Abstract
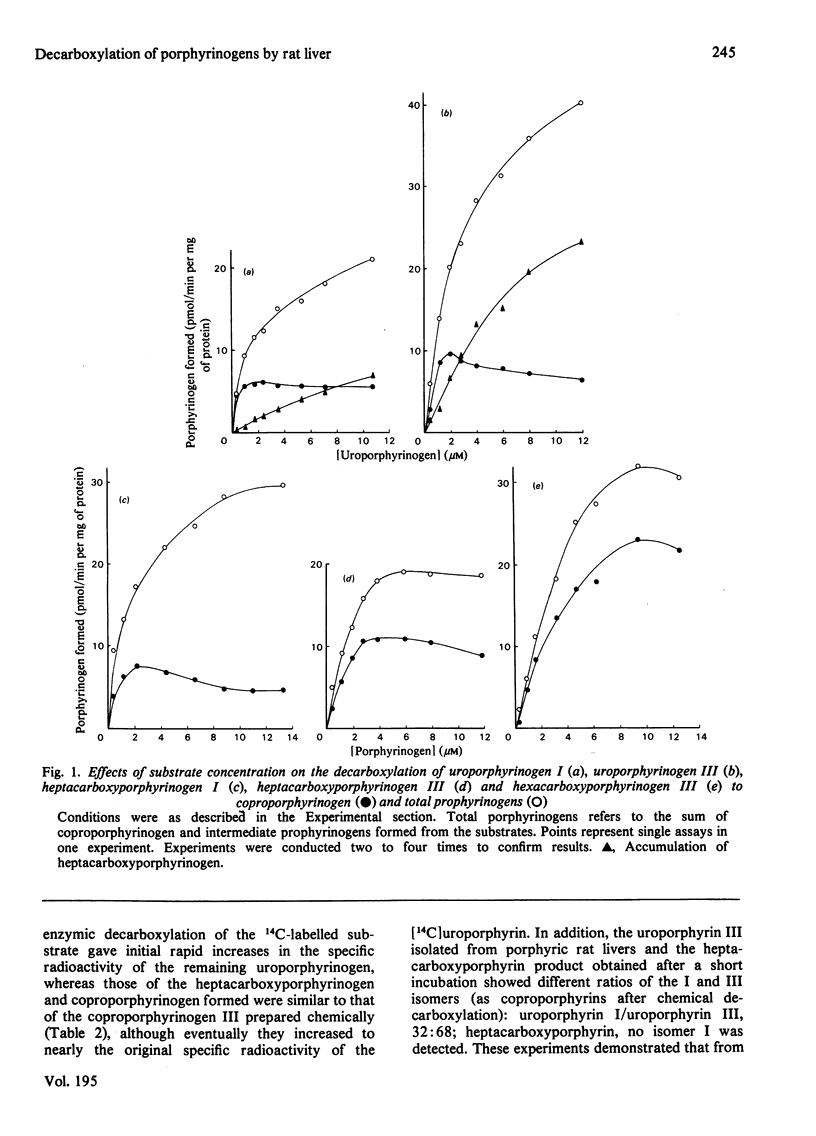
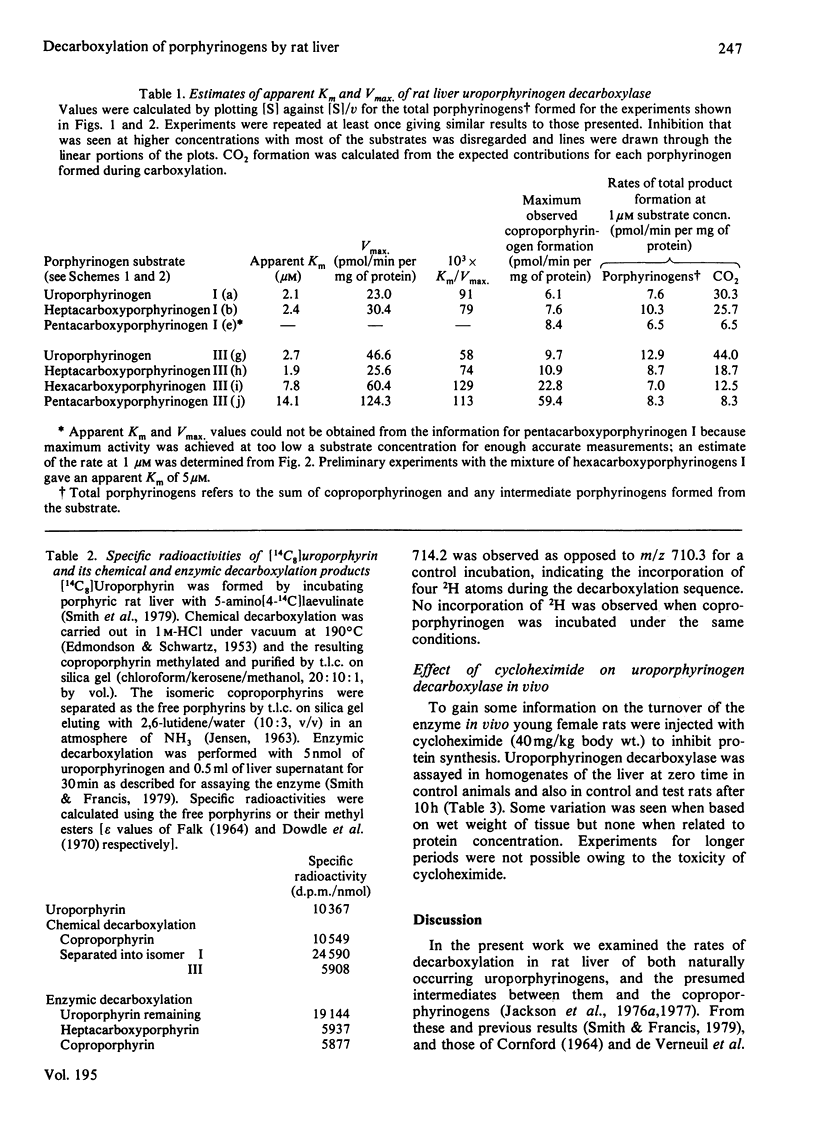
1. The decarboxylations of uroporphyrinogens, hepta-, hexa- and penta-carboxyporphyrinogens I and III by porphyrinogen carboxy-lyase (EC 4.1.1.37) in rat liver supernatant have been compared as functions of substrate concentrations. Although Km and Vmax. (for total porphyrinogens formed) were estimated, prophyrinogens and CO2 produced at 1 microM were considered to be a better indication of real relative rates, owing to substrate/product inhibitions. Uroporphyrinogen III was the best substrate by the criteria of Km/Vmax. and decarboxylation at 1 microM and was converted into coproporphyrinogen more quickly than its series-I isomer. 2. The difference between uroporphyrinogens I and III as substrates was confirmed by using a mixture of [14C8]uroporphyrinogens, the discrimination occurring principally in the first decarboxylation. 3. Porphyrins, especially oxidation products of the substrates, inhibited the enzyme. Heptacarboxyporphyrin III was the most effective inhibitor of both uroporphyrinogen III and heptacarboxyporphyrinogen III conversion into coproporphyrinogen. 4. Rapid analysis of the livers from rats made porphyric with hexachlorobenzene demonstrated that substantial quantities of the tetrapyrroles were present in vivo as the porphyrinogens (21-42%). 5. Enzymic decarboxylation of uroporphyrinogen III in 2H2O-containing buffer gave [2H4]coproporphyrinogen. 6. Rats treated with cycloheximide for 10h showed no decrease in uroporphyrinogen decarboxylase activity/mg of protein, suggesting a relatively slow turnover of the enzyme.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard G. F., Akhtar M. Stereochemical and mechanistic studies on the decarboxylation of uroporphyrinogen III in haem biosynthesis. J Chem Soc Perkin 1. 1979;10:2354–2360. doi: 10.1039/p19790002354. [DOI] [PubMed] [Google Scholar]
- Cornford P. Transformation of porphobilinogen into porphyrins by preparations from human erythrocytes. Biochem J. 1964 Apr;91(1):64–73. doi: 10.1042/bj0910064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle E., Goldswain P., Spong N., Eales L. The pattern of porphyrin isomer accumulation and excretion in symptomatic porphyria. Clin Sci. 1970 Aug;39(2):147–158. doi: 10.1042/cs0390147. [DOI] [PubMed] [Google Scholar]
- EDMONDSON P. R., SCHWARTZ S. Studies of the uroporphyrins. III. An improved method for the decarboxylation of uroporphyrin. J Biol Chem. 1953 Dec;205(2):605–609. [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Matlin S. A. The effect of the porphyrogenic compound, hexachlorobenzene, on the activity of hepatic uroporphyrinogen decarboxylase in the rat. Clin Sci Mol Med. 1976 Jul;51(1):71–80. doi: 10.1042/cs0510071. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Tovey J. A. Uroporphyrinogen decarboxylase activity of human tissues [proceedings]. Biochem Soc Trans. 1977;5(5):1470–1472. doi: 10.1042/bst0051470. [DOI] [PubMed] [Google Scholar]
- Garcia R. C., San Martin de Viale L. C., Tomio J. M., Grinstein M. Porphyrin biosynthesis. X. Porphyrinogen carboxy-lyase from avian erythrocytes: further properties. Biochim Biophys Acta. 1973 May 5;309(1):203–210. doi: 10.1016/0005-2744(73)90332-x. [DOI] [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Jackson A. H., Sancovich H. A., Ferramola A. M., Evans N., Games D. E., Matlin S. A., Elder G. H., Smith S. G. Macrocyclic intermediates in the biosynthesis of porphyrins. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):191–206. doi: 10.1098/rstb.1976.0009. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Steinmuller D. P., Lee G. R. The role of iron in the pathogenesis of porphyria cutanea tarda. II. Inhibition of uroporphyrinogen decarboxylase. J Clin Invest. 1975 Sep;56(3):661–667. doi: 10.1172/JCI108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. Porphyrin biosynthesis in erythrocytes. III. Uroporphyrinogen and its decarboxylase. J Biol Chem. 1958 Jun;232(2):1141–1162. [PubMed] [Google Scholar]
- Rasmussen G. L., Kushner J. P. The enzymatic decarboxylation of the naturally occurring isomers of uroporphyrinogen by human erythrocytes. J Lab Clin Med. 1979 Jan;93(1):54–59. [PubMed] [Google Scholar]
- Romeo G., Levin E. Y. Uroporphyrinogen decarboxylase from mouse spleen. Biochim Biophys Acta. 1971 Feb 23;230(2):330–341. doi: 10.1016/0304-4165(71)90220-0. [DOI] [PubMed] [Google Scholar]
- San Martin de Viale L. C., Viale A. A., Nacht S., Grinstein M. Experimental porphyria induced in rats by hexachlorobenzene. A study of the porphyrins excreted by urine. Clin Chim Acta. 1970 Apr;28(1):13–23. doi: 10.1016/0009-8981(70)90155-5. [DOI] [PubMed] [Google Scholar]
- San Martín De Viale L. C., Ríos De Molina M. D., De Calmanovici R. W., Tomio J. M. Porphyrins and porphyrinogen carboxy-lase in hexachlorobenzene-induced porphyria. Biochem J. 1977 Dec 15;168(3):393–400. doi: 10.1042/bj1680393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Brooks C. J. The substrate specificity and stereochemistry, reversibility and inhibition of the 3-oxo steroid delta 4-delta 5-isomerase component of cholesterol oxidase. Biochem J. 1977 Oct 1;167(1):121–129. doi: 10.1042/bj1670121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Cabral J. R., De Matteis F. A difference between two strains of rats in their liver non-haem iron content and in their response to the porphyrogenic effect of hexachlorobenzene. Chem Biol Interact. 1979 Oct;27(2-3):353–363. doi: 10.1016/0009-2797(79)90138-8. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Decarboxylation of porphyrinogens by rat liver uroporphyrinogen decarboxylase. Biochem J. 1979 Nov 1;183(2):455–458. doi: 10.1042/bj1830455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Grandchamp B., Nordmann Y. Some kinetic properties of human red cell uroporphyrinogen decarboxylase. Biochim Biophys Acta. 1980 Jan 11;611(1):174–186. doi: 10.1016/0005-2744(80)90053-4. [DOI] [PubMed] [Google Scholar]


