed] [Google Scholar]
- Beale D., Feinstein A. Structure and function of the constant regions of immunoglobulins. Q Rev Biophys. 1976 May;9(2):135–180. doi: 10.1017/s0033583500002390. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. G., Williams A. F., Bayley P. M., Reid K. B. Structural similarities between Thy-1 antigen from rat brain and immunoglobulin. Nature. 1979 Nov 15;282(5736):341–342. doi: 10.1038/282341a0. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Sternberg M. J., Taylor W. R. Analysis and prediction of protein beta-sheet structures by a combinatorial approach. Nature. 1980 Jun 5;285(5764):378–382. doi: 10.1038/285378a0. [DOI] [PubMed] [Google Scholar]
- Colomb M., Porter R. R. Characterization of a plasmin-digest fragment of rabbit immunoglobulin gamma that binds antigen and complement. Biochem J. 1975 Feb;145(2):177–183. doi: 10.1042/bj1450177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. XI. Functional implications. Biochemistry. 1970 Aug 4;9(16):3197–3205. doi: 10.1021/bi00818a012. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. An improved method of testing for evolutionary homology. J Mol Biol. 1966 Mar;16(1):9–16. doi: 10.1016/s0022-2836(66)80258-9. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. The relation between frequencies of amino acids and ordered trinucleotides. J Mol Biol. 1966 Mar;16(1):1–8. doi: 10.1016/s0022-2836(66)80257-7. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Connell G. E., Gotch F. M. Effector activating determinants on IgG. II. Differentiation of the combining sites for C1q from those for cytotoxic K cells and neutrophils by plasmin digestion of rabbits IgG. Immunology. 1974 Feb;26(2):303–310. [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Monoclonal antibodies to Ia antigens from rat thymus: cross reactions with mouse and human and use in purification of rat Ia glycoproteins. Immunol Rev. 1979;47:117–137. doi: 10.1111/j.1600-065x.1979.tb00291.x. [DOI] [PubMed] [Google Scholar]
- McNabb T., Koh T. Y., Dorrington K. J., Painter R. H. Structure and function of immunoglobulin domains. V. Binding, University of immunoglobulin G and fragments to placental membrane preparations. J Immunol. 1976 Sep;117(3):882–888. [PubMed] [Google Scholar]
- Metzger H. The IgE-mast cell system as a paradigm for the study of antibody mechanisms. Immunol Rev. 1978;41:186–199. doi: 10.1111/j.1600-065x.1978.tb01465.x. [DOI] [PubMed] [Google Scholar]
- Moore G. W., Goodman M. Alignment statistic for identifying related protein sequences. J Mol Evol. 1977 Apr 29;9(2):121–130. doi: 10.1007/BF01732744. [DOI] [PubMed] [Google Scholar]
- Novotný J., Franek F. Different degrees of interspecies homology in immunoglobulin lamda chain constant domain correlated with three-dimensional structure. Nature. 1975 Dec 18;258(5536):641–643. doi: 10.1038/258641a0. [DOI] [PubMed] [Google Scholar]
- O'Donnell I. J., Frangione B., Porter R. R. The disulphide bonds of the heavy chain of rabbit immunoglobulin G. Biochem J. 1970 Jan;116(2):261–268. doi: 10.1042/bj1160261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poger M. E., Lamm M. E. Localization of free and bound secretory component in human intestinal epithelial cells. A model for the assembly of secretory IgA. J Exp Med. 1974 Mar 1;139(3):629–642. doi: 10.1084/jem.139.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S., Getzoff E. D., Richardson D. C. The beta bulge: a common small unit of nonrepetitive protein structure. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2574–2578. doi: 10.1073/pnas.75.6.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. Distribution of immunoglobulin G receptors in the small intestine of the young rat. J Cell Biol. 1980 Apr;85(1):18–32. doi: 10.1083/jcb.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul F. A., Amzel L. M., Poljak R. J. Preliminary refinement and structural analysis of the Fab fragment from human immunoglobulin new at 2.0 A resolution. J Biol Chem. 1978 Jan 25;253(2):585–597. [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Wasserman R. L., Capra J. D. Amino acid sequence of the Fc region of a canine immunoglobulin M: interspecies homology for the IgM class. Science. 1978 Jun 9;200(4346):1159–1161. doi: 10.1126/science.653360. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Letarte-Muirhead M., Morris R. J. Rat thy-1 antigens from thymus and brain: their tissue distribution, purification, and chemical composition. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):51–61. doi: 10.1101/sqb.1977.041.01.009. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]


