Key Teaching Points.
-
•
Sustained ventricular arrhythmias require a triad of an arrhythmogenic trigger, abnormal substrate, and appropriate autonomic conditions. Effective management involves consideration of all 3.
-
•
Patients who present with ventricular tachycardia or after an implantable cardioverter-defibrillator shock may have unique triggers. It is critical for physicians to evaluate for and understand any potential arrhythmogenic source.
-
•
Careful analysis of electrograms from a device recording is essential to delineate the underlying rhythm and allow for an appropriate treatment strategy.
Introduction
Ventricular tachycardia (VT) leading to an implantable cardioverter-defibrillator (ICD) shock is often unanticipated. It can be difficult to find a precise reason why that moment in time gave rise to a malignant arrhythmia and, fortunately, lifesaving therapy. The golden triad of arrhythmogenesis includes a trigger to start the tachycardia, substrate that maintains, and autonomic tone that provides ideal conditions for the perfect storm. Often in electrophysiology (EP) practice, our focus is on substrate identification and modification in patients with sustained shocks owing to monomorphic VT. However, modification of unique triggers and alterations in autonomic tone may be of just as much benefit to patients. We report a unique case of a patient who had an investigational cardiac resynchronization therapy (CRT) pacing device who developed VT during an echocardiogram that was maintained from abnormal substrate.
Case report
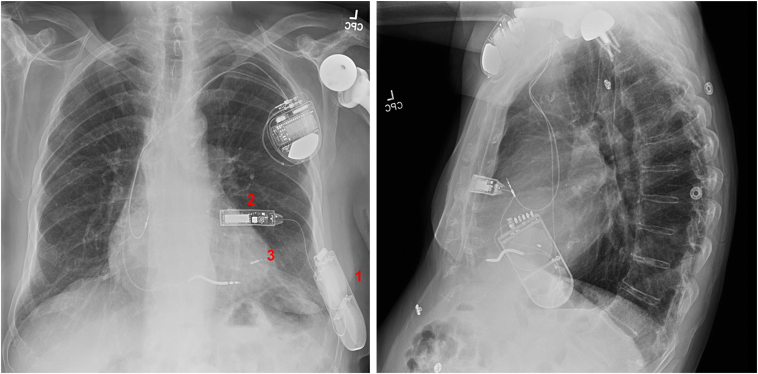
An 84-year-old male patient with ischemic cardiomyopathy, prior myocardial infarction, VT, and atrial flutter had an ICD for secondary prevention placed in 2018. He subsequently developed worsening ventricular function and left bundle branch block requiring an upgrade to a CRT defibrillator. However, his left subclavian vein was occluded. After informed discussion, it was decided to implant a novel system, the Wireless Stimulation Endocardially for Cardiac Resynchronization (WiSE CRT) (EBR Systems, Sunnyvale, CA), as part of the SOLVE-CRT trial.1 This system uses a subcutaneously placed ultrasound (US) emitter and an endocardially placed US receiver to achieve left ventricular pacing and cardiac resynchronization. In 2019, he underwent successful implantation (Figure 1) of the WiSE CRT system.
Figure 1.
Posteroanterior and lateral chest radiographs depicting the novel pacing system. WiSE CRT (EBR Systems, Sunnyvale, CA) components include the subcutaneous battery (1), transmitter (2), and endocardial electrode (3).
The patient did well for many years, with his left ventricular ejection fraction improving from 33% to 45%. However, in the summer of 2023 he developed symptomatic atrial arrhythmias. As part of his evaluation, a standard transthoracic echocardiogram was done. During the study, he developed sudden-onset profound weakness and shortly thereafter received an ICD shock.
Device interrogation
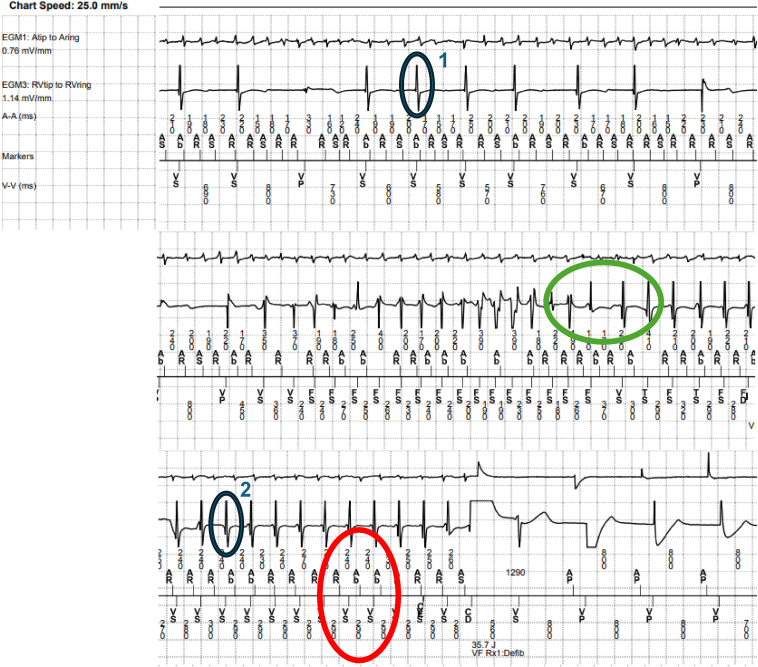
On review of his device tracings, the initial rhythm is atrial fibrillation with slow ventricular rates. An abrupt transition is seen, with irregular rapid tachycardia with markedly varying bipolar electrograms. This then transitions to a monomorphic tachycardia with electrogram morphology distinctly different from conducted rhythm that terminates with an ICD shock and restoration to atrioventricular sequential pacing (Figure 2). The initial polymorphic VT coincided with the time of echocardiographic imaging. Careful electrogram analysis of the event shows different ventricular morphologies between conducted beats and tachycardia. The VT cycle length is most consistently 290 ms. The atrial tachycardia cycle length is most consistently 240 ms, without a clear relationship factor. The preponderance of evidence suggests the regular tachycardia is most consistent with monomorphic VT.
Figure 2.
Device tracings representative of the event in question. Initially there is an atrial fibrillation with slowly conducted ventricular beats. Subsequently, we see initiation of a polymorphic ventricular tachycardia (VT) followed by the more regular monomorphic VT, which is ultimately terminated with defibrillation shock. Close electrogram analysis reveals different ventricular morphologies between conducted beats (blue circle 1) and the tachycardia (blue circle 2). Immediately following the polymorphic VT is an R-R interval increase, which is inconsistent with the described Wedensky effect. If the Wedensky effect is to be invoked, rapid facilitated conduction would begin immediately without the initial ventricular slowing (green circle). Further, the cycle length of the VT is most consistent at 290 ms, while the most consistent cycle length of the atrial tachycardia is 240 ms, suggesting no clear relationship factor, such as a 3:2 or 5:4 pattern (red circle). In summary, the preponderance of evidence suggests the regular tachycardia is most consistent with a monomorphic VT.
EP study
For further evaluation of the ICD shock, the patient was admitted to the telemetry unit and referred for EP study along with antiarrhythmic drug loading and possible ablation.
Ventricular extrastimulation showed no inducible sustained monomorphic VT. US imaging was then done with direct visualization of the left ventricular endocardial US receiver at about 6 cm from the probe. Nonsustained polymorphic VT was reliably inducible. No VT was seen when the probe was removed or the US beam was directed away from the device. Systematic changes in US and emitter frequency did not impact the inducibility of ventricular arrhythmia. However, when the mechanical index of the US emitter was reduced to below 0.6 dB, ectopy was no longer induced. Continuous-wave Doppler imaging of the device did not induce ectopy. No ablation was done.
Discussion
We present a case with the unusual occurrence of an ICD shock triggered by cardiac US examination. Understanding the triangle of arrhythmogenesis relevant to the specific nature of possible triggers and substrate in this given patient allows for an appropriate management strategy.
Ventricular tachycardia
Meticulous analysis of a patient’s history and previous procedures may provide clues to the presence of unusual potential triggering mechanisms for VT. These may include retained intracardiac fragments, cardiac tumors, intravenous lines placed too deeply into the cardiac chambers, pacemaker and ICD leads, or left ventricular assist devices.2,3 In this patient, the specific triggering mechanism involves the left ventricular endocardial implant (receiver electrode) as part of the WiSE CRT system. The transducer transmits acoustic energy, which is converted into an electrical impulse by the left ventricular endocardial receiver electrode.
The production of ventricular extrasystoles when the receiver electrode is directly targeted by a diagnostic US beam is a known phenomenon and is likely the triggering event in this case. Several safety features and protocols exist, and education is conducted with an abundance of caution by the manufacturer and investigators. Specifically, patients with the WiSE CRT device are informed that direct US imaging of the device may be hazardous as part of the consent process for the investigational study. Further, patients receive an implant card describing the risk and are advised to show future health care providers an accompanying medical alert bracelet that directs to the WiSE echo protocol website for further information. Specific study investigators are also informed of the potential risk and trained to mitigate the hazard. Indeed, this patient had previous uneventful echocardiography studies post WiSE CRT implantation.
The substrate for VT, retained fragments, leads, or—as in this case—acoustic energy transduced to an electrical impulse may produce ectopy and nonsustained VT. However, this patient received an ICD shock as a result of sustained monomorphic tachycardia. The Wedensky phenomenon occurs when an otherwise subthreshold impulse reaches threshold to induce a response shortly after another, stronger impulse reaches an area of block and enhances excitability, facilitating conduction. One might invoke this effect in this scenario, suggesting that nonsustained premature ventricular contractions accelerated conduction in the AV node and allowed for more rapid conduction of an atrial flutter/fibrillation; however, the distinct change in bipolar electrograms makes this mechanism unlikely.4 The sustained VT that resulted in an ICD shock was not noted during the exact time of US-instigated nonsustained polymorphic VT but rather was a result of underlying substrate from ischemic cardiomyopathy. The sustained VT was, however, initiated by the brief polymorphic tachycardia during echocardiography, an important distinction.
Unique interactions and the perfect storm
The patient had an identifiable trigger but not one that should necessarily result in sustained VT without a concurrent underlying arrhythmogenic substrate. However, programmed stimulation during the EP study with right ventricular pacing did not initiate monomorphic VT despite triple extrastimulation at 2 cycle lengths and 2 sites. Understanding the interaction between trigger and substrate specifically occurring with a unique site of stimulation and a given autonomic milieu helps explain the observed ICD shock during echocardiography in this patient.
Why did prior echocardiograms not result in ICD shock?
It is feasible that no ventricular arrhythmia was noticed at prior echocardiograms, perhaps from the uniqueness of the imaging planes used. It is also possible that the receiver electrode, anchored on the lateral wall, had not been directly targeted during previous echocardiograms. Further, at EP study, we noted a mechanical index threshold at which we could induce polymorphic arrhythmia. However, even if nonsustained changing-morphology tachycardia had been induced transiently, without a specific substrate allowing for induction and maintenance of sustained VT of sufficient duration, the shock may not have occurred.
Why was VT not inducible at EP study?
In patients with highly abnormal substrate, any trigger inducing commonplace random premature ventricular contractions or unique specific triggers, as in this patient or those with mechanical left ventricular assist devices, mitral valve redundancy, tumors, etc, should have inducible VT with carefully conducted programmed stimulation, whereas this patient did not. Even when substrate is present, the critical timing, location, and entrance wavefronts to the arrhythmogenic substrate may be critical in inducing tachycardia that sustains and is not terminated by continuous activity from the trigger itself.5,6 Thus, in our patient, the endocardial site and proximity to abnormal substrate with the ischemic cardiomyopathy, in addition in the absence of sedation, may have resulted in the perfect storm during the US exam.
Conclusion
We present a case of ICD shock during an echocardiographic exam that provides unique insights and reminders that patient care teams must be fully acquainted with the details of potential triggering mechanisms and underlying substrate to fully address a potentially malignant arrhythmia and prevent recurrence. This is especially relevant when mechanical and biophysical triggering mechanisms, including foreign bodies, endocardial tumors, unique pacing systems (as in our patient), or other indwelling devices, are present.
Disclosures
Speaking and research for Dr Asirvatham: Johnson & Johnson, Biotronik, Boston Scientific, Medtronic. Mayo Clinic has pursued protection of intellectual property in the form of patents and patent applications naming Dr Asirvatham as an inventor related to devices for cardiac ablation, rhythm management, and autonomic modulation, including the use of deep learning in cardiology.
Acknowledgments
Funding Sources
None.
References
- 1.Singh J.P., Abraham W.T., Auricchio A., et al. Design and rationale for the Stimulation Of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy in non-responders and previously untreatable patients (SOLVE-CRT) trial. Am Heart J. 2019;217:13–22. doi: 10.1016/j.ahj.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Murata H., Miyauchi Y., Nitta T., et al. Electrophysiological and histopathological characteristics of ventricular tachycardia associated with primary cardiac tumors. JACC Clin Electrophysiol. 2024;10:43–55. doi: 10.1016/j.jacep.2023.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Nakahara S., Chien C., Gelow J., et al. Ventricular arrhythmias after left ventricular assist device. Circ Arrhythm Electrophysiol. 2013;6:648–654. doi: 10.1161/CIRCEP.113.000113. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg H.D. Mechanism of the Wedensky phenomena in the left bundle branch. Am J Cardiol. 1971;27:698–702. doi: 10.1016/0002-9149(71)90240-2. [DOI] [PubMed] [Google Scholar]
- 5.Robertson J.F., Cain M.E., Horowitz L.N., et al. Anatomic and electrophysiologic correlates of ventricular tachycardia requiring left ventricular stimulation. Am J Cardiol. 1981;48:263–268. doi: 10.1016/0002-9149(81)90606-8. [DOI] [PubMed] [Google Scholar]
- 6.Doherty J.U., Kienzle M.G, Waxman H.L., Buxton A.E., Marchlinski F.E., Josephson M.E. Programmed ventricular stimulation at a second right ventricular site: an analysis of 100 patients, with special reference to sensitivity, specificity and characteristics of patients with induced ventricular tachycardia. Am J Cardiol. 1983;52:1184–1189. doi: 10.1016/0002-9149(83)90571-4. [DOI] [PubMed] [Google Scholar]