Test your knowledge!
-
•
Take an interactive quiz related to this article: https://www.heartrhythmcasereports.com/content/quiz_archive
Introduction
Pathogenic variants of the SCN5A gene encoding the cardiac sodium channel, NaV 1.5, are well known to be associated with Brugada syndrome or long QT syndrome. However, less frequently they can cause other phenotypes including arrhythmogenic right ventricular cardiomyopathy, atrial stand-still, dilated cardiomyopathy, atrial fibrillation, sick sinus syndrome, and other cardiac conduction diseases.1 We report a case of an SCN5A gene mutation presenting with stroke in a 28-year-old patient found to have ARVC and atrial myopathy.
Case report
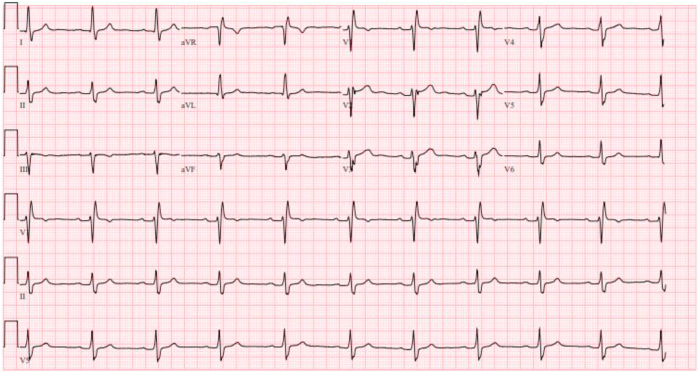
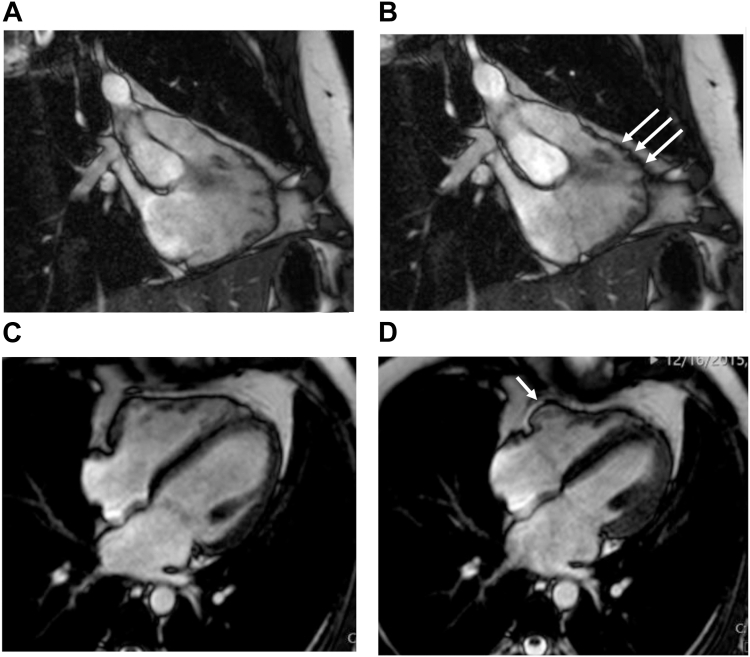
A 28-year-old male patient with history of tobacco and moderate alcohol use presented in 2015 with aphasia, ataxia, and left arm weakness. Imaging revealed acute infarcts in the bilateral cerebellar region consistent with embolic stroke. A computed tomography angiogram showed no vascular etiology. Echocardiography with contrast showed dilated left ventricle (LV) with LV ejection fraction (LVEF) 50%, right ventricular (RV) dysfunction, and mild left atrial dilatation with left atrial volume index 36 mL/m2. There was no evidence of intracardiac shunt. Electrocardiogram (ECG) showed sinus rhythm with borderline first-degree atrioventricular block (AVB), right bundle branch block (RBBB), and a suggestion of epsilon waves in leads V2 and V3 (Figure 1), although the suspicious deflection occurs within the QRS in the presence of RBBB. Cardiac magnetic resonance imaging (MRI) met a major criterion for ARVC with RV ejection fraction (RVEF) 39% and regional wall motion abnormality (Figure 2) and showed mild left atrial dilatation with a left atrial end-systolic area of 27 cm2. There was no personal history of cardiac symptoms, syncope, or venous thrombosis. Family history was notable for a stroke in his paternal grandfather at age 62. Stress test and 24-hour ECG monitor results were normal. Genetic testing showed the patient to be heterozygous for a pathogenic missense variant, pD1275N, in SCN5A, previously reported to be associated with sinus node dysfunction, atrial standstill, frequent need for ventricular pacing, and dilated cardiomyopathy with significant arrhythmic risk.1 Electrophysiologic testing with voltage mapping of the RV showed no inducible ventricular tachycardia or RV scar; however, extensive scarring was seen in the right atrium. Considering his history of thromboembolic strokes and atrial myopathy, he was treated with anticoagulation. Because of his elevated risk of sudden cardiac death (SCD), he was counseled on exercise restriction and, after shared decision making, he underwent implantation of a single-chamber primary prevention implantable cardioverter-defibrillator (ICD). He subsequently received 2 inappropriate shocks for atrial fibrillation with rapid ventricular response, and due to further episodes of symptomatic paroxysmal atrial fibrillation, after declining ablation he was treated with sotalol with good arrhythmia suppression. He has not had any ventricular tachyarrhythmia or recurrent stroke.
Figure 1.
The proband’s electrocardiogram at presentation shows normal sinus rhythm with first-degree atrioventricular block, right bundle branch block, and a suggestion of epsilon waves in V2 and V3.
Figure 2.
Proband cardiac magnetic resonance imaging. A: Steady State Free Precession (SSFP) end-diastolic still frame of the right ventricle (RV) in an inflow-outflow view showing a mildly dilated RV and irregular wall border. B: SSFP end-systolic still frame of the RV in an inflow-outflow view showing microaneurysms at the anterior free wall region below the pulmonary valve. C: SSFP end-diastolic still frame of heart in a 4-chamber view showing mild RV dilation. D: SSFP end-systolic still frame of heart in a 4-chamber view showing hypokinesia of the basal RV free wall.
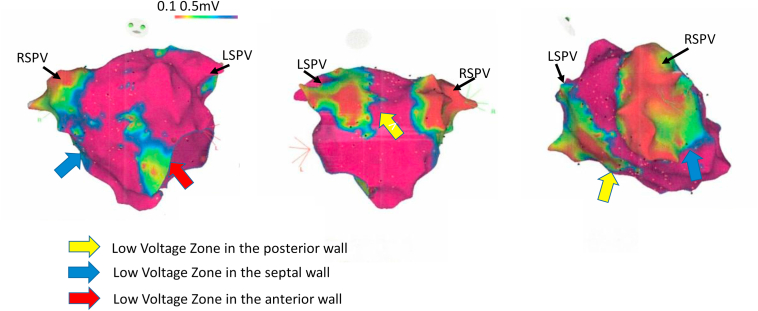
Screening of family members revealed that the patient’s father, then age 59, carried the pathogenic variant. The father’s baseline ECG showed sinus bradycardia with borderline first-degree atrioventricular block (AVB), intraventricular conduction delay with QRS width 118 ms, and an epsilon wave in lead V1. Signal-averaged ECG was performed and was abnormal by all 3 criteria, although arguably it was uninterpretable because of a simultaneous unfiltered QRS duration of 125 ms. The father’s stress test and 24-hour ECG monitor results were normal. His cardiac MRI showed a dilated RV with normal function. Electrophysiologic study with voltage mapping showed extensive left atrial scarring (FIGURE 3). Based on his elevated stroke risk, he chose to initiate anticoagulation. He was counseled on exercise restriction. Over 8 years of follow-up, he has had disease progression as evidenced by progressive sinus bradycardia and first-degree AVB, a hospital admission for acute decompensated heart failure, and repeated cardiac MRI showing RVEF 39% and LVEF 45%. He remains stroke-free with anticoagulation medication and will undergo shared decision making regarding whether to proceed to ICD implantation.
Figure 3.
Father’s atrial voltage mapping. The father’s atrial voltage mapping shows extensive areas of left atrial low voltage consistent with fibrosis. LSPV = left superior pulmonary vein; RSPV = right superior pulmonary vein.
Discussion
Our proband had an unusual presentation of the pathogenic SCN5A variant pD1275N in that he presented with stroke and criteria for ARVC. Previous reports of this variant describe typical presentation with predominant conduction system disease1 or atrial standstill,2 with ARVC a later development.1 In addition, investigators have reported pacemaker failure to capture,3 left ventricular cardiomyopathy,1 and stroke.4,5 Our proband had RBBB. The patient’s father, in addition to extensive atrial scarring, has shown progressive conduction disease manifesting as bradycardia and first-degree AVB, as well as progressive cardiomyopathy.
The mechanism by which SCN5A variants lead to cardiomyopathy remains uncertain. Watanabe and colleagues6 showed in a murine model that the SCN5A variant pD1275N was associated with loss of sodium channel function and led to development of cardiomyopathy; they also suggested that the mechanism might be electromechanical dyssynchrony or impaired interaction with cytoskeletal proteins.6 In a systematic review of 18 dilated cardiomyopathy-associated SCN5A variants across 29 kindreds, Peters and colleagues7 found no cardiomyopathy in the absence of arrhythmias, which were always the presenting symptom. In contrast, our proband and his father presented with evidence of cardiomyopathy and no symptomatic arrhythmia.
This case illustrates that the phenotype in individuals with SCN5A mutations can vary within a family and, over time, in a single individual. In this family the proband’s first manifestation of disease was embolic stroke because of thrombogenic atrial myopathy, recognizable as atrial fibrosis prior to development of clinically apparent atrial fibrillation. His RV structural abnormality met a major ARVC criterion by age 28. In contrast, his father remained asymptomatic at age 59, and he did not develop significant RV dysfunction until age 67.
Because of the temporal variability of the phenotype, careful follow-up over time is necessary to identify disease progression. At the time of diagnosis, our proband had a definite diagnosis of ARVC by the 2010 revised task force criteria8 (at least two major task force criteria): regional RV akinesia on MRI with RVEF ≤ 40%, (major criterion), possible epsilon waves (major criterion), and identification of a pathogenic mutation associated with ARVC (major criterion). He met class 2a indications for a primary prevention ICD.9 In contrast, despite also meeting 2010 revised task force criteria8 for a diagnosis of ARVC with epsilon waves (major criterion) and a pathogenic mutation associated with ARVC (major criterion), the father did not have an ICD indication at first assessment with only mild global RV dilatation. The abnormal findings on his current MRI including RVEF 39% and LVEF 45% now give him a class 2a indication for implantation of an ICD.9,10 It should be noted that his 5-year risk of sustained ventricular events based on the ARVC risk-calculator (arvcrisk.com) is relatively low (2.9% over 5 years) compared with his son’s (6.1% over 5 years calculated with his initial data) since the risk of SCD is lower in older adults.
While Kato and colleagues11 previously described a case of atrial standstill with biopsy-proven atrial fibrosis associated with an SCN5A variant, our case demonstrates that atrial fibrosis and increased thromboembolic stroke risk can precede clinical atrial fibrillation in this syndrome. The role of atrial low voltage as a predictor of atrial fibrillation is an area of active investigation. Our group previously showed that atrial low voltage is associated with left atrial appendage dysfunction,12 which itself is associated with thromboembolic risk.13
This case also illustrates the importance of screening to identify affected family members. Because the father was identified, he was able to undergo serial screening for SCD risk and counseling on exercise restriction. Importantly, he chose to undergo atrial voltage mapping, and, when this was abnormal, to initiate anticoagulation for stroke prevention. In the context of his son’s and father’s early strokes, it is possible that this intervention protected him from a cardioembolic stroke.
In summary, our case illustrates an unusual phenotype associated with the pD1275N SCN5A variant, including atrial myopathy, stroke risk, atrial fibrillation, conduction system disease, and ARVC phenotype. As in other genetic arrhythmic disorders, it is essential to establish a diagnosis and to assess individual risk. Careful follow-up over time is necessary to identify disease progression and further inform therapy. Screening to identify affected family members can lead to tailored treatment and improve outcomes.
Acknowledgments
We appreciate the assistance of Ashish Aneja, MD with interpretation of the cardiac MRI.
References
- 1.McNair W.P., Ku L., Taylor M.R.G., et al. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–2167. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 2.Groenewegen W.A., Firouzi M., Bezzina C.R., et al. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003;92:14–22. doi: 10.1161/01.res.0000050585.07097.d7. [DOI] [PubMed] [Google Scholar]
- 3.Chiang D.Y., Kim J.J., Valdes S.O., et al. Loss-of-function SCN5A mutations associated with sinus node dysfunction, atrial arrhythmias, and poor pacemaker capture. Circ Arrhythm Electrophysiol. 2015;8:1105–1112. doi: 10.1161/CIRCEP.115.003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laitinen-Forsblom P.J.L., Mäkynem P., Mäkynem H., et al. SCN5A mutation associated with cardiac conduction defect and atrial arrhythmias. J Cardiovasc Electrophysiol. 2006;17:480–485. doi: 10.1111/j.1540-8167.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 5.Kang D.-S., Khmao P., Oh J., Pak H.-N. A case of SCN5A mutation-associated isolated left atrial standstill and ischemic stroke. Korean Circ J. 2022;52:717–719. doi: 10.4070/kcj.2022.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe H., Yang T., Myers Stroud D., et al. Striking in vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro. Circulation. 2011;124:1001–1011. doi: 10.1161/CIRCULATIONAHA.110.987248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters S., Bryony A.T., Perrin M., et al. Arrhythmic phenotypes are a defining feature of dilated cardiomyopathy-associated SCN5A variants: a systematic review. Circ Genom Precis Med. 2022;15:44–54. doi: 10.1161/CIRCGEN.121.003432. [DOI] [PubMed] [Google Scholar]
- 8.Marcus F.I., McKenna W.J., Sherrill D., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrado D., Wichter T., Link M.S., et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015;132:441–453. doi: 10.1161/CIRCULATIONAHA.115.017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krahn A.D., Wilde A.A.M., Calkins H., et al. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol EP. 2022;8:533–553. doi: 10.1016/j.jacep.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Kato Y., Nozaki Y., Takahashi-Igari M., Sugano M., Makita N., Horigome H. Progressive atrial myocardial fibrosis in a 4-year-old girl with atrial standstill associated with an SCN5A gene mutation. HeartRhythm Case Rep. 2022;8:636–638. doi: 10.1016/j.hrcr.2022.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y., Sattayaprasert P., Dhanvanthari S., Ziv O. Left atrial scar predicts left atrial appendage dysfunction. Circulation. 2017;136 [Google Scholar]
- 13.Handke M., Harloff A., Hetzel A., Olschewski M., Bode C., Geibel A. Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation—a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J M Soc Echocardiogr. 2005;18:1366–1372. doi: 10.1016/j.echo.2005.05.006. [DOI] [PubMed] [Google Scholar]