Abstract
Objective
In this study, we aimed to obtain real-life data on the use of antimyeloma agents, which significantly increase overall survival (OS) in multiple myeloma (MM) patients, in primary plasma cell leukemia (pPCL) patients with poor prognosis.
Materials and Methods
Data from 53 patients who were diagnosed with pPCL between 2011 and 2020 and who used at least one proteasome inhibitor (PI) and/or immunomodulatory (IMID) agent were analyzed retrospectively. Depending on the year of the pPCL diagnosis, 20% leukocytes or ≥2x109/L plasma cells in the peripheral blood was used as a diagnostic criterion.
Results
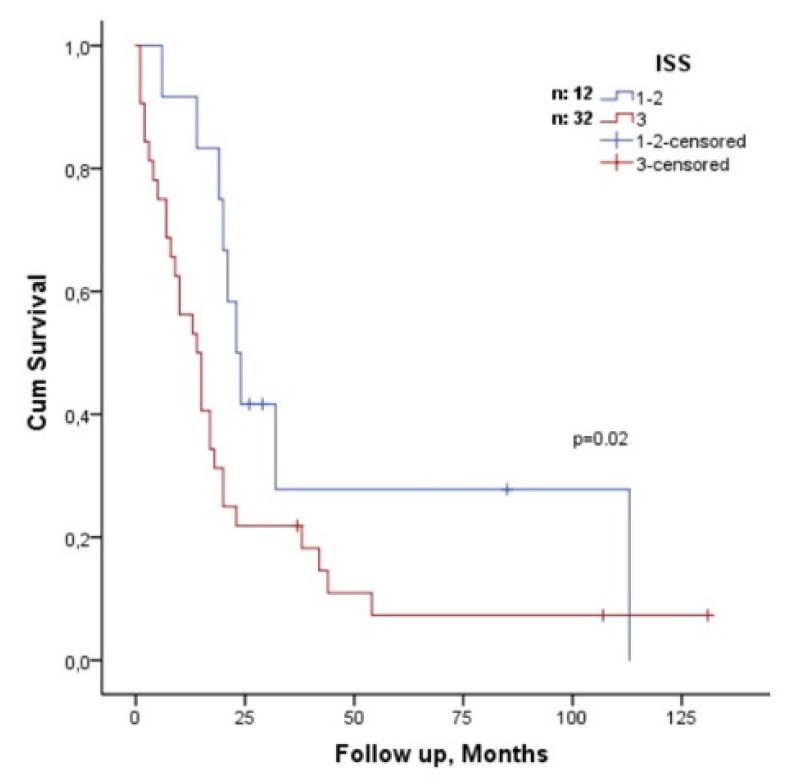
The median age of the patients was 58 years and 23 (43.4%) patients were over 65 years of age. For first-line treatment, PI or IMID alone was used by 31 (58.5%) patients, and PI and IMID were used simultaneously by 15 (28.3%) patients. Additionally, 21 (39.6%) patients received transplantation and 13 (24.5%) patients received maintenance treatment. The median progression-free survival was 4 (range: 1-42) months. When patients whose primary disease was refractory to first-line therapy were excluded, the duration of treatment was 6.5 months. The median OS was 15 months with a median follow-up duration of 15 months. Only 7 (13.2%) of the patients were alive at the last follow-up visit. Those with higher β2-microglobulin levels and International Staging System stage 3 and non-transplant patients receiving first-line treatment had shorter OS (p=0.005, p=0.02, and p=0.008, respectively). The concomitant use of PIs and IMIDs, the addition of chemotherapy to induction therapy, and the response to induction therapy or maintenance therapy did not affect OS.
Conclusion
In this study, as in previous similar studies, we could not see an increased survival trend in pPCL, which is observed in MM. New studies are needed for pPCL, which is likely to increase with new diagnostic criteria, based on current agents and information from MM.
Keywords: Primary plasma cell leukemia, Antimyeloma agents, Proteasome inhibitors, Immunomodulatory agents, Hematopoietic stem cell transplantation
Abstract
Amaç
Bu çalışma ile multipl miyelom (MM) hastalarında genel sağkalımda (OS) anlamlı bir artış sağlayan antimiyeloma ajanlarının, prognozu daha kötü olan primer plazma hücreli lösemi (pPHL) hastalarında kullanımına ilişkin gerçek hayat verilerini ortaya koymak istedik.
Gereç ve Yöntemler
2011-2020 yılları arasında pPHL tanısı alan ve en az bir proteazom inhibitörü (PI) ve/veya immünomodülatör (IMID) ajan kullanan 53 hastanın verileri retrospektif olarak analiz edildi. Hastaların tanı yıllarından kaynaklı olarak, periferik kanda plazma hücresinin lökositlerin %20’sinden fazla veya ≥2x109/L olması pPHL tanı kriteri kabul edildi.
Bulgular
Hastaların ortanca yaşı 58 olup, 23 (%43,4) hasta 65 yaş üzerindeydi. İlk sıra tedavide 31 (%58,5) hastada PI veya IMID tek başına kullanılırken, 15 (%28,3) hastada PI ve IMID eş zamanlı kullanıldı. Ayrıca 21 (%39,6) hastaya nakil, 13 (%24,5) hastaya ise idame tedavisi uygulandı. Hastaların ortanca progresyonsuz sağkalım süresi 4 (1-42) aydı. İlk sıra tedaviye primer refrakter hastalar dışlandığında ise 6,5 aydı. Ortanca takip süresi 15 ay olan hastaların, ortanca OS süresi de 15 aydı. Son kontrolde hastaların sadece 7’si (%13,2) hayattaydı. β2 mikroglobulin düzeyi yüksek, Uluslararası Evreleme Sistemi skoru 3 olan ve birinci basamak tedavide nakil yapılmayan hastalarda OS daha kısaydı (sırasıyla, p=0,005, p=0,02 ve p=0,008). Öte yandan indüksiyon tedavisinde PI ve IMID ajanlarının birlikte kullanılmasının, kemoterapi eklenmesinin, indüksiyon tedavisine yanıtın ve idame tedavisinin OS üzerine etkisi olmadığı görüldü.
Sonuç
Önceki benzer çalışmalarda olduğu gibi, çalışmamızda pPHL’de MM’de gözlenen artan sağkalım eğilimini göremedik. Yeni tanı kriteri ile birlikte artması olası pPHL hastaları için, MM’daki güncel ajanlar ve bilgiler dahilinde, yapılacak yeni çalışmalara ihtiyaç vardır.
Keywords: Primer plazma hücreli lösemi, Antimiyelom ajanlar, Proteazom inhibitörleri, İmmünomodülatör ajanlar, Hematopoietik kök hücre nakli
Introduction
The diagnostic criterion for plasma cell leukemia (PCL), which was first defined in 1974 based on the detection of more than 20% leukocytes or ≥2x109/L plasma cells with monoclonal gammopathy in the peripheral blood, was revised in 2018 to the detection of more than 5% leukocytes or ≥0.5x109/L plasma cells in peripheral blood [1,2,3]. Since the prognosis of high-risk multiple myeloma (MM) patients with circulating plasma cells in the peripheral blood is as poor as that of primary PCL (pPCL) patients, >2% has been suggested as the optimal prognostic threshold for flow cytometry [4,5]. PCL, which accounts for approximately 1%-2% of all plasma cell dyscrasias, occurs in two forms, primary (de novo) and secondary, the latter of which develops in patients who have previously been diagnosed with MM. Patients with pPCL, which accounts for approximately 60% of PCL cases, are younger than patients with secondary PCL (sPCL) [2,6]. Both types have worse prognosis than MM, but sPCL has worse prognosis than pPCL [7,8].
Over the last 20 years, the use of proteasome inhibitors (PIs), immunomodulatory agents (IMIDs), and targeted drugs in various combinations, as well as autologous hematopoietic stem cell transplantation (HSCT) and maintenance therapy, in conjunction with a clearer treatment algorithm, has resulted in a significant improvement in the overall survival (OS) of MM patients [9]. Although agents and treatments that are effective for MM have been administered to pPCL patients in recent years, no substantial improvement in terms of OS has been reported compared to MM patients. The 4-year OS rate of pPCL patients is still approximately 30% despite the use of HSCT [10,11]. According to analysis based on the Surveillance, Epidemiology, and End Results database by Gonsalves et al. [12], the median survival times of patients with pPCL were 5, 6, and 4 months in 1973-1995, 1996-2000, and 2001-2005, respectively. The median OS of patients diagnosed between 2006 and 2009, which coincided with the use of the first antimyeloma agent, was 12 months [12]. The use of bortezomib, thalidomide, lenalidomide, and HSCT has been reported to improve OS and progression-free survival (PFS) [13,14,15,16,17,18,19]. However, with the increasing use of antimyeloma agents in the following period, it is not clear whether a trend similar to that of MM occurred in PCL, whose treatment algorithm is not yet clear.
Therefore, with this multicenter retrospective study, we aimed to analyze current real-life data of pPCL patients using new PIs, IMIDs, and monoclonal antibodies, which are increasingly being used in MM.
Materials and Methods
The archival records and clinical and laboratory data of patients diagnosed with PCL at 19 centers in Türkiye between January 2011 and December 2020 were retrospectively analyzed. Patients with pPCL who met the 2003 International Myeloma Working Group (IMWG) diagnostic criteria for PCL (detection of more than 20% leukocytes or ≥2x109/L plasma cells in peripheral blood) instead of the new diagnostic criteria due to the years of diagnosis were included in the study [2,3,4,5]. The included patients were over 18 years of age and received at least one series of PI and/or IMID treatment. Additionally, 5 patients who received the vincristine, adriamycin, and dexamethasone (VAD) protocol, which did not include PI and/or IMID agents in induction, were included in the study because these agents were used in subsequent treatment processes. Patients with sPCL were excluded.
For each patient, baseline data were collected at the time of diagnosis and information on all-line therapies and patient responses was noted. Responses to treatments were evaluated according to the IMWG response criteria [20]. The primary outcome evaluated was OS, which was measured from the date of diagnosis to the time of last follow-up or death. The impacts of HSCT and maintenance therapy on OS and PFS were the secondary outcomes of interest. Death within the first 3 months due to disease or treatment side effects was defined as early death.
This study was approved by the Ethics Committee of the Akdeniz University Faculty of Medicine and was conducted in accordance with the principles outlined in the Declaration of Helsinki and all applicable regulations (date and approval number: 22/07/2020, KAEK-537). Informed written consent was not obtained because of the retrospective nature of the study.
Statistical Analysis
IBM SPSS Statistics 24 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. Descriptive statistics were used to present the data. Categorical data are presented as numbers and ratios while numerical data are presented as median, minimum, and maximum values. Significant differences between the data were analyzed using the Mann-Whitney U test for independent variables. OS was defined as the duration from the date of the first day of treatment to the date of death or time to the survivors’ last follow-up appointments. Kaplan-Meier survival analysis was applied for OS and log-rank tests were used to examine the factors affecting survival. Cox regression analysis was applied to evaluate factors affecting survival. Differences with values of p≤0.05 were considered statistically significant.
Results
Patients
Records were obtained for a total of 54 patients from 19 different centers in Türkiye. One patient was excluded from the study due to death without any treatment and the data of 53 patients who used at least one series of PIs and/or IMIDs were analyzed. The demographic and clinical characteristics of the patients are presented in Table 1.
Table 1. Demographic and clinical characteristics of the patients.
|
Characteristics |
Number |
|
|
Age, years, median (range) |
58 (24-84) |
|
|
>65 |
23 (43.4%) |
|
|
<65 |
30 (56.6%) |
|
|
Sex, n (%) |
Female |
20 (37.7%) |
|
Male |
33 (62.3%) |
|
|
Comorbidities, n (%) |
Hypertension |
14 (26.4%) |
|
Chronic kidney disease |
11 (20.8%) |
|
|
Coronary artery disease |
7 (13.2%) |
|
|
Chronic obstructive pulmonary disease |
6 (11.3%) |
|
|
Diabetes mellitus |
4 (7.5%) |
|
|
Heart failure |
2 (3.8%) |
|
|
Chronic liver disease |
2 (3.8%) |
|
|
Alzheimer’s disease |
2 (3.8%) |
|
|
Solid cancer |
2 (3.8%) |
|
|
M-protein type, n (%) |
IgG kappa |
16 (30.1%) |
|
IgG lambda |
9 (16.9%) |
|
|
IgA kappa |
4 (7.5%) |
|
|
IgA lambda |
3 (5.7%) |
|
|
Light chain (kappa/lambda) |
16 (9/7) (30.1%) |
|
|
Non-secreting |
3 (5.7%) |
|
|
Unspecified |
2 (3.8%) |
|
|
Laboratory results |
Hemoglobin, g/dL, median (range) |
8.68 (4.6-12.8) |
|
Creatinine, mg/dL, median (range) |
1.34 (0.4-11.6) |
|
|
Calcium, mg/dL, median (range) |
9.96 (7.4-17.6) |
|
|
β2-microglobulin, mg/L, median (range) |
8.4 (2.0-38.7) |
|
|
ISS staging |
ISS 1 |
5 (9.4%) |
|
ISS 2 |
7 (13.2%) |
|
|
ISS 3 |
32 (60.3%) |
|
|
Not available |
9 (16.9%) |
Ig: Immunoglobulin, ISS: International Staging System.
All patients received at least one line of treatment, with a maximum of 4 lines. During first-line therapy, 21 (39.6%) patients underwent HSCT and 13 (24.5%) patients received maintenance therapy. Thirty-two (60.4%) patients underwent second-line treatment and 19 (35.8%) patients underwent SCT after second-line treatment. Sixteen (30.2%) patients received third-line treatment and 5 (9.4%) patients received fourth-line treatment.
During the entire treatment period, 51 (96.2%) of 53 patients received bortezomib, 34 (64.2%) received lenalidomide, 16 (30.2%) received thalidomide, 9 (16.9%) received carfilzomib, 5 (9.4%) received pomalidomide, 5 (9.4%) received daratumumab, 2 (3.7%) received ixazomib, 2 (3.7%) received venetoclax, and 26 (49%) received chemotherapy. Chemotherapy entailed cisplatin, doxorubicin, cyclophosphamide, and etoposide (PACE) combined with antimyeloma agents in 25 cases and dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP) in 1 case.
First-line Treatment
First-line treatments comprised 9 different treatment protocols. Except for five patients who were given VAD treatment, bortezomib was used as the PI, and/or thalidomide and lenalidomide were used as the IMID. The distribution of patients and their responses to these treatments according to the regimens used in induction treatment and the distribution of agents used in maintenance treatment are given in Table 2. The mean duration from diagnosis to transplantation was 5.5 (range: 3-10) months. Nine out of the 13 patients who received maintenance treatment had to be discontinued because of side effects (1 patient) and disease progression (8 patients). Three patients were still alive and continued to receive maintenance treatment (one other patient was lost to follow-up). The median duration of maintenance therapy was 6.5 (range: 1-20) months. The median time to progression after first-line treatment was 4 (range: 1-42) months.
Table 2. Distribution of induction regimens, consolidative transplants, maintenance therapy, and treatment responses.
|
Number of patients (%) |
||
|
53 |
||
|
Induction treatment, n (%) |
Only PI-based |
29 (54.7) |
|
Only IMID-based |
2 (3.7) |
|
|
PI- and IMID-based |
8 (15.1) |
|
|
PI and/or IMIDs + PACE |
9 (16.9) |
|
|
*Only VAD |
5 (9.4) |
|
|
Response to induction therapy, n (%) |
CR/VGPR |
8 (15.1)/13 (24.5) |
|
PR/MR |
7 (13.2)/5 (9.4) |
|
|
SD/progression |
6 (11.3)/3 (5.7) |
|
|
Unspecified |
11 (20.8) |
|
|
HSCT, n (%) |
Yes (autologous/allogeneic) |
21 (20/1) (39.6) |
|
No |
32 (60.4) |
|
|
Maintenance, n (%) |
13 (24.5) |
|
|
Lenalidomide |
7 (13.2) |
|
|
Bortezomib |
3 (5.6) |
|
|
PI and IMID combination |
3 (5.6) |
|
|
Final status after first-line treatment, n (%) |
51 |
|
|
Refractory |
22 (41.5) |
|
|
Relapse |
20 (37.7) |
|
|
Remission |
9 (16.9) |
CR: Complete response; HSCT: hematopoietic stem cell transplantation; IMID: immunomodulatory agent; MR: minimal response; PACE: cisplatin, doxorubicin, cyclophosphamide, etoposide; PI: proteasome inhibitor; PR: partial response; SD: stable disease; VAD: vincristine, adriamycin, dexamethasone; VGPR: very good partial response.
Only PI-based: VD: Bortezomib, dexamethasone; VCD: bortezomib, cyclophosphamide, dexamethasone. Only IMID-based: MPT: Melphalan, prednisone, thalidomide.
PI- and IMID-based: VRD: Bortezomib, lenalidomide, dexamethasone; VTD: bortezomib, thalidomide, dexamethasone. PI and/or IMIDs + PACE: VCD or VTD + PACE.
*: PI (bortezomib) and/or IMIDs (lenalidomide and thalidomide) were used in the subsequent treatment processes of the patients.
Treatment of Relapsed/Refractory Patients
Thirty-two patients (60.3%) received second-line treatment with 9 different treatment protocols. After second-line treatment, the median time to progression after transplantation was 6 (range: 1-31) months. The 16 (30.2%) patients who were alive with or without transplantation after second-line treatment and who received third-line treatment due to progression received 11 different protocols. The median duration of third-line treatment was 4 (range: 1-24) months. Four different treatment protocols were applied for 5 (9.4%) patients who received fourth-line treatment. With respect to fourth-line treatment, one patient who achieved a partial response (PR) to pomalidomide and dexamethasone treatment survived for 55 months, whereas progression and death occurred within 2 months for the other 4 patients. Table 3 shows the treatment distribution and response statuses of the patients with relapsed/refractory disease.
Table 3. Distribution of second-, third- and fourth-line treatment regimens as well as transplants after second-line treatment and response to treatments.
|
Number of patients (%) |
||
|
Second-line treatment |
32 |
|
|
Only PI-based |
5 (15.6) |
|
|
Only IMID-based |
7 (21.8) |
|
|
PI- and IMID-based |
12 (37.5) |
|
|
PI and IMIDs + PACE |
6 (18.7) |
|
|
DRd |
1 (3.1) |
|
|
Venetoclax |
1 (3.1) |
|
|
Response to second-line treatment |
CR/VGPR |
6 (18.7)/8 (25) |
|
PR/MR |
5 (15.6)/1 (3.1) |
|
|
SD/progression |
1 (3.1)/2 (6.2) |
|
|
Unspecified |
9 (28.1) |
|
|
HSCT after second-line treatment |
19 (59.3) |
|
|
Autologous |
5 (15.6) |
|
|
Allogeneic |
11 (34.3) |
|
|
Autologous and allogeneic |
3 (9.3) |
|
|
Response after HSCT |
CR/VGPR |
5 (26.3)/6 (31.6) |
|
PR/MR |
0 (0)/0 (0) |
|
|
SD/progression |
1 (5.3)/3 (15.8) |
|
|
Unspecified |
4 (21.1) |
|
|
Third-line treatment |
16 |
|
|
Only PI-based |
2 (12.5) |
|
|
Only IMID-based |
7 (43.7) |
|
|
PI- and IMID-based |
3 (18.8) |
|
|
DVd |
2 (12.5) |
|
|
Venetoclax |
1 (6.3) |
|
|
DCEP |
1 (6.3) |
|
|
Response to third-line treatment |
CR/VGPR |
1 (6.3)/0 (0) |
|
PR/MR |
0 (0)/0 (0) |
|
|
SD/progression |
2 (12.5)/8 (50) |
|
|
Unspecified |
5 (31.2) |
|
|
Fourth-line treatment |
5 |
|
|
Only PI-based |
1 (20) |
|
|
Only IMID-based |
2 (40) |
|
|
PI- and IMID-based |
1 (20) |
|
|
DVd |
1 (20) |
|
|
Response to fourth-line treatment |
CR/VGPR |
0 (0)/0 (0) |
|
PR/MR |
1 (20)/0 (0) |
|
|
SD/progression |
1 (20)/3 (60) |
|
|
Unspecified |
0 (0) |
CR: Complete response; DCEP: dexamethasone, cyclophosphamide, etoposide, cisplatin; DRd: daratumumab, lenalidomide, dexamethasone; DVd: daratumumab, bortezomib, dexamethasone; HSCT: hematopoietic stem cell transplantation; IMID: immunomodulatory agent; MR: minimal response; PACE: cisplatin, doxorubicin, cyclophosphamide, etoposide; PI: proteasome inhibitor; PR: partial response; SD: stable disease; VGPR: very good partial response. Only PI-based: VD: Bortezomib, dexamethasone; VCD: bortezomib, cyclophosphamide, dexamethasone; KD: carfilzomib, dexamethasone; KCD: carfilzomib, cyclophosphamide, dexamethasone. Only IMID-based: RD: Lenalidomide, dexamethasone; PD: pomalidomide, dexamethasone; PCD: pomalidomide, cyclophosphamide, dexamethasone. PI- and IMID-based: VRD: Bortezomib, lenalidomide, dexamethasone; VTD: bortezomib, thalidomide, dexamethasone; IRD: ixazomib, lenalidomide, dexamethasone; KPD: carfilzomib, pomalidomide, dexamethasone; KRD: carfilzomib, lenalidomide, dexamethasone. PI and/or IMIDs + PACE: VCD or VTD + PACE.
Survival
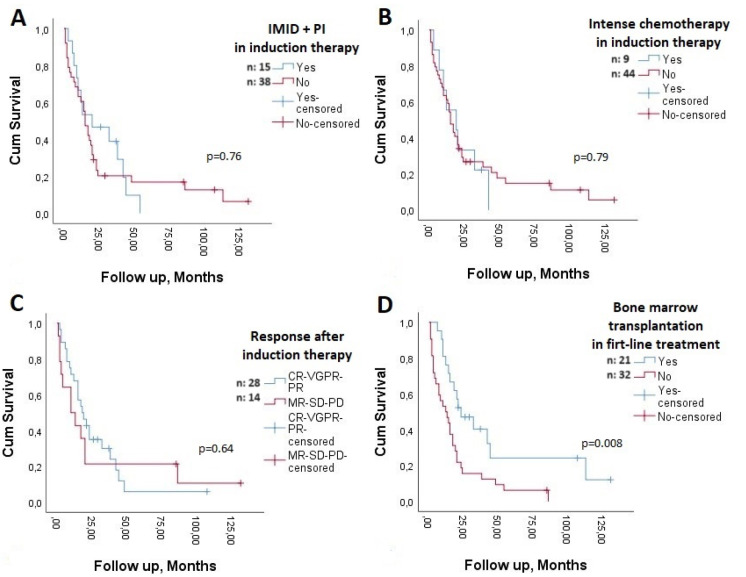
The median PFS was 4 (range: 1-42) months. When early deaths were excluded, the median PFS was 5 months, and when patients whose primary disease was refractory to first-line therapy were excluded, it was 6.5 months. PFS was similar in patients aged ≥65 years and younger patients (p=0.11), those with International Staging System (ISS) stage 3 and stage 1-2 disease (p=0.54), those who received and did not receive PI and IMID combinations as first-line therapy (p=0.45), and those who received and did not receive intensive chemotherapy with antimyeloma agents as first-line therapy (p=1.0). However, the median PFS of patients who were able to receive transplantation as first-line therapy was longer than that of patients who were not able to undergo transplantation (p<0.001) (Figure 1).
Figure 1.
Progression-free survival in patients with and without bone marrow transplantation in first-line therapy. n: Number of patients.
The median OS was 15 months (95% confidence interval [CI]: 10-19), with a median follow-up of 15 (range: 1-131) months. The OS rate was 13.2% after a median follow-up of 15 months. Only 7 (13.2%) of the patients were alive at the last follow-up visit and 46 (86.3%) patients (30 due to disease and 16 due to non-disease causes) had died. In the first 3 months, nine patients died, for an early mortality rate of 17%. The median OS of patients other than these patients was 19.5 (range: 1-131) months.
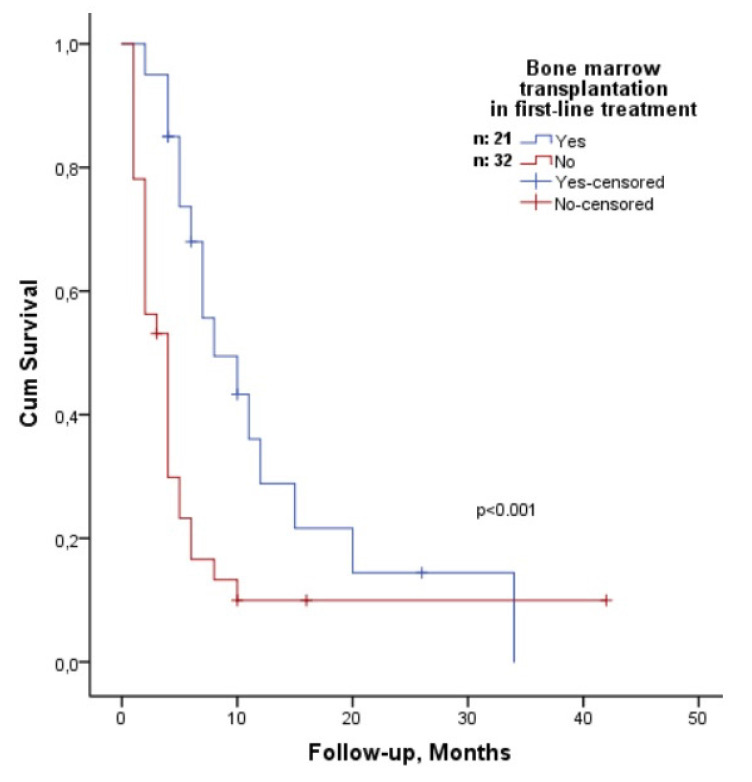
According to univariate analysis, the OS of patients older than 65 years was 12 months (95% CI: 2-21) and the OS of patients younger than 65 years was 19 months (95% CI: 14-23); however, the difference was not statistically significant (p=0.076). There was no statistically significant difference in OS between the sexes (p=0.054). Those with high β2-microglobulin levels had shorter OS (p=0.005) than those with low β2-microglobulin levels. In addition, patients with ISS stage 3 disease had shorter OS compared to patients with ISS stage 1-2 disease (p=0.02) (Figure 2). In terms of induction therapy, 15 patients (28.3%) who received IMID and PI drugs simultaneously and those who did not (p=0.76) had similar OS (Figure 3A), as did 9 patients (16.9%) who received intense chemotherapy with antimyeloma agents and those who did not (p=0.79) (Figure 3B). Although the OS of 28 (52.8%) patients who achieved at least PR (complete response, very good PR, and PR) after induction therapy was 18 months (95% CI: 16-36) and that of 14 patients who achieved <PR (minimal response, stable disease, and progression) was 10 months (95% CI: 7-53), the difference was not statistically significant (p=0.64) (Figure 3C). Although non-transplant patients had shorter OS than transplant recipients did in first-line treatment (p=0.008; hazards ratio: 2.3) (Figure 3D), maintenance therapy was not correlated with OS (p=0.24). Only β2-microglobulin was found to be correlated with OS (p=0.04; hazards ratio: 1.05) in multivariate analysis that included β2-microglobulin and transplantation during first-line treatment.
Figure 2.
Comparison of overall survival according to International Staging System (ISS) stages 1-2 and 3. n: Number of patients.
Figure 3.
(A) Proteasome inhibitor (PI) and/or immunomodulatory (IMID) agents used together and not used in induction therapy; (B) in induction treatment, with or without intensive chemotherapy with an antimyeloma agent; (C) after induction therapy, patients with or without at least partial response; (D) patients with or without consolidative bone marrow transplantation in first-line therapy. n: Number of patients.
Discussion
A mean OS of 15 months was revealed by our retrospective analysis of 53 pPCL patients who were diagnosed between 2011 and 2020 at 19 different sites. We identified different potential therapeutic regimens comprising various drug combinations of both induction therapy and second-line treatment. Additionally, new drugs that are more potent and have different mechanisms, which contribute positively to OS in patients with MM, were administered to a small number of patients.
Few studies in the international literature have attempted to determine the OS of pPCL patients during the period when antimyeloma drugs were more widely employed. To our knowledge, the numbers of patients included in studies conducted in the period when new first-line agents were being used were lower than the number of patients included in our study, except for two studies. In those studies involving more than 10 (11-117) pPCL patients, the mean OS was reported to be between 14 and 33 months [21,22,23,24,25,26,27,28]. In the study conducted by Mina et al. [28], who reported the longest OS of 33 months, the combined use of PI and IMID during induction (92%), HSCT (74%), and maintenance therapy (60%) rates were higher than those reported in other studies. Ganzel et al. [27], who reported that the OS time of 39 patients was the same as that in our study, reported early death in 18% of patients while the OS of patients who did not die in the first 3 months was 22.5 months. In addition to age and/or morbidity, early death is another reason for consolidative transplantation not being performed, likely affecting OS.
Previous studies have shown that pPCL patients who use new agents, particularly bortezomib and lenalidomide, which are antimyeloma agents that are increasingly used and are the most discussed antimyeloma agents in this field, experience longer OS than those who do not [13,18,19,22,23,27,29,30,31]. Although all patients in our analysis used a PI (96.2% bortezomib) and/or IMID (64.2% lenalidomide) at least once, the percentage of patients who used PIs and IMIDs together (triplet) for induction therapy (28.3%) was low. This may be the reason why we were unable to demonstrate its advantage in terms of OS. In a recent phase 2 study (EMN12/HOVON129), PFS and OS were found to be longer in both young and older PCL patients after induction, consolidation, and maintenance treatment with carfilzomib, a more potent PI, and lenalidomide than in previous studies. However, as with other studies, the results were not satisfactory with respect to improvements in MM. In addition, despite the greater responses obtained after induction with carfilzomib, lenalidomide, and dexamethasone (KRd), no significant relationship could be shown between the depth of response and OS, as in our study [32].
Mina et al. [28] reported that adding intensive chemotherapy to new agents during induction treatment did not improve PFS or OS, as in our study. On the other hand, Peña et al. [22] reported that adding intensive chemotherapy provided an OS advantage [22]. Information on whether the use of intensive chemotherapy, which was also used in the years when antimyeloma agents were not used and the OS was less than 12 months, in combination with antimyeloma agents, which are more potent today, will improve OS is contradictory, and more studies are needed in this direction. However, the addition of intensive chemotherapy to new agents is still recommended, especially for young patients [33].
Many studies have revealed that patients who undergo consolidative upfront HSCT experience longer OS than those who do not [21,27,34]. However, a small number of studies failed to demonstrate that advantage. Although Peña et al. [22] reported an advantage of HSCT in univariate analysis, that advantage was lost in multivariate analysis, as in our study. Additionally, Mina et al. [28] demonstrated the PFS advantage of upfront autologous HSCT but failed to demonstrate the OS advantage. According to the records of the European Society for Blood and Marrow Transplantation, there was an increase in transplantation rates for pPCL patients from 1998 to 2014 and the median OS of all patients was 33 months, regardless of the type of transplantation. In addition, complete remission before transplantation has been shown to provide a major OS benefit [35]. Providing a deep response with more potent antimyeloma agents, such as KRd as used in the EMN12/HOVON 129 study, and determining the appropriate transplantation strategy are considered the most appropriate approaches [32,35].
In the study conducted by Mina et al. [28], although maintenance therapy improved PFS, it did not significantly improve OS. In contrast, in our study, in which a single agent was largely utilized in maintenance therapy (77% of patients) and no OS advantage could be demonstrated, we suggest that the use of dual agents (PI and IMID) in maintenance therapy, as in the EMN12/HOVON 129 study, was advantageous for a majority of patients (87%) [28,32].
Almost all studies similar to our study were conducted with pPCL patients who met the diagnostic criteria determined in 2003 [2]. Among the studies that compared the OS durations of patients with ≥20% peripheral blood plasma cells with those between 5% and 19% and found them to be similar, a Spanish study reported OS of 6 months and 14 months, respectively, while a Mayo Clinic study reported 13 months in both groups [3,36]. Subsequently, in 2021, the IMWG lowered the threshold from 20% to 5% to better reflect the high-risk nature of PCL patients [37]. After the new definition, researchers have wondered whether similar results are being obtained. In this regard, some studies re-evaluated patients diagnosed in the past, and the survival times of patients with ≥20% plasma cells in peripheral blood and those with 5%-19% plasma cells were found to be similar [30,31,34]. However, the latest recommendation in this direction is that it should be above 2% [4,5].
In addition to being a retrospective study, the most important limitation of our study was that we could not access demographic data and also failed to obtain cytogenetic features such as t(11;14), which is common in pPCL; therefore, we could not perform risk classification with the revised MM ISS or evaluate the patients in this respect because patients from different years and centers were included. Additionally, patients diagnosed with pPCL with the diagnostic criteria of 2003 were included in the study depending on the year of diagnosis. Therefore, since patients with lower peripheral blood plasma cell ratios were not included in our study and data on the number and ratio of plasma cells in peripheral blood were not available, analysis in this direction could not be performed. Finally, treatment protocols were quite different due to center experience and differences in access to agents due to the year of diagnosis, age, and performance. This prevented us from performing adequate analysis in terms of response status and survival according to treatments. Therefore, patients had to be grouped and analyzed according to their use of PIs and IMIDs.
Conclusion
In most retrospective studies, as in our study, there are non-standardized treatment approaches applied for different reasons that were developed based on MM treatment. Although these studies have different results and direct comparisons cannot be made, new antimyeloma agents and HSCT seem to provide partially positive results for the survival of pPCL patients. However, there is insufficient information on the necessity of using intensive chemotherapy in addition to new agents and which agent(s) should be used for maintenance therapy. The new definition is likely to increase the number of pPCL patients. Current studies and good registries, with the support of historical information in the literature and current MM approaches, may be helpful in identifying the optimal treatment approaches for pPCL.
Ethics
Ethics Committee Approval: This study was approved by the Ethics Committee of the Akdeniz University Faculty of Medicine and was conducted in accordance with the principles outlined in the Declaration of Helsinki and all applicable regulations (date and approval number: 22/07/2020, KAEK-537).
Informed Consent: Informed written consent was not obtained because of the retrospective nature of the study.
Footnotes
Authorship Contributions
Concept: O.S., U.I., Ü.A., O.K.Y., L.Ü.; Design: Ü.A., U.I., O.S., O.K.Y., L.Ü.; Data Collection or Processing: All authors; Analysis or Interpretation: T.U., Ü.A., U.I., O.S., L.Ü.; Literature Search: U.A., U.I., O.S., O.K.Y.; Writing: Ü.A., U.I., T.U., O.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974;133(5):813–818. doi: 10.1001/archinte.133.5.813. [DOI] [PubMed] [Google Scholar]
- 2.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 3.Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, Lacy MQ, Go RS, Dispenzieri A, Kapoor P, Lust JA, Dingli D, Lin Y, Russell SJ, Leung N, Gertz MA, Kyle RA, Bergsagel PL, Rajkumar SV. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018;8(12):116. doi: 10.1038/s41408-018-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Donk NWCJ. How we manage newly diagnosed multiple myeloma with circulating tumor cells. J Clin Oncol. 2023;41(7):1342–1349. doi: 10.1200/JCO.22.02114. [DOI] [PubMed] [Google Scholar]
- 5.Jelinek T, Bezdekova R, Zihala D, Sevcikova T, Anilkumar Sithara A, Pospisilova L, Sevcikova S, Polackova P, Stork M, Knechtova Z, Venglar O, Kapustova V, Popkova T, Muronova L, Chyra Z, Hrdinka M, Simicek M, Garcés JJ, Puig N, Cedena MT, Jurczyszyn A, Castillo JJ, Penka M, Radocha J, Mateos MV, San-Miguel JF, Paiva B, Pour L, Rihova L, Hajek R. More than 2% of circulating tumor plasma cells defines plasma cell leukemia-like multiple myeloma. J Clin Oncol. 2023;41(7):1383–1392. doi: 10.1200/JCO.22.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bladé J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13(6):1259–1272. doi: 10.1016/s0889-8588(05)70125-8. [DOI] [PubMed] [Google Scholar]
- 7.Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, Van Wier SA, Chng WJ, Ketterling RP, Gertz MA, Henderson K, Greipp PR, Dispenzieri A, Lacy MQ, Rajkumar SV, Bergsagel PL, Stewart AK, Fonseca R. Genetic aberrations and survival in plasma cell leukemia. Leukemia. 2008;22(5):1044–1052. doi: 10.1038/leu.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey H, Goel H, Verma S, Gupta S, Tanwar K, Rahul E, Kapoor G, Vasantharaman J, Ranjan A, Tanwar P, Chopra A. Clinicopathological and laboratory parameters of plasma cell leukemia among Indian population. Am J Blood Res. 2022;12(6):190–195. [PMC free article] [PubMed] [Google Scholar]
- 9.Goel U, Usmani S, Kumar S. Current approaches to management of newly diagnosed multiple myeloma. Am J Hematol. 2022;97(Suppl 1):3–25. doi: 10.1002/ajh.26512. [DOI] [PubMed] [Google Scholar]
- 10.Dhakal B, Patel S, Girnius S, Bachegowda L, Fraser R, Davila O, Kanate AS, Assal A, Hanbali A, Bashey A, Pawarode A, Freytes CO, Lee C, Vesole D, Cornell RF, Hildebrandt GC, Murthy HS, Lazarus HM, Cerny J, Yared JA, Schriber J, Berdeja J, Stockerl-Goldstein K, Meehan K, Holmberg L, Solh M, Diaz MA, Kharfan-Dabaja MA, Farhadfar N, Bashir Q, Munker R, Olsson RF, Gale RP, Bayer RL, Seo S, Chhabra S, Hashmi S, Badawy SM, Nishihori T, Gonsalves W, Nieto Y, Efebera Y, Kumar S, Shah N, Qazilbash M, Hari P, D'Souza A. Hematopoietic cell transplantation utilization and outcomes for primary plasma cell leukemia in the current era. Leukemia. 2020;34(12):3338–3347. doi: 10.1038/s41375-020-0830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge X, Meng W, Wang W, Ma H, Zhao S, Cui K. Causes of death in primary plasma cell leukemia differ from multiple myeloma: a STROBE-compliant descriptive study based on SEER database. Medicine (Baltimore) 2022;101(29):e29578. doi: 10.1097/md.0000000000029578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, Singh PP, Buadi FK, Lacy MQ, Kapoor P, Dingli D, Lust JA, Zeldenrust SR, Hayman SR, Kyle RA, Gertz MA, Kumar SK. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124(6):907–912. doi: 10.1182/blood-2014-03-565051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Arena G, Valentini CG, Pietrantuono G, Guariglia R, Martorelli MC, Mansueto G, Villani O, Onofrillo D, Falcone A, Specchia G, Semenzato G, Di Renzo N, Mastrullo L, Venditti A, Ferrara F, Palumbo A, Pagano L, Musto P. Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party. Ann Oncol. 2012;23(6):1499–1502. doi: 10.1093/annonc/mdr480. [DOI] [PubMed] [Google Scholar]
- 14.Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C, Arnulf B, Macro M, Cailleres S, Brion A, Brechignac S, Belhadj K, Chretien ML, Wetterwald M, Chaleteix C, Tiab M, Leleu X, Frenzel L, Garderet L, Choquet S, Fuzibet JG, Dauriac C, Forneker LM, Benboubker L, Facon T, Moreau P, Avet-Loiseau H, Marolleau JP. Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase II study of the Intergroupe Francophone du Myelome. J Clin Oncol. 2016;34:2125–2132. doi: 10.1200/JCO.2015.63.1929. [DOI] [PubMed] [Google Scholar]
- 15.Iriuchishima H, Ozaki S, Konishi J, Matsumoto M, Murayama K, Nakamura F, Yamamoto G, Handa H, Saitoh T, Nagura E, Shimizu K, Nojima Y, Murakami H. Primary plasma cell leukemia in the era of novel agents: a multicenter study of the Japanese Society of Myeloma. Acta Haematol. 2016;135(2):113–121. doi: 10.1159/000439424. [DOI] [PubMed] [Google Scholar]
- 16.Pagano L, Valentini CG, De Stefano V, Venditti A, Visani G, Petrucci MT, Candoni A, Specchia G, Visco C, Pogliani EM, Ferrara F, Galieni P, Gozzetti A, Fianchi L, De Muro M, Leone G, Musto P, Pulsoni A, for GIMEMA-ALWP (Gruppo Italiano Malattie EMatologiche dell'Adulto, Acute Leukemia Working Party: coordinator Sergio Amadori) Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 2011;22(7):1628–1635. doi: 10.1093/annonc/mdq646. [DOI] [PubMed] [Google Scholar]
- 17.Mahindra A, Kalaycio ME, Vela-Ojeda J, Vesole DH, Zhang MJ, Li P, Berenson JR, Bird JM, Dispenzieri A, Gajewski JL, Gale RP, Holmberg L, Kumar S, Kyle RA, Lazarus HM, Lonial S, Mikhael J, Milone GA, Munker R, Nath R, Saccaro S, To LB, Vogl DT, Wirk B, Hari P. Hematopoietic cell transplantation for primary plasma cell leukemia: results from the Center for International Blood and Marrow Transplant Research. Leukemia. 2012;26(5):1091–1097. doi: 10.1038/leu.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musto P, Simeon V, Martorelli MC, Petrucci MT, Cascavilla N, Di Raimondo F, Caravita T, Morabito F, Offidani M, Olivieri A, Benevolo G, Mina R, Guariglia R, D'Arena G, Mansueto G, Filardi N, Nobile F, Levi A, Falcone A, Cavalli M, Pietrantuono G, Villani O, Bringhen S, Omedè P, Lerose R, Agnelli L, Todoerti K, Neri A, Boccadoro M, Palumbo A. Lenalidomide and low-dose dexamethasone for newly diagnosed primary plasma cell leukemia. Leukemia. 2014;28(1):222–225. doi: 10.1038/leu.2013.241. [DOI] [PubMed] [Google Scholar]
- 19.Katodritou E, Terpos E, Kelaidi C, Kotsopoulou M, Delimpasi S, Kyrtsonis MC, Symeonidis A, Giannakoulas N, Stefanoudaki A, Christoulas D, Chatziaggelidou C, Gastari V, Spyridis N, Verrou E, Konstantinidou P, Zervas K, Dimopoulos MA. Treatment with bortezomib-based regimens improves overall response and predicts for survival in patients with primary or secondary plasma cell leukemia: analysis of the Greek Myeloma Study Group. Am J Hematol. 2014;89(2):145–150. doi: 10.1002/ajh.23600. [DOI] [PubMed] [Google Scholar]
- 20.Fernández de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, Hajek R, San Miguel JF, Sezer O, Sonneveld P, Kumar SK, Mahindra A, Comenzo R, Palumbo A, Mazumber A, Anderson KC, Richardson PG, Badros AZ, Caers J, Cavo M, LeLeu X, Dimopoulos MA, Chim CS, Schots R, Noeul A, Fantl D, Mellqvist UH, Landgren O, Chanan-Khan A, Moreau P, Fonseca R, Merlini G, Lahuerta JJ, Bladé J, Orlowski RZ, Shah JJ, International Myeloma Working Group. Leukemia. 2013;27(4):780–791. doi: 10.1038/leu.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurczyszyn A, Radocha J, Davila J, Fiala MA, Gozzetti A, Grząśko N, Robak P, Hus I, Waszczuk-Gajda A, Guzicka-Kazimierczak R, Atilla E, Mele G, Sawicki W, Jayabalan DS, Charliński G, Szabo AG, Hajek R, Delforge M, Kopacz A, Fantl D, Waage A, Avivi I, Rodzaj M, Leleu X, Richez V, Knopińska-Posłuszny W, Masternak A, Yee AJ, Barchnicka A, Druzd-Sitek A, Guerrero-Garcia T, Liu J, Vesole DH, Castillo JJ. Prognostic indicators in primary plasma cell leukaemia: a multicentre retrospective study of 117 patients. Br J Haematol. 2018;180(6):831–839. doi: 10.1111/bjh.15092. [DOI] [PubMed] [Google Scholar]
- 22.Peña C, Riva E, Schutz N, Ramírez A, Vásquez J, Del Carpio D, Seehaus C, Ochoa P, Vengoa R, Duarte P, Martínez-Cordero H, Figueredo Y, Ríos RO, Ramírez J, Bove V, Roa M, Russo M, Espinoza M, Rodriguez G, Remaggi G, Enciso ME, Chandía M, Fantl D, Grupo de Estudio Latinoamericano de Mieloma Múltiple (GELAMM) Primary plasma cell leukemia in Latin America: demographic, clinical, and prognostic characteristics. A study of GELAMM group. Leuk Lymphoma. 2023;64(4):816–821. doi: 10.1080/10428194.2023.2171266. [DOI] [PubMed] [Google Scholar]
- 23.Yu T, Xu Y, An G, Tai YT, Ho M, Li Z, Deng S, Zou D, Yu Z, Hao M, Anderson KC, Qiu L. Primary plasma cell leukemia: real-world retrospective study of 46 patients from a single-center study in China. Clin Lymphoma Myeloma Leuk. 2020;20(10):e652–e659. doi: 10.1016/j.clml.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Li WJ, Wang FR, Wen L, Chen Y, Chen H, Huang XJ, Lu J. The clinical characteristics of patients with primary plasma cell leukemia and the efficacy of novel agents and hematopoietic stem cell transplantation. Zhonghua Nei Ke Za Zhi. 2020;59:801–806. doi: 10.3760/cma.j.cn112138-20200306-00201. [DOI] [PubMed] [Google Scholar]
- 25.Nakaya A, Yagi H, Kaneko H, Kosugi S, Kida T, Adachi Y, Shibayama H, Kohara T, Kamitsuji Y, Fuchida SI, Uoshima N, Kawata E, Uchiyama H, Shimura Y, Takahashi T, Urase F, Ohta K, Hamada T, Miyamoto K, Kobayashi M, Shindo M, Tanaka H, Shimazaki C, Hino M, Kuroda J, Kanakura Y, Takaoari-Kondo A, Nomura S, Matsumura I, Kansai Myeloma Forum Investigators. Leuk Res Rep. 2018;10:7–10. doi: 10.1016/j.lrr.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav N, Aggarwal M, Mehta P, Kapoor J, Thekkudan SF, Bhandari P, Soni P, Ahmed R, Bhurani D, Agrawal N. Primary plasma cell leukemia: a retrospective study of a rare disease from tertiary cancer centre from India. Indian J Hematol Blood Transfus. 2019;35(4):649–654. doi: 10.1007/s12288-019-01114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganzel C, Rouvio O, Avivi I, Magen H, Jarchowsky O, Herzog K, Cohen Y, Tadmor T, Horwitz NA, Leiba M, Nagler A, Cohen Y, Bulvik S, Polliack A, Rowe JM, Gatt ME, Israeli Multiple Myeloma Study Group. Primary plasma cell leukemia in the era of novel agents for myeloma - a multicenter retrospective analysis of outcome. Leuk Res. 2018;68:9–14. doi: 10.1016/j.leukres.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Mina R, Joseph NS, Kaufman JL, Gupta VA, Heffner LT, Hofmeister CC, Boise LH, Dhodapkar MV, Gleason C, Nooka AK, Lonial S. Survival outcomes of patients with primary plasma cell leukemia (pPCL) treated with novel agents. Cancer. 2019;125(3):416–423. doi: 10.1002/cncr.31718. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Zhou H, Zhang Z, Geng C, Chen W. Bortezomib-based regimens improve the outcome of patients with primary or secondary plasma cell leukemia: a retrospective cohort study. Turk J Hematol. 2020;37:91–97. doi: 10.4274/tjh.galenos.2019.2019.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nandakumar B, Kumar SK, Dispenzieri A, Buadi FK, Dingli D, Lacy MQ, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Muchtar E, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Siddiqui M, Go RS, Jevremovic D, Kyle RA, Gertz MA, Rajkumar SV, Gonsalves WI. Clinical characteristics and outcomes of patients with primary plasma cell leukemia in the era of novel agent therapy. Mayo Clin Proc. 2021;96(3):677–687. doi: 10.1016/j.mayocp.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katodritou E, Kastritis E, Dalampira D, Delimpasi S, Spanoudakis E, Labropoulou V, Ntanasis-Stathopoulos I, Gkioka AI, Giannakoulas N, Kanellias N, Papadopoulou T, Sevastoudi A, Michalis E, Papathanasiou M, Kotsopoulou M, Sioni A, Triantafyllou T, Daiou A, Papadatou M, Kyrtsonis MC, Pouli A, Kostopoulos I, Verrou E, Dimopoulos MA, Terpos E. Improved survival of patients with primary plasma cell leukemia with VRd or daratumumab-based quadruplets: a multicenter study by the Greek Myeloma Study Group. Am J Hematol. 2023;98(5):730–738. doi: 10.1002/ajh.26891. [DOI] [PubMed] [Google Scholar]
- 32.van de Donk NWCJ, Minnema MC, van der Holt B, Schjesvold F, Wu KL, Broijl A, Roeloffzen WWH, Gadisseur A, Pietrantuono G, Pour L, van der Velden VHJ, Lund T, Offidani M, Grasso M, Giaccone L, Razawy W, Tacchetti P, Mancuso K, Silkjaer T, Caers J, Zweegman S, Hájek R, Benjamin R, Vangsted AJ, Boccadoro M, Gay F, Sonneveld P, Musto P. Treatment of primary plasma cell leukaemia with carfilzomib and lenalidomide-based therapy (EMN12/HOVON-129): final analysis of a non-randomised, multicentre, phase 2 study. Lancet Oncol. 2023;24(10):1119–1133. doi: 10.1016/S1470-2045(23)00405-9. [DOI] [PubMed] [Google Scholar]
- 33.Visram A, Suska A, Jurczyszyn A, Gonsalves WI. Practical management and assessment of primary plasma cell leukemia in the novel agent era. Cancer Treat Res Commun. 2021;28:100414. doi: 10.1016/j.ctarc.2021.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung SH, Kim K, Yoon SE, Moon JH, Kim D, Kim HJ, Kim MK, Kim KH, Lee HJ, Lee JH, Kim SH, Yoo KH, Lee JH, Lee JJ. Validation of the revised diagnostic criteria for primary plasma cell leukemia by the Korean Multiple Myeloma Working Party. Blood Cancer J. 2022;12(11):157. doi: 10.1038/s41408-022-00755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawless S, Iacobelli S, Knelange NS, Chevallier P, Blaise D, Milpied N, Foà R, Cornelissen JJ, Lioure B, Benjamin R, Poiré X, Minnema MC, Collin M, Lenhoff S, Snowden JA, Santarone S, Wilson KMO, Trigo F, Dreger P, Böhmer LH, Putter H, Garderet L, Kröger N, Yaukoub-Agha I, Schönland S, Morris C. Comparison of autologous and allogeneic hematopoietic cell transplantation strategies in patients with primary plasma cell leukemia, with dynamic prediction modeling. Haematologica. 2023;108(4):1105–1114. doi: 10.3324/haematol.2021.280568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granell M, Calvo X, Garcia-Guiñón A, Escoda L, Abella E, Martínez CM, Teixidó M, Gimenez MT, Senín A, Sanz P, Campoy D, Vicent A, Arenillas L, Rosiñol L, Sierra J, Bladé J, de Larrea CF, GEMMAC (Grup per l’estudi del mieloma i l’amiloïdosi de Catalunya) Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition. Haematologica. 2017;102(6):1099–1104. doi: 10.3324/haematol.2016.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández de Larrea C, Kyle R, Rosiñol L, Paiva B, Engelhardt M, Usmani S, Caers J, Gonsalves W, Schjesvold F, Merlini G, Lentzch S, Ocio E, Garderet L, Moreau P, Sonneveld P, Badros A, Gahrton G, Goldschmidt H, Tuchman S, Einsele H, Durie B, Wirk B, Musto P, Hayden P, Kaiser M, Miguel JS, Bladé J, Rajkumar SV, Mateos MV. Primary plasma cell leukemia: Consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021;11(12):192. doi: 10.1038/s41408-021-00587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]