Abstract
Performing a pulmonary segmentectomy is a complex process, with precise localization of pulmonary nodules and recognition of intraoperative anatomical variations posing significant challenges. This study aims to assess the advantages of preoperative three-dimensional reconstruction (3D-RE) in thoracoscopic segmentectomy. The study, at Fujian Medical University Cancer Hospital, analyzed data from segmentectomy patients from January 2016 to February 2022. It compared 3D-RE and two-dimensional computed tomography (2D-CT) preoperative scans, focusing on perioperative complications within30 days to identify any differences. This investigation encompassed a total of 265 instances, with 148 belonging to the 3D-RE group and 117 aligned with the 2D-CT group. The 3D-RE group showed reduced intraoperative blood loss and shorter postoperative hospital stays (P < 0.001). They also had higher rates of lymph node sampling and combined subsegmentectomy and segmentectomy procedures (P < 0.01). Postoperative complications, particularly pneumonia and lung fistula, were lower in the 3D-RE group (P = 0.041). The rates of minimally invasive adenocarcinoma (MIA) and invasive adenocarcinoma (IAC) were significantly higher in the 3D-RE group, while adenocarcinoma in situ (AIS) and benign cases were less common (P = 0.006). Surgical duration, chest tube duration, chest drainage volume, surgery complexity, and pathological diagnoses showed no significant differences between the groups. Utilization of preoperative 3D-RE holds potential to minimize both intraoperative and postoperative complications, thereby enhancing the safety and feasibility of undertaking segmentectomy procedures.
Keywords: Advantages, Three-dimensional reconstruction (3D-RE), Two-dimensional computed tomography (2D-CT), Thoracoscopic segmentectomy
Introduction
The freshly published Global Cancer Statistics 2020 indicates that lung cancer ranks second in terms of diagnosis prevalence and first in terms of cancer-induced mortality for the year 2020, constituting roughly 11.4% of cancer diagnoses and contributing to 18.0% of deaths [1]. Lately, the utilization of low-dose chest computed tomography (CT) witnessing a rise, consequently escalating the detection rate of minuscule pulmonary nodules. The standard procedure for patients with stage I non-small cell lung cancer (NSCLC) is lobectomy and mediastinal lymph node sampling or none.
The ongoing exploration of segmentectomy is unearthing mounting evidence consolidating the equivalency of its prognosis to lobectomy in the context of early-stage lung cancer, with the former displaying superior preservation of lung function [2–6]. Compared to wedge resection, segmentectomy ensures sufficient tumor staging alongside the achievement of surgical margin and lymph node sampling, thereby significantly outperforming the former in prognosis [7–9].Hence, lobectomy-intolerant patients diagnosed with early lung cancer, benign lesions, and metastatic tumors should prioritize segmentectomy.
Conversely, pulmonary segmentectomy embodies a highly challenging procedure, particularly when considering the accurate positioning of pulmonary nodules and therein identification of intraoperative anatomical variation. Preoperative 2D-CT image accuracy in detailing the intricate anatomical structures of lungs such as arteries, bronchi and veins falls short, inherently escalating the risk of segmentectomy precision. As medical imaging technology advances, 3D-RE is gradually seeing application in thoracoscopic segmentectomy. Its abilities to precisely locate pulmonary nodules, estimate the resection's scope and determine the intersegmental plane's position, as well as detect variant bronchi and blood vessels, render surgery more accurate and safe. Hence, the pivotal role that preoperative 3D-RE plays in accurate segmentectomy becomes clear [10–14].
In this study, we looked back over the in-depth clinical characteristics of segmentectomy patients from our department and juxtaposed preoperative 3D-RE with 2D-CT in thoracoscopic surgery. The purpose of this article lies in assessing the impact of preoperative 3D-RE in thoracoscopic segmentectomy, thereby offering a point of reference for clinical application.
Material & methods
Patients
In this retrospective analysis, patients who underwent anatomical segmentectomy via video-assisted thoracoscopic surgery (VATS) at the Fujian Cancer Hospital’s Department of Thoracic Surgery, from January 2016 through February 2022, were considered. All the surgical procedures were uniformly performed by one designated thoracic surgeon who had performed over 300 lung segmentations.
Inclusion criteria
-
(I)
the feasibility of the segmentectomy, as dictated by a safe resection margin which was larger than 2 cm or the diameter of the pulmonary nodule;
-
(II)
the execution of all anatomical segmentectomies via uniport or two-port VATS;
-
(III)
the necessity of mediastinal lymph node sampling or none for malignant pulmonary nodules;
-
(IV)
the allowance of benign or malignant pulmonary nodules for inclusion;
-
(V)
no restrictions based on age and gender.
Exclusion criteria
-
(I)
the presence of pulmonary nodules located centrally within the lobe that couldn't be targeted with segmentectomy;
-
(II)
localization within the middle lobe;
-
(III)
diagnosis of severe emphysema;
-
(IV)
presence of severe whole-chest adhesions;
-
(V)
history of lung surgery;
-
(VI)
occurrence of distant metastasis;
-
(VII)
insufficiency of essential clinical data.
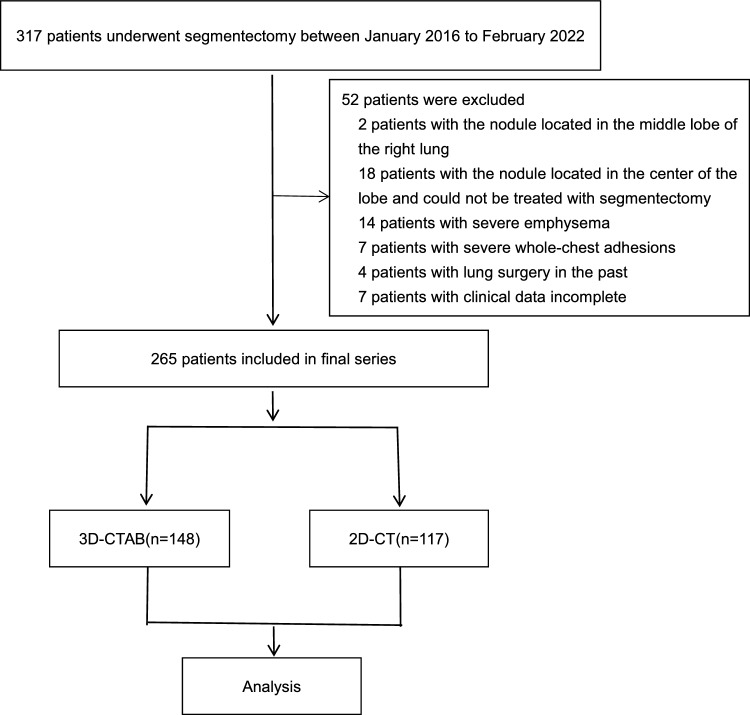
Upon applying these eligibility parameters, a total of 264 patients were listed for the retroactive analysis (Fig. 1).
Fig. 1.
Study flowchart
3D-RE
Employing a 256-channel Multi-detector Computed Tomography (MDCT, General Electric Healthcare, Boston, MA, USA), lung imaging was executed with a slice thickness specification of 0.625 mm. As part of the procedure, an intravenous injection of 35 ml contrast medium was administered at a velocity of 5 ml/s, instantly succeeded by 20 ml of normal saline, utilizing an automatic injector to amplify vessel enhancement. The acquired thin-slice two-dimensional CT images, in Digital Imaging and Communications in Medicine (DICOM) format, were transitioned to dedicated software (IQQA-Lung, EDDA Technology, Princeton, NJ, USA). This software facilitated the reconstruction of a three-dimensional model encompassing the bronchial and vessel system and the coordinates of the nodule. Potential errors and perceived defects predominantly affecting the distal bronchial and vessel imaging were subsequently manually rectified.
Case grouping
3D-RE required an additional cost, so we do it on a voluntary basis. The patients were categorized into two groups based on the utilization of 3D-RE: the 3D-RE group and the 2D-CT group.
The 3D-RE group
Preoperative surgical simulation
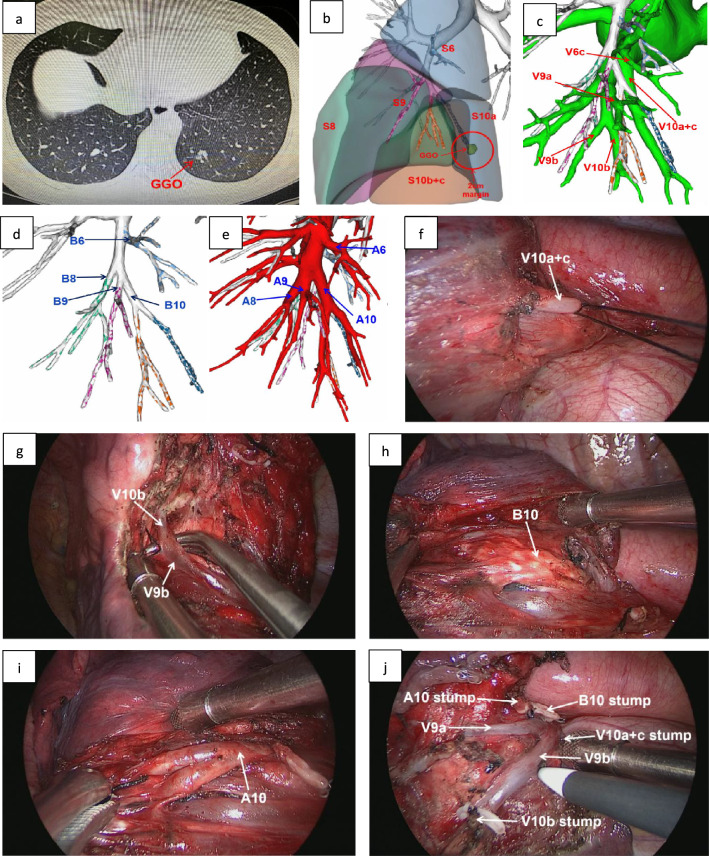
Utilising 3D-RE imaging, we can identify the precise location of the nodules and their respective lung segment. Following this determination, we verified the target segmental bronchus, arteries, segmental veins, and intersegmental veins, subsequently formulating the surgical approach. Furthermore, it's imperative to highlight that the quantification of the pulmonary nodule's maximum diameter was conducted via the CT lung window. An essential stipulation for resection margins was that they must be a minimum of 2 cm or exceed the maximum diameter of the pulmonary nodule, as illustrated by Fig. 2.
Fig. 2.
The CT scan and 3D-CTAB of a 48-year-old female patient. One GGO was found in the left lower lobe. According to the 3D-CTAB, anatomical segmentectomy with left S10 was performed; a the CT scan showed that a GGO was located in the left lobe (S10); b according to the results of 3D-CTAB and the safe margin of 2 cm, the confirmed target lung segment was LS10; c 3D-CTAB showed pulmonary vein anatomy; d 3D-CTAB showed the pulmonary bronchial anatomy; e 3D-CTAB showed the pulmonary artery anatomy; f V10a + c was showed in the operation; g V9b and V10b was showed in the operation; h B10 was showed in the operation; i A10 was showed in the operation; j the surgical wound after LS10 segmentectomy
Surgical procedures
Utilizing general anesthesia in conjunction with double-lumen endotracheal intubation, all patients underwent a one-lung ventilation procedure on the contralateral side and were positioned laterally. The ensuing surgical procedures were executed by one seasoned thoracic surgeon, with the assistance of either uniport or two-port video assisted thoracic surgery.
The single-port surgery entailed establishing a 4 cm utility incision within the anterior axillary line through the fourth intercostal space (ICS). In contrast, the two-port surgery procedure additionally incorporated a 10 mm trocar into the midaxillary line inside the ICS to enable the access of a 30-degree thoracoscope. In alignment with the predetermined surgical simulation, the intended arteries, veins, and bronchi were severed, while the intersegmental veins were preserved. The differentiation of the targeted and non-targeted segment borders were subsequently identified using the “inflation-deflation” approach. The interface between the inflation and deflation was dissected along the intersegmental veins towards the outer one third of the pulmonary parenchyma using ultrasonic scalpels. The remaining pulmonary parenchyma was then resected using staplers. Utilizing an ultrasonic knife, the pulmonary parenchyma was separated along the inflation-deflation line and the intersegmental veins from the segment hilum to the outer one-third of the lung tissue. Linear cutting staplers were then employed for the removal of remaining lung tissue. A pulmonary leakage test was conducted post-procedure to assess lung durability. In cases of evident air leakage, suturing with a 3-0 or 4-0 Prolene suture was performed. If air leakage was minimal, the wound was covered with hemostatic materials for closure. In operative scenarios involving malignant nodules, intra-pulmonary and mediastinal lymph nodes were isolated for examination via intraoperative frozen section testing. Signs of malignancy within the lymph nodes prompted the execution of a lobectomy along with a systematic lymph node dissection.
The surgical approach encompassed segmentectomy, subsegmentectomy, and combined segmentectomy. Segmentectomy was characterized as excision of a single lung segment. Subsegmentectomy was described as excision of a solitary lung subsegment. Combined segmentectomy was typified as resection of a minimum of two subsegments, a segment and a subsegment, or two or more pulmonary segments.
As delineated in a previous study [3], the categorization of segmentectomy was orchestrated into simple and intricate segmentectomy, dependent on the number of intersegmental planes excised. In instances when a solo intersegmental plane was subject to resection, it was classified as a simple segmentectomy, evidenced in the superior segment of the right or left lower lobe (S6), basal segment, left superior, and the lingular segment. The remaining variants were organized under complex segmentectomy.
The 2D-CT group
Reliant on empirical knowledge and preoperative thin-section 2D-CT sans, the thoracic surgeon delineated the location of pulmonary nodules and the designated pulmonary segment, alongside the vascular and bronchial structures intended for resection. Ancillary procedures remained congruent with those executed in the 3D-RE group.
Statistical analysis
Analytical procedures of all accrued clinical data were undertaken utilizing SPSS (IBM SPSS Statistics for Windows, Version 22.0, IBM Corp., Armonk, NY, USA). Continuous data presentations adhered to the mean ± standard deviation (SD) model, and the t-test or Wilcoxon test enacted comparisons of quantitative continuous data. Usage of Chi-square or Fishers exact tests was prompted by the necessity for categorical variables. A P value of < 0.05 was the threshold for statistical significance.
Results
Clinical characteristics of the patients
In this investigation, a total of 265 subjects were considered eligible, including 148 within the 3D-RE cohort and 117 falling into the 2D-CT group. Table 1 provides comprehensive delineation of the clinical profiles for both study groups.
Table 1.
presents the clinical characteristics of each group, with the data reported as n (%) and means (SD) unless otherwise specified
| Variable | 3D-CTAB(148) | 2D-CT(117) | t/χ2 | P |
|---|---|---|---|---|
| Age(years)(SD) | 56.0 ± 10.7 | 54.5 ± 11.8 | 1.047 | 0.296 |
| Gender | 1 | 0.981 | ||
| Male | 66(44.6%) | 52(44.4%) | ||
| Female | 82(55.4%) | 65(55.6%) | ||
| Smoking | 0.030 | 0.862 | ||
| Yes | 13(8.8%) | 11(9.4%) | ||
| No | 135(91.2%) | 106(90.6%) | ||
| Maximum lesion diameter(mm) (SD) | 12.54 ± 5.30 | 13.88 ± 6.67 | -1.823 | 0.069 |
| Nodule location | 0.996 | 0.802 | ||
| LUL | 48(32.4%) | 32(27.4%) | ||
| LLL | 31(20.9%) | 25(21.4%) | ||
| RUL | 38(25.7%) | 35(29.9%) | ||
| RLL | 31(20.9%) | 25(21.4%) | ||
| Pulmonary function | ||||
| FEV1 | 2.59 ± 0.61 | 2.47 ± 0.54 | 1.690 | 0.092 |
| FEV1 (%) | 105.12 ± 89.78 | 90.80 ± 14.01 | 1.650 | 0.100 |
| Hypertension | 0.000 | 0.990 | ||
| Yes | 29(19.6%) | 23(19.7%) | ||
| No | 119(80.4%) | 94(80.3%) | ||
| Diabetes | 0.590 | 0.442 | ||
| Yes | 14(9.5%) | 8(6.8%) | ||
| No | 134(90.5%) | 109(93.2%) | ||
| COPD | 0.578 | 0.447 | ||
| Yes | 18(12.2%) | 18(15.4%) | ||
| No | 130(87.8%) | 99(84.6%) |
Intraoperative and postoperative data
Thoracoscopic segmentectomies were successfully executed for all individuals, circumventing any need for conversion to thoracotomy or lobectomy. It should be noted that within the 2D-CT arm, five subjects underwent combined segmentectomy due to varying issues, one being an inadequate resection margin, two due to missection of the pulmonary artery and another two due to bronchial miscuts. Importantly, lymph nodes from all individuals showed no sign of pathology, and the surgical margins were within acceptable limits according to the established surgical parameters.
The 3D-RE group exhibited a decrease in intraoperative blood loss and shorter hospital stays post-surgery relative to their 2D-CT counterparts (77.5 ± 29.0 mL versus 120 ± 68.5 mL, and 5.7 ± 3.5 days versus 7.3 ± 3.6 days respectively), with statistical significance denoted (P < 0.05). Lymph node sampling was also notably higher within the 3D-RE group, constituting 89.2% in comparison to 78.6% in the 2D-CT group. Regarding surgical modalities, a significantly higher occurrence of subsegmentectomy and combined segmentectomy was noted in the 3D-RE group (8.8% and 48.6% respectively) when juxtaposed with the 2D-CT group (0% and 42.7% respectively). Moreover, the postoperative complications in the 3D-RE cohort were markedly lower (8.1% vs. 16.2%), with particular reference to incidences of pneumonia and lung fistula. Lung fistula was defined as a leak of air in the lungs lasting more than 5 days. Stratified analysis carried out among adenocarcinoma subjects demonstrated that MIA was significantly higher within the 3D cohort (53.7% compared to 38.8%), while AIS was less prevalent (9.1% versus 24.7%). Contrasts between the groups in terms of average operative duration, length of chest tube placement, total chest drainage, level of surgical difficulty or pathological diagnosis did not show a significant statistical difference (P > 0.05)—details depicted in Table 2.
Table 2.
Intraoperative and postoperative data of the two group
| Variable | 3D-CTAB(148) | 2D-CT(117) | t/χ2 | P |
|---|---|---|---|---|
| Average operative duration (minutes) | 170.5 ± 47.4 | 176 ± 52.3 | – 0.925 | 0.356 |
| Intraoperative blood loss(mL) | 77.5 ± 29.0 | 120 ± 68.5 | – 6.336 | 0.000 |
| The duration of chest tube placement (days) | 3.2 ± 3.4 | 3.8 ± 3.3 | – 1.488 | 0.138 |
| Postoperative hospital stays(days) | 5.7 ± 3.5 | 7.3 ± 3.6 | – 3.524 | 0.001 |
| Postoperative complications | 12(8.1%) | 19(16.2%) | 4.182 | 0.041 |
| Pneumonia | 2(1.4%) | 3(2.6%) | ||
| Atelectasis | 0(0%) | 2(1.7%) | ||
| Chylothorax | 2(1.4%) | 0(0%) | ||
| Lung fifistula | 10(6.8%) | 16(13.7%) | ||
| Reinsertion of chest drain | 4(2.7%) | 3(2.6%) | ||
| Mediastinal lymph node | 5.568 | 0.018 | ||
| None | 16(10.8%) | 25(21.4%) | ||
| Sampling | 132(89.2%) | 92(78.6%) | ||
| Total chest drainage (mL) | 835.9 ± 712.1 | 957.9 ± 605.2 | – 1.479 | 0.140 |
| Mode of surgery | 13.651 | 0.001 | ||
| Segmentectomy | 63(42.6%) | 67(57.3%) | ||
| Subsegmentectomy | 13(8.8%) | 0(0%) | ||
| Combined Segmentectomy | 72(48.6%) | 50(42.7%) | ||
| surgical difficulty level | 0.998 | 0.318 | ||
| simple segmentectomy | 63(42.6%) | 57(48.7%) | ||
| complex segmentectomy | 85(57.4%) | 60(51.3%) | ||
| Pathological diagnosis | 5.816 | 0.121 | ||
| Adenocarcinoma | 121(81.8%) | 85(72.6%) | ||
| Squamous cell Carcinoma | 0(0%) | 2(1.7%) | ||
| Benign | 27(18.2%) | 29(24.8%) | ||
| Carcinoid | 0(0%) | 1(0.9%) | ||
| Adenocarcinoma | 10.172 | 0.006 | ||
| AIS | 11(9.1%) | 21(24.7%) | ||
| MIA | 65(53.7%) | 33(38.8%) | ||
| IAC | 45(37.2%) | 31(36.5%) |
Discussion
The 1995 LCSG study established lobectomy as the standard surgical method for T1N0 NSCLC [15]. Segmentectomy's efficacy as an alternative to lobectomy for early NSCLC is debated. The JCOG0802 trial compared segmentectomy with lobectomy for ≤ 2 cm peripheral NSCLC. Segmentectomy had higher air leakage [3] but superior 5-year survival rates with less decline in FEV1 at 6 and 12 months. Segmentectomy is recommended for patients with small peripheral NSCLC [4]. The CALGB 140503 Trial confirmed segmentectomy's non-inferiority to lobectomy with advantages in lung function preservation [2, 6, 16]. NCCN guidelines suggest segmentectomy or wedge resection for patients with poor pulmonary reserve or comorbidities contraindicating lobectomy and nodules meeting specific criteria [17]. Segmentectomy is recognized as a viable option for early NSCLC treatment [18].
Nevertheless, it should be noted that segmentectomy posed inherent challenges as a surgical procedure. To commence, a key hurdle involved precise localization of the pulmonary nodule and identification of the targeted segment. This not only ensured an adequate surgical margin but also facilitated efficient lesion detection, ultimately reducing operation time. Additionally, the anatomy of lung segments is considerably more intricate compared to the structure of lung lobes. There exists a wide range of anatomical variations pertaining to bronchial and vascular structures, thereby necessitating thoracic surgeons' comprehensive familiarity with the specific lung anatomy of each patient.
Previous practices involved the utilization of 2D-CT for locating pulmonary nodules and identifying pulmonary anatomical structures. However, this approach presented challenges in accurately discerning the anatomical structures of target segments, pulmonary arteries, veins, and bronchi. Consequently, there was a higher risk of misplanning the target lung segment. Inadvertent damage to the pulmonary vessels or bronchi during surgery could necessitate combined segmentectomy or lobectomy, potentially resulting in severe consequences. Notably, several studies have demonstrated that more than one-third of p-T1N0M0 NSCLC tumors extend beyond a single segment [19]. Reliance solely on 2D-CT for locating pulmonary nodules in such cases would make it significantly more arduous to determine the targeted segment and ensure adequate surgical margins. In the 2D-CT group of the present study, insufficient resection margin was observed in one case, mis-cutting of the pulmonary artery occurred in two cases, and mis-cutting of the bronchus occurred in two cases, necessitating expanded surgery and combined segmental resection. Conversely, no similar occurrences were reported in the 3D-RE group. Additionally, we observed a higher incidence of pneumonia and atelectasis in the 2D-CT group, possibly due to accidental damage to the bronchi or vessels during surgery.
The advancement of medical imaging technology has facilitated the gradual incorporation of 3D-RE technology in thoracoscopic segmentectomy procedures. 3D-RE enables the precise restoration of the lung's true anatomical structure with remarkable clarity. It offers the capability to accurately locate nodules, estimate resection ranges, determine target segments, and provide clear visualization of lung segment anatomical structures along with diverse bronchial and vascular anatomical variations. In light of these advantages, our medical center has also adopted 3D-RE technology for analyzing lung anatomical structures, uncovering rare anatomical variations [20–22]. Leveraging preoperative 3D-RE data, personalized and precise pulmonary segment resection plans are developed for each patient, and intraoperative navigation is performed. This integrated approach enhances surgical accuracy and safety throughout the procedure [12, 14, 23, 24].In our study, the 3D-RE group showed a higher lymph node sampling rate and fewer surgery-related complications. This further demonstrates the advantages of the 3D group, leading to the decision not to conduct a matching analysis. Moreover, we believe that the 3D-RE group displayed clear and precise delineation between target and non-target segments through preoperative 3D planning, thereby reducing the incidence of air leakage. Additionally, the 3D-RE group underwent more subsegmentectomy or combined segmentectomy procedures than the 2D-CT group, which are technically challenging and involve a larger surgical injury surface. Nevertheless, patients in the 3D-RE group experienced shorter surgical times, less blood loss, fewer postoperative complications, and shorter hospital stays, highlighting the benefits of 3D-RE.
Limitation
The present investigation was subject to certain constraints. Initially, the failure to achieve maximum deep aspiration during a CT scan could engender inadequate expansion, leading to an incomplete bronchial reconstruction. Secondary to this, there is the likelihood that smaller bronchi, arteries or veins may have been overlooked, generating potential bias in our findings. Finally, the limits of the collective sample size solicits the need for an extended, multi-center investigation to fortify these outcomes.
Conclusion
Engaging in preoperative 3D-RE offers substantial benefits in discerning pulmonary nodule locations and identifying anatomical lung variations prior to surgery. This technology permits comprehensive strategic planning and intraoperative navigation to facilitate an accurate process of pulmonary segmentectomy, thus decreasing risks during and post-operation. For pulmonary segmentectomy, the application of preoperative 3D-RE is both safe and practical.
Abbreviations
- 3D-RE
Three-dimensional reconstruction
- 2D-CT
Two-dimensional computed tomography
- COPD
Chronic obstructive pulmonary disease
- MIA
Minimally invasive adenocarcinoma
- IAC
Invasive adenocarcinoma
- AIS
Adenocarcinoma in situ
- CT
Computed tomography
- NSCLC
Non-small cell lung cancer
- VATS
Video-assisted thoracoscopic surgery
- DICOM
Digital Imaging and Communications in Medicine
- ICS
Intercostal space
- SD
Standard deviation
- LCSG
Lung Cancer Study Group
- NCCN
National Comprehensive Cancer Network
- GGO
Ground-glass opacity
- LUL
Left upper lobe
- LLL
Left lower lobe
- RUL
Right upper lobe
- RLL
Right lower lobe
Author contributions
Hao He: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Writing-original draft. Peiyuan Wang: Conceptualization; Methodology; Writing-original draft preparation. Hang Zhou: Data curation. Wenwei Wei: Data curation. Junpeng Lin: Data curation. Yujie Chen: Data curation. Feng Wang: Conceptualization; Methodology; Formal analysis; writing-original draft preparation; Data curation; Supervision. Shuoyan Liu: Data curation; Conceptualization; Methodology; Formal analysis; writing-original draft preparation.
Funding
This work was supported by the Natural Science Foundation of Fujian Province, China (No. 2023J011270).
Data availability
The data will be made available on reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
This study was conducted in alignment with the revised Declaration of Helsinki (2013). It received ethical approval from the regional ethics board at Fujian Cancer Hospital (permit No: K20220-017-01).
Research involving human participants and/or animals and Informed consent
Research involving human participants and/or animals was conducted acquiring written informed consents from all participants
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hao He, Peiyuan Wang have contribute equally to this work.
Contributor Information
Feng Wang, Email: swfmd120@163.com.
Shuoyan Liu, Email: shuoyanliu2010@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249 [DOI] [PubMed] [Google Scholar]
- 2.Altorki NK, Wang X, Wigle D et al (2018) Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 6(12):915–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Saji H, Aokage K et al (2019) Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg 158(3):895–907 [DOI] [PubMed] [Google Scholar]
- 4.Saji H, Okada M, Tsuboi M et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Watanabe SI, Wakabayashi M et al (2022) A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 163(1):289–301.e2 [DOI] [PubMed] [Google Scholar]
- 6.Altorki N, Wang X, Kozono D et al (2023) Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med 388(6):489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsutani Y, Handa Y, Shimada Y et al (2021) Comparison of cancer control between segmentectomy and wedge resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 162(4):1244-1252.e1 [DOI] [PubMed] [Google Scholar]
- 8.Smith CB, Swanson SJ, Mhango G, Wisnivesky JP (2013) Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 8(1):73–78 [DOI] [PubMed] [Google Scholar]
- 9.Ye T, Deng L, Xiang J et al (2018) Predictors of pathologic tumor invasion and prognosis for ground glass opacity featured lung adenocarcinoma. Ann Thorac Surg 106(6):1682–1690 [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Gan F, Xia C et al (2019) Discovery of lung surface intersegmental landmarks by three-dimensional reconstruction and morphological measurement. Transl Lung Cancer Res 8(6):1061–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao F, Wang J, Yao J, Hang F, Lei X, Cao Y (2017) Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int J Surg 39:16–22 [DOI] [PubMed] [Google Scholar]
- 12.Xu G, Chen C, Zheng W, Zhu Y, Chen H, Cai B (2019) Application of the IQQA-3D imaging interpretation and analysis system in uniportal video-assisted thoracoscopic anatomical segmentectomy: a series study. J Thorac Dis 11(5):2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu WB, Xu XF, Wen W et al (2016) Three-dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 8(Suppl 9):S710–S715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu WB, Xia Y, Pan XL et al (2019) Three-dimensional navigation-guided thoracoscopic combined subsegmentectomy for intersegmental pulmonary nodules. Thorac Cancer 10(1):41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg RJ, Rubinstein LV (1995) Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 60(3):615–622 (discussion 622-3) [DOI] [PubMed] [Google Scholar]
- 16.N. Altorki ea (2022) Lobar or sub-lobar resection for peripheral clinical stage IA = 2 cm non-small cell lung cancer (NSCLC): results from an international randomized phase III Trial (CALGB 140503 [Alliance]). In: 2022 World Conference on Lung Cancer. . PL03.06.
- 17.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) (2022) Non-small cell lung cancer. Version 5.ed
- 18.Shiono S, Okumura T, Boku N et al (2017) Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 51(3):504–510 [DOI] [PubMed] [Google Scholar]
- 19.Horinouchi H, Nomori H, Nakayama T et al (2011) How many pathological T1N0M0 non-small cell lung cancers can be completely resected in one segment? Special reference to high-resolution computed tomography findings. Surg Today 41(8):1062–1066 [DOI] [PubMed] [Google Scholar]
- 20.He H, Chen P, Chen X, Wang PY, Liu SY, Wang F (2021) Analysis of anatomical variations of the lingular artery of the left upper lobe using 3D computed tomography angiography and bronchography. J Thorac Dis 13(8):5035–5041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He H, Wang F, Wang PY et al (2022) Anatomical analysis of variations in the bronchus pattern of the left upper lobe using three-dimensional computed tomography angiography and bronchography. Ann Transl Med 10(6):305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H, Wei W, He H et al (2022) A cross-sectional study: analysis of anatomical variation in the right upper lung intersegmental vein V2a based on a 3D reconstruction technique. J Thorac Dis 14(11):4460–4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W, Zhang K, Han X et al (2021) Three-dimensional computed tomography angiography and bronchography combined with three-dimensional printing for thoracoscopic pulmonary segmentectomy in stage IA non-small cell lung cancer. J Thorac Dis 13(2):1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.She XW, Gu YB, Xu C et al (2018) Three-dimensional (3D)- computed tomography bronchography and angiography combined with 3D-video-assisted thoracic surgery (VATS) versus conventional 2D-VATS anatomic pulmonary segmentectomy for the treatment of non-small cell lung cancer. Thorac Cancer 9(2):305–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be made available on reasonable request.