Abstract
Background and Purpose
The role of high‐resolution nerve ultrasound (HRUS) in the diagnosis of chemotherapy‐induced polyneuropathy is unclear. The present prospective longitudinal controlled study evaluates the utility of HRUS in vincristine‐induced polyneuropathy (VIPN).
Methods
Twelve patients receiving vincristine and 12 healthy age‐matched controls were included. Visits before and 3 weeks, 8 weeks, and 6 months after the start of vincristine treatment included clinical examination, the total neuropathy score (TNS), nerve conduction studies (NCSs), and HRUS of the bilateral median, ulnar, radial, tibial, peroneal, and sural nerve cross‐sectional areas (CSAs).
Results
Median TNS increased from 0 points (interquartile range [IQR] 0) to 0.5 points (IQR 1, p = .26) at Week 3 and to 4 points (IQR 2.5, p < .001) at Week 8. At 6 months, there was a nonsignificant decrease to 2 points (IQR 2, p = .66). HRUS of individual nerve sites showed no significant changes in CSA and intranerve variability. The total CSA of all entrapment sites increased significantly (p = .007) at Week 8. Sensory nerve action potentials decreased significantly after 6 months (sural nerve, p = .001; radial nerve, p = .004; ulnar and median nerve, p < .001). The tibial nerve compound muscle action potential (p = .006) and nerve conduction velocity (p < .001) were reduced.
Conclusions
At mid‐treatment, there is an increase in the total CSA at entrapment sites parallel to an increase in clinical symptoms. In individual nerve sites, HRUS does not detect significant signs of VIPN. NCSs exhibit signs of a predominantly sensory axonal polyneuropathy. The clinical examination remains the most sensitive tool in the early detection of VIPN.
Keywords: chemotherapy‐induced polyneuropathy, high‐resolution nerve ultrasound, lymphoma, nerve conduction studies, total neuropathy score, vincristine
INTRODUCTION
Vincristine‐induced polyneuropathy (VIPN) is a frequent toxic undesirable effect of vincristine chemotherapy occurring both in children and in adults. 1 , 2 Early diagnosis of chemotherapy‐induced polyneuropathy (CIPN) is increasingly recognized as an interdisciplinary task concerning neurologists and oncologists. 3 High‐resolution ultrasound (HRUS) of peripheral nerves has been established as a useful tool in the diagnosis and follow‐up of polyneuropathies. 4
The role of HRUS in the early detection of CIPN is unclear. A small number of published studies covering HRUS reported on different types of CIPN. Lycan et al 5 found a decrease in sural nerve cross‐sectional area (CSA) in 20 patients with taxane‐induced polyneuropathy (TIPN) and an increased median nerve CSA in the carpal tunnel. Katz et al 6 described an increase of sural and median nerve CSA in 15 patients with TIPN. Pitarokoili et al 7 reported an increased CSA in 13 patients in oxaliplatin‐induced polyneuropathy (OIPN), especially at entrapment sites as first described by Briani et al 8 in 15 patients treated with oxaliplatin. VIPN has not been characterized sufficiently both in terms of clinical standardization and evaluation of nerve conduction studies (NCSs). 2 Recently, a combined prospective and posttreatment study of 57 VIPN patients reported important clinical features using the total neuropathy score (TNS) and the natural history of VIPN including NCSs. 9 Nerve ultrasound results in VIPN have not been reported in the literature.
The present prospective longitudinal controlled observational pilot study reports HRUS measurements in VIPN in lymphoma patients within the first 6 months after starting vincristine chemotherapy.
METHODS
The local ethics committee of the Ruhr University of Bochum, Germany, approved the study protocol compliant with the declaration of Helsinki (Approval number: 2020–609a).
Participants
Participants were adult (>18 years) lymphoma patients treated in the oncology department of the Johannes Wesling University Hospital Minden, Germany, and scheduled for chemotherapy with vincristine, or healthy‐age matched controls (relatives or hospital employees). Participants were examined between November 2020 and January 2023.
Exclusion criteria
Preexisting conditions with a potential impact on nerve CSA and NCS results (eg, known diabetes, alcohol abuse, history of polyneuropathy, nerve entrapment syndromes, other peripheral nerve lesions, radiculopathies, peripheral neurosurgical procedures, major trauma to the extremities, or end‐stage cancer) led to exclusion.
Study design
Patients were examined at baseline (Visit 0) before chemotherapy with vincristine, after 3 weeks (Visit 1), 8 weeks (Visit 2), and 6 months (Visit 3) after the beginning of chemotherapy with vincristine. Vincristine chemotherapy had a duration of 18‐20 weeks. Tumor staging and chemotherapy are displayed in Table 1. Healthy controls were examined only once. NCSs and HRUS were performed by different raters blinded to each other's results. Clinical examination and participant status (patient or control) were not blinded. The study design is summarized in Figure 1.
TABLE 1.
Demographic and clinical characteristics of individual patients.
| Patient number | Age (years) | Height (cm) | Weight (kg) | Lymphoma histology and Ann Arbor stage | Chemotherapy scheme and frequency of application | Additional systemic therapy | Vincristine cumulative dose (mg/m2) |
|---|---|---|---|---|---|---|---|
| 1 | 59 | 183 | 66.0 | DLBCL Stage IV A | R‐CHOP every 3 weeks | Rituximab (twice) | 3.78 |
| 2 | 55 | 170 | 115.0 | DLBCL Stage II A | R‐CHOP every 3 weeks | None | 2.71 |
| 3 | 51 | 179 | 83.0 | FBCL Stage I A | R‐CHOP every 2 weeks | None | 2.97 |
| 4 | 58 | 158 | 59.0 | PTCL Stage II AE | CHOP every 3 weeks | None | 5.03 |
| 5 | 71 | 180 | 85.0 | DLBCL Stage IV B | R‐CHOP every 3 weeks | None | 2.93 |
| 6 | 39 | 178 | 90.7 | DLBCL Stage IV B | R‐CHOP every 3 weeks | None | 2.93 |
| 7 | 44 | 182 | 85.0 | LBCL Stage IV A | R‐CHOP every 4 weeks | None | 6.76 |
| 8 | 71 | 183 | 106.0 | DLBCL Stage I A | R‐CHOP every 4 weeks | None | 5.36 |
| 9 | 43 | 181 | 90.0 | DLBCL Stage II A | R‐CHOP every 3 weeks | None | 3.79 |
| 10 | 69 | 184 | 95.0 | DLBCL Stage III A | R‐CHOP every 3 weeks | None | 2.74 |
| 11 | 55 | 163 | 67.0 | DLBCL Stage I BE | R‐CHOP every 3 weeks | None | 3.50 |
| 12 | 60 | 184 | 104.5 | DLBCL Stage I A | R‐CHOP every 3 weeks | None | 2.63 |
| Median (patients) [IQR] | 56.5 [21.00] | 180.5 [11.0] | 87.5 [31.13] | n.a. | n.a. | n.a. | 3.3 [1.93] |
| Median (controls) [IQR] | 49.5 [14.50], p = .163 | 172.5 [16.0], p = .203 | 81.8 [23.25], p = .198 | n.a. | n.a. | n.a. | n.a. |
Abbreviations: CHOP, Cyclophosphamide, Doxorubicin, Vincristine, Prednisolone; DLBCL, diffuse large B‐cell lymphoma; FBCL, follicular B‐cell lymphoma; IQR, interquartile range; LBCL, large B‐cell lymphoma; n.a., not applicable; PTCL, peripheral T‐cell lymphoma; R‐CHOP, CHOP + Rituximab.
FIGURE 1.
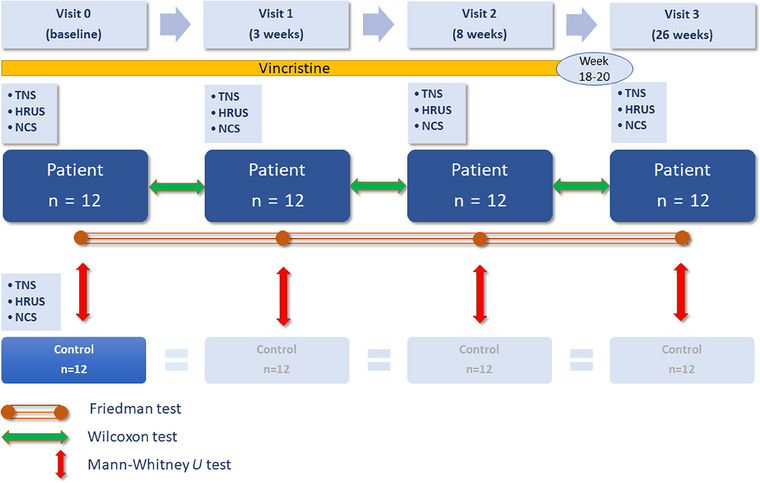
Study design. HRUS, high‐resolution nerve ultrasound; n, number; NCS, nerve conduction studies; TNS, total neuropathy score.
Clinical examination
Participants underwent a neurological examination. Sensory testing for touch and temperature was performed using a 10‐g filament (Twin‐Tip), and vibratory sensation at both ankles and wrists was assessed using a Rydel‐Seiffer 64 Hz tuning fork. The clinical part of the TNS was measured to determine the presence and severity of polyneuropathy. The TNS has been validated for CIPN. 10 It consists of six items scored with 0 (normal) to 4 (severely abnormal): sensory and motor symptoms, pinprick and vibration sensation, muscle strength, and deep tendon reflexes. The sum ranges from 0 (normal) to 24 (clinical signs of severe neuropathy).
Ultrasound examination
Ultrasound was performed with an Affiniti 50 (Philips) device using a 5‐18 MHz linear array transducer. Ultrasound pictures and measurements were agreed upon by M. Knaup and H. Mork. Measurements were supervised and validated by J. Philipps (board‐certified neurophysiologist with more than 10 years of experience in nerve ultrasound). For interrater reliability testing, J. Philipps, M. Knaup, M. Katz, and H. Mork performed 42 blinded CSA measurements of upper limb nerves in seven healthy volunteers before the start of the present study. These blind measurements were repeated in the same volunteers after 14 days in order to calculate the intrarater reliabilities of M. Knaup, M. Katz, and H. Mork. The transducer was held at a perpendicular angle to the nerve to obtain the correct CSA. Zoom was not used to avoid alterations in CSA measurement. For CSA measurements in 1/10 mm2, nerves were measured in a transverse plane within the inner border of the hyperechoic epineurium using the free‐hand tracer.
Selection of nerve sites
Each nerve was measured bilaterally at predefined sites. The median nerve was measured in the carpal tunnel, in the distal third of the forearm, and in the upper arm. The ulnar nerve was measured at the wrist proximally to Guyon's canal, in the distal third of the forearm, at the ulnar sulcus, and in the upper arm at an equal distance between the medial epicondyle and the axilla. The median and ulnar nerve were traced from the wrist to the proximal upper arm to measure the minimum and the maximum CSA. The radial nerve was measured in the upper arm at the spiral groove. The intranerve CSA variability (INV), defined as the ratio of the largest to the smallest CSA of one nerve, was calculated for the median and ulnar nerve. 11 The tibial nerve was localized in the popliteal fossa and at the ankle. The peroneal nerve was measured in the popliteal fossa and at the fibular head. The sural nerve was measured between the gastrocnemius heads.
Nerve conduction studies
NCSs of the median, ulnar, radial, tibial, peroneal, and sural nerves were performed bilaterally using a Natus Synergy EDX device with NicView Synergy software (v. 20.1, Natus Medical Ltd, Planegg, Germany, https://www.natus.com/de/neuro/nicolet‐edx/). Temperature of the extremities was adjusted to a skin temperature goal of 32‐34 centigrade using warm water if necessary. NCS parameters included the distal motor latency, compound muscle action potential (CMAP), nerve conduction velocity (NCV), and F‐wave latencies for the median, ulnar, tibial, and peroneal nerve. For the median, ulnar, radial, and sural nerves, sensory nerve action potential (SNAP) and NCV were measured. In the case of an SNAP amplitude of 0 µV, the sensory NCV was considered incalculable and excluded from the analysis.
Statistical analyses
Results of bilateral testing of HRUS and NCSs were considered as 24 separate data pairs in 12 patients and 12 controls. Considering four comparisons to the same control group and multiple post hoc tests between different visits, Bonferroni correction was applied and p < .01 was considered statistically significant for differences between groups and visits. For regression analyses of all data collected at the four visits, p < .05 was accepted as significant. Normal distribution of the data was tested using the Shapiro‐Wilk test.
Intraclass correlation coefficients for repeated single measurements with fixed observers and absolute agreement were calculated to assess interrater and intrarater reliabilities of HRUS.
The Mann‐Whitney U test was used to compare the results of clinical, NCS, and HRUS examinations at different visits of patients with the results of one single examination of controls (Figure 1).
The Friedman test and post hoc paired samples Wilcoxon tests were used to compare the results of the TNS, NCS, and HRUS examinations between different visits in the patient group (Figure 1). Wilcoxon tests were used to compare the total CSA of the entrapment nerve sites (defined as the sum of all measured CSA values of the median nerve at the carpal tunnel, the ulnar nerve at Guyon's canal and at the ulnar sulcus, the peroneal nerve at the fibular head, and the tibial nerve at the ankle) at different visits. Wilcoxon tests were used to compare the total CSA of the nonentrapment nerve sites at different visits.
Correlation coefficients were calculated for variables independently of the time of visit in the patient group. Correlations were tested for TNS with CSA, INV and NCS results.
Statistical analysis was performed using IBM SPSS Statistics for Windows (v. 28.0, IBM, Armonk, NY, USA, https://www.ibm.com).
RESULTS
Demographic characteristics of participants
Twelve patients and 12 healthy controls were included. Although a formal screening log was not kept, difficulties in recruitment were observable: Around 1 out of 5 eligible and informed patients accepted inclusion. Demographic characteristics of patients including cumulative vincristine dose are shown in Table 1. There were no significant differences regarding weight, height, and age between lymphoma patients at baseline (visit 0) and healthy controls. The sex distribution between groups was not significantly different (Fisher's exact test, p = .21).
Complete data were obtained in nerve ultrasound (1248 HRUS measurements in 26 bilateral nerve sites in patients at four visits, 312 HRUS measurements in controls). HRUS, TNS, and NCS data were not normally distributed.
Interrater reliability
The intraclass coefficient (ICC) compared to J. Philipps was .77 (.60‐.87) for M. Knaup, .68 (.47‐.81) for M. Katz, and .78 (.62‐.88) for H. Mork.
Intrarater reliability
The ICC of repeated HRUS examinations after 14 days was .72 (.54‐.84) for M. Knaup, .73 (.55‐.85) for M. Katz, and .89 (.81‐.94) for H. Mork.
Clinical examination
All patients reported neuropathic symptoms or exhibited signs of polyneuropathy relevant to the TNS during the observation period of 6 months. After 6 months, 25% reported sensory symptoms including painful sensations, 67% had a reduced vibration sensation, 16% had a reduced pin sensitivity, 16% reported autonomic symptoms, and 67% had reduced tendon reflexes. The evolution of clinical symptoms over time is represented in Figure 2.
FIGURE 2.
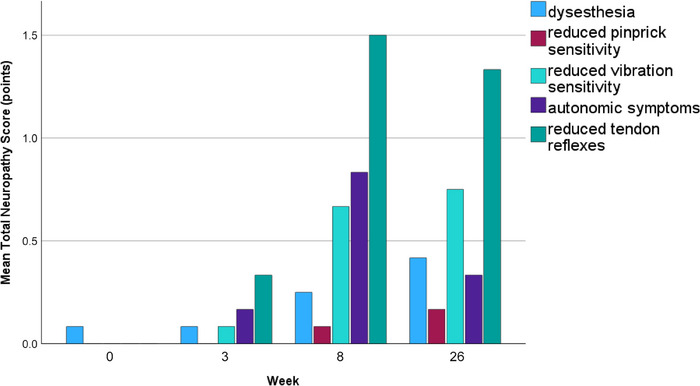
Evolution of clinical symptoms after vincristine treatment in lymphoma patients (N = 12).
Comparison between patients and healthy controls at different visits
TNS, NCS, and HRUS (except CSA of the fibular nerve at the fibular head and sural nerve sensory NCV) showed no difference between groups at baseline.
Comparing patients to controls, there was a significantly higher TNS at Week 3 (p = .006), Week 8 (p < .001), and 6 months (p < .001) in the patient group.
The sural nerve NCV was significantly reduced after Week 3. However, it was excluded from the analysis due to a difference at baseline. At 6 months, there was a significant reduction in motor NCV in the median (p = .025), ulnar (p = .002), tibial (p < .001), and peroneal (p < .001) nerves, as well as a reduction in sensory NCV in the ulnar (p = .013) and radial (p < .006) nerves. CMAP was reduced in the peroneal (p < .030) nerve. SNAP was reduced in the ulnar (p = .010), radial (p = .003), and sural (p < .001) nerves. Prolonged distal latencies were found in the median (p = .016), tibial (p < .001), and peroneal (p < .003) nerves. F‐waves were prolonged in the median (p = .001), ulnar (p = .001), tibial (p < .002), and peroneal (p < .015) nerves.
HRUS showed no Bonferroni‐corrected CSA difference between patients and controls at 3 weeks, 8 weeks, and 6 months. The INV of the median and ulnar nerve was not different between groups at all visits.
Comparison between different visits in the patient group
Median TNS increased from 0 points (interquartile range [IQR] 0) at baseline and 0.5 points (IQR 1, p = .26) at week 3 to 4 points (IQR 2.5, p < .001) at Week 8. At 6‐month follow‐up, there was a nonsignificant decrease to 2 points (IQR 2, p = .66; Figures 2 and 3).
FIGURE 3.
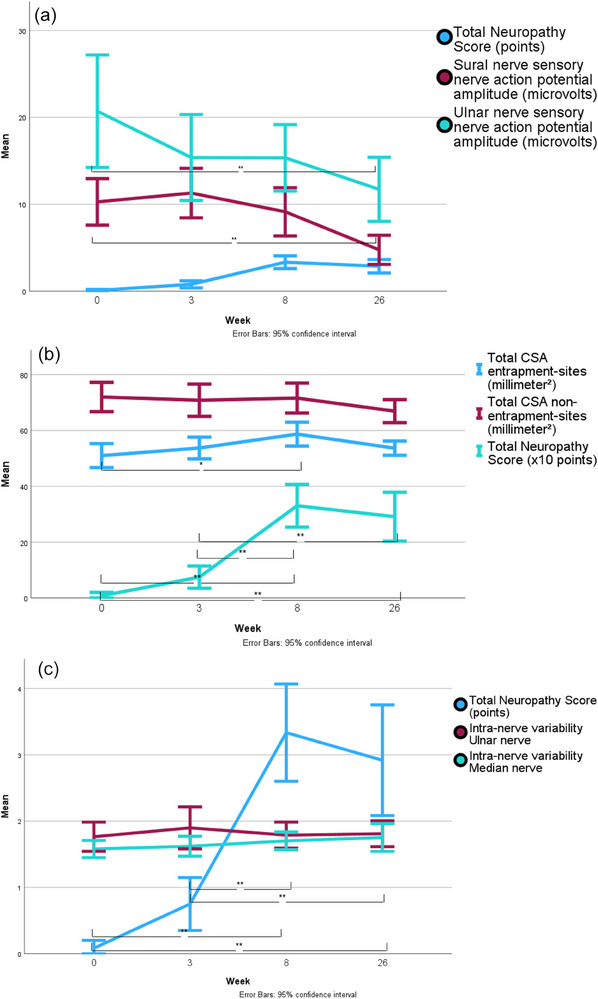
Evolution of the sural and ulnar nerve sensory action potential and the total neuropathy score (TNS) at different visits (A). Evolution of the total cross‐sectional area (CSA) calculated for entrapment sites and nonentrapment sites and the TNS at different visits (B). Evolution of the intranerve variability of the ulnar and median nerve and the TNS at different visits (C). Significant differences for the TNS at different visits are indicated only in panels (B) and (C). *p < .01; **p < .001. N = 12.
Sural nerve SNAP decreased significantly after 6 months (p = .001; Table 2; Figure 3A). Radial (p = .004), ulnar (p < .001), and median nerve (p < .001) SNAPs were significantly reduced after 6 months compared to baseline. The tibial nerve CMAP (p < .006) and motor NCV (p < .001) were reduced after 6 months. The peroneal nerve motor NCV was reduced (p < .001) after 6 months. F‐waves were prolonged in the tibial nerve (p = .003); distal latencies were not significantly different between visits.
TABLE 2.
Results of high‐resolution nerve ultrasound, nerve conduction studies, and total neuropathy score in lymphoma patients treated with vincristine at different visits.
| Baseline | 3 weeks | 8 weeks | 6 months | |
|---|---|---|---|---|
| Median nerve CSA, carpal tunnel [mm2] (IQR) | 10.6 (3.8) | 10.9 (3.8) | 11.5 (3.7) | 10.9 (2.6) |
| Median nerve CSA, forearm [mm2] (IQR) | 7.7 (2.1) | 7.5 (2.5) | 7.7 (2.6) | 7.4 (3.6) |
| Median nerve CSA, upper arm [mm2] (IQR) | 9.6 (4.9) | 9.7 (3.1) | 10.9 (6.4) | 10.1 (3.4) |
| Ulnar nerve CSA, Guyon's canal [mm2] (IQR) | 6.1 (1.8) | 5.9 (2.3) | 6.4 (1.4) | 5.9 (1.5) |
| Ulnar nerve CSA, forearm [mm2] (IQR) | 7.2 (2.5) | 6.4 (2.7) | 6.8 (2.2) | 5.8 (1.4) |
| Ulnar nerve CSA, ulnar sulcus [mm2] (IQR) | 7.6 (3.4) | 8.3 (3.2) | 9.0 (3.5) | 8.1 (3.7) |
| Ulnar nerve CSA, upper arm [mm2] (IQR) | 8.0 (2.3) | 7.7 (3.5) | 8.4 (2.8) | 7.5 (1.8) |
| Radial nerve CSA, upper arm [mm2] (IQR) | 5.7 (2.7) | 5.2 (3.2) | 4.9 (2.0) | 5.0 (2.4) |
| Peroneal nerve popliteal fossa [mm2] (IQR) | 11.5 (6.3) | 13.0 (7.9) | 13.6 (3.4) | 11.4 (2.1) |
| Peroneal nerve fibular head [mm2] (IQR) | 12.3 (4.0) | 13 (5.4) | 14.4 (7.3) | 13.5 (5.7) |
| Tibial nerve CSA, popliteal fossa [mm2] (IQR) | 18.8 (5.6) | 20.0 (10.6) | 17.3 (6.8) | 19.0 (8.5) |
| Tibial nerve CSA, ankle [mm2] (IQR) | 11.8 (7.5) | 13.2 (10.2) | 14.3 (7) | 13.2 (5) |
| Sural nerve CSA [mm2] (IQR) | 3.2 (2) | 3.1 (2.1) | 3.2 (2.5) | 2.7 (1.5) |
| Sural nerve SNAP [µv] (IQR) | 8.9 (3) | 11.1 (10) | 6.9 (10) | 4.6 (5.3), p = .001 |
| Median nerve SNAP [µv] (IQR) | 16.9 (7.3) | 15.1 (10) | 13.9 (5.3) | 12.6 (6.9), p < .001 |
| Ulnar nerve SNAP [µv] (IQR) | 18.1 (11.9) | 12.7 (17.2) | 13.1 (16.7) | 10.6 (12.8), p < .001 |
| Radial nerve SNAP [µv] (IQR) | 5.1 (3.7) | 3.7 (3.2) | 4.7 (3.1) | 4.1 (3.0), p = .004 |
| Tibial nerve CMAP [mV] (IQR) | 12.1 (8.2) | 12.4 (6.6) | 12.9 (5.1) | 10.8 (9.7), p = .006 |
| TNS [points] (IQR) | 0 (0) | 0.5 (1) | 4 (2.5), p < .001 | 2 (2.5), p < .001 |
Note: N = 12. Median and the interquartile range (IQR) are indicated. The p‐values for Wilcoxon tests compared to baseline are indicated. Only significant p‐values are indicated.
Abbreviations: CMAP, compound muscle action potential; CSA, cross‐sectional area; SNAP, sensory nerve action potential; TNS, total neuropathy score.
Nerve CSA showed no significant difference between visits in the Friedman test across all individual nerve sites. There was a nonsignificant CSA maximum in most nerve sites and at all entrapment sites at Week 8 (Table 2). The total CSA of entrapment nerve sites increased parallel to an increase of clinical symptoms reaching a significant Wilcoxon test compared to baseline (p = .007) at Week 8, whereas the total CSA of nonentrapment nerve sites did not change significantly (Figure 3B). Ulnar and median nerve INV were not different between visits (Figure 3C). TNS, a selection of NCV, and all HRUS results at different visits are displayed in Table 2. Ultrasound images of different nerve sites representing two points in time (follow‐up) are displayed in Figure 4.
FIGURE 4.
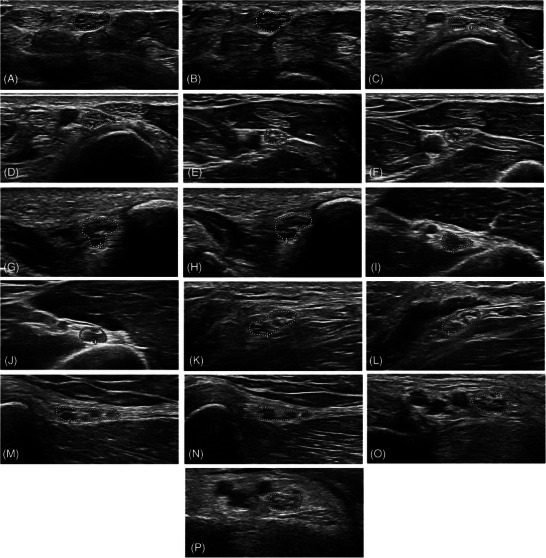
Ultrasound images of different nerve sites (region of interest) in patients with lymphoma receiving chemotherapy with vincristine representing two points in time (follow‐up) of the same nerve site. (a) Median nerve at the carpal tunnel, baseline. (b) Median nerve at the carpal tunnel, 8 weeks. (c) Ulnar nerve at Guyon's canal, baseline. (d) Ulnar nerve at Guyon's canal, 6 months. (e) Ulnar nerve in the forearm, baseline. (f) Ulnar nerve in the forearm, 6 months. (g) Ulnar nerve in the ulnar sulcus, baseline. (h) Ulnar nerve in the ulnar sulcus, 6 months. (i) Radial nerve in the upper arm, baseline. (j) Radial nerve in the upper arm, 6 months. (k) Peroneal nerve in the popliteal fossa, baseline. (l) Peroneal nerve in the popliteal fossa, 6 months. (m) Peroneal nerve at the fibular head, baseline. (n) Peroneal nerve at the fibular head, 8 weeks. (o) Tibial nerve in the tarsal tunnel, baseline. (p) Tibial nerve in the tarsal tunnel, baseline 8 weeks.
Correlations
We found no correlation between nerve CSA and the TNS. The INV of the ulnar and median nerve was not correlated with the TNS. The sural nerve SNAP (r = .32, beta = −.94, p = .008) was correlated with the TNS. The tibial F‐wave latency was correlated with the TNS (r = .25, beta = .77, p = .042).
DISCUSSION
The present prospective longitudinal pilot study investigates the capability of HRUS to detect VIPN during and shortly after treatment with vincristine in lymphoma patients. Patients developed clinical symptoms of VIPN detectable as an increase in the clinical part of the TNS beginning at Week 3 with a further significant increase at Week 8. This is in line with the literature showing that primary clinical symptoms of neurotoxicity occur early in VIPN around 7 weeks after the start of treatment. 9 Afterward, a nonsignificant decrease of the TNS was observed at 6 months, approximately 2 months after the end of vincristine chemotherapy. This is in line with the observation of Li et al, 9 who reported a chronic impairment in patients with VIPN with a tendency toward a beginning recovery at the end of the observation period; thus, a coasting phenomenon was not observed. Although the present study was focused on the early phase of VIPN, it is unlikely that a significant clinical deterioration of VIPN might have occurred after the observation period following the observed improvement after 6 months. Coasting defined as progressive worsening of clinical neuropathic symptoms after the end of chemotherapy has been described in polyneuropathies associated with platinum‐derived chemotherapies 12 and in TIPN as well. 6 , 13 Its occurrence in VIPN is controversial even at higher dosages 14 , 15 ; the most recent study with vincristine treatment capped at 2 mg/m2 per dosage has not found coasting in VIPN. 9
Nerve ultrasound results suggest that up to 6 months after starting vincristine treatment, CSA and INV do not change significantly in any of the analyzed individual nerve sites. However, a nonsignificant maximum CSA was observed at Week 8, especially at the entrapment sites, in parallel with the maximum TNS. Calculating the total CSA of all entrapment sites, this CSA maximum was significant in contrast to the total CSA of all nonentrapment nerve sites. This is in line with HRUS findings in other forms of CIPN: Pitarokoili et al 7 and Briani et al 8 reported a CSA increase in the leg nerves after 3 months and a trend toward a CSA increase in entrapment sites in the upper extremity in OIPN. Lycan et al 5 reported an increase in median nerve CSA in the carpal tunnel and a decrease in sural nerve CSA compared to historical controls in TIPN. Katz et al 6 found an increase in nerve CSA in TIPN. All these studies were able to observe significant HRUS changes at individual nerve sites despite a limited number of patients (n ≤ 20), so a milder CSA increase at the entrapment sites in the present sample might not only be attributable to the low number of participants. A mid‐treatment mean clinical TNS score of 2.6/24 points in VIPN reported by Li et al 9 is comparable to a mean of 3.3 and median of 4/24 points at Week 8 in the present study and signifies a milder polyneuropathy than observed in TIPN 6 (TNS 10.6/32 points) and OIPN 7 (mean neuropathy symptom score 5.6/10 points). Another reason might be the early onset of recovery as indicated by the TNS reduction found in the present study and reported by Li et al 9 at follow‐up. Hypothetically, the presence of coasting observed in platinum derivatives and taxanes might facilitate the observability of morphological changes related to OIPN and TIPN, whereas the absence of coasting in VIPN might be associated with a milder CSA increase observable in HRUS at the entrapment sites during mid‐treatment. A CSA increase at the entrapment sites and an unchanged INV can be interpreted as sonographic signs of axonal neuropathy.
In the present study, the clinical examination revealing mostly distal‐symmetrical sensory symptoms and NCS exhibiting signs of a predominantly sensory axonal polyneuropathy are in line with older 16 and recent 9 data on VIPN.
Recruitment proved to be very difficult in the present study; only 1 in 5 eligible patients accepted to participate. Reasons for this low recruitment response were mostly acute malaise due to lymphoma and the fact of being overwhelmed by a life‐changing diagnosis, the urgent need for diagnostic procedures, port implantation, and urgent chemotherapy treatment. The study design necessitated an extensive clinical, NCS, and HRUS exam before treatment. This differs from treatment protocols in neoadjuvant breast cancer treatment with taxanes and pretreatment with epirubicin, giving the patient more time to participate in study‐related exams. For further HRUS studies of VIPN in lymphoma patients, a mixed prospective and posttreatment approach 9 in a multicenter setting might be more appropriate than the present prospective‐longitudinal protocol. The long‐term effects of VIPN in terms of regression of clinical symptoms, changes of NCSs and HRUS results cannot be investigated in a 6‐month follow‐up period, as vincristine treatment frequently lasts up to 5 months or even longer. Therefore, a longer follow‐up period of at least 1 year might be recommended for future studies.
A major limitation of the present study is the very low number of participants. The conclusions should only be regarded as hypotheses that need further evaluation in studies with larger patient samples. A further limitation is the lack of data on nerve echogenicity and HRUS of cervical roots and brachial plexus. The limitation of follow‐up to 6 months after baseline was a result of the focus on the early phase of VIPN but constitutes a limitation concerning the evaluation of VIPN in its further course. A strength is the combination of a longitudinal and a controlled design.
At mid‐treatment with vincristine, there is an increase in the total CSA at entrapment sites parallel to an increase in clinical symptoms. In individual nerve sites, HRUS does not detect significant signs of VIPN. NCSs exhibit signs of a predominantly sensory axonal polyneuropathy. The clinical examination remains the most sensitive tool in the early detection of VIPN in lymphoma patients.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank the participants of the present study for their patience and cooperation despite a life‐changing diagnosis.
Open access funding enabled and organized by Projekt DEAL.
Philipps J, Knaup M, Katz M, Axton K, Mork H, Treichel J, et al. Nerve cross‐sectional area in vincristine‐induced polyneuropathy: A nerve ultrasound pilot study. J Neuroimaging. 2025;35:e13255. 10.1111/jon.13255
A part of the material has been contained within a poster presentation at the national meeting “Wissenschaftliche Jahrestagung der Deutschen Gesellschaft für Klinische Neurophysiologie und Funktionelle Bildgebung” (DGKN) 2023 in Hamburg, Germany.
REFERENCES
- 1. Burgess J, Ferdousi M, Gosal D, et al. Chemotherapy‐induced peripheral neuropathy: epidemiology, pathomechanisms and treatment. Oncol Ther. 2021;9:385‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madsen ML, Due H, Ejskjaer N, et al. Aspects of vincristine‐induced neuropathy in hematologic malignancies: a systematic review. Cancer Chemother Pharmacol. 2019;84:471‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desforges AD, Hebert CM, Spence AL, et al. Treatment and diagnosis of chemotherapy‐induced peripheral neuropathy: an update. Biomed Pharmacother. 2022;147:112671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaidman CM, Al‐Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve. 2009;40:960‐66. [DOI] [PubMed] [Google Scholar]
- 5. Lycan TW, Hsu FC, Ahn CS, et al. Neuromuscular ultrasound for taxane peripheral neuropathy in breast cancer. Muscle Nerve. 2020;61:587‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Katz M, Mork H, Baghdasaryan N, et al. High‐resolution nerve ultrasound and corneal confocal microscopy in taxane‐induced polyneuropathy. Eur J Neurol. 2024;31:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pitarokoili K, Hoffken N, Lonneker N, et al. Prospective study of the clinical, electrophysiologic, and sonographic characteristics of oxaliplatin‐induced neuropathy. J Neuroimaging. 2019;29:133‐139. [DOI] [PubMed] [Google Scholar]
- 8. Briani C, Campagnolo M, Lucchetta M, et al. Ultrasound assessment of oxaliplatin‐induced neuropathy and correlations with neurophysiologic findings. Eur J Neurol. 2013;20:188‐192. [DOI] [PubMed] [Google Scholar]
- 9. Li T, Trinh T, Bosco A, et al. Characterising vincristine‐induced peripheral neuropathy in adults: symptom development and long‐term persistent outcomes. Support Care Cancer. 2024;32:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavaletti G, Frigeni B, Lanzani F, et al. The total neuropathy score as an assessment tool for grading the course of chemotherapy‐induced peripheral neurotoxicity: comparison with the national cancer institute‐common toxicity scale. J Peripher Nerv Syst. 2007;12:210‐215. [DOI] [PubMed] [Google Scholar]
- 11. Padua L, Martinoli C, Pazzaglia C, et al. Intra‐ and internerve cross‐sectional area variability: new ultrasound measures. Muscle Nerve. 2012;45:730‐733. [DOI] [PubMed] [Google Scholar]
- 12. Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14(Suppl 4):iv45‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen Hammond E, Pitz M, Shay B. Neuropathic pain in taxane‐induced peripheral neuropathy: evidence for exercise in treatment. Neurorehabil Neural Repair. 2019;33:792‐799. [DOI] [PubMed] [Google Scholar]
- 14. Verstappen CC, Koeppen S, Heimans JJ, et al. Dose‐related vincristine‐induced peripheral neuropathy with unexpected off‐therapy worsening. Neurology. 2005;64:1076‐1077. [DOI] [PubMed] [Google Scholar]
- 15. Haim N, Epelbaum R, Ben‐Shahar M, et al. Full dose vincristine (without 2‐mg dose limit) in the treatment of lymphomas. Cancer. 1994;73:2515‐2519. [DOI] [PubMed] [Google Scholar]
- 16. Bradley WG, Lassman LP, Pearce GW, et al. The neuromyopathy of vincristine in man. Clinical, electrophysiological and pathological studies. J Neurol Sci. 1970;10:107‐131. [DOI] [PubMed] [Google Scholar]


