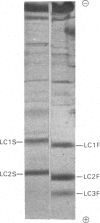
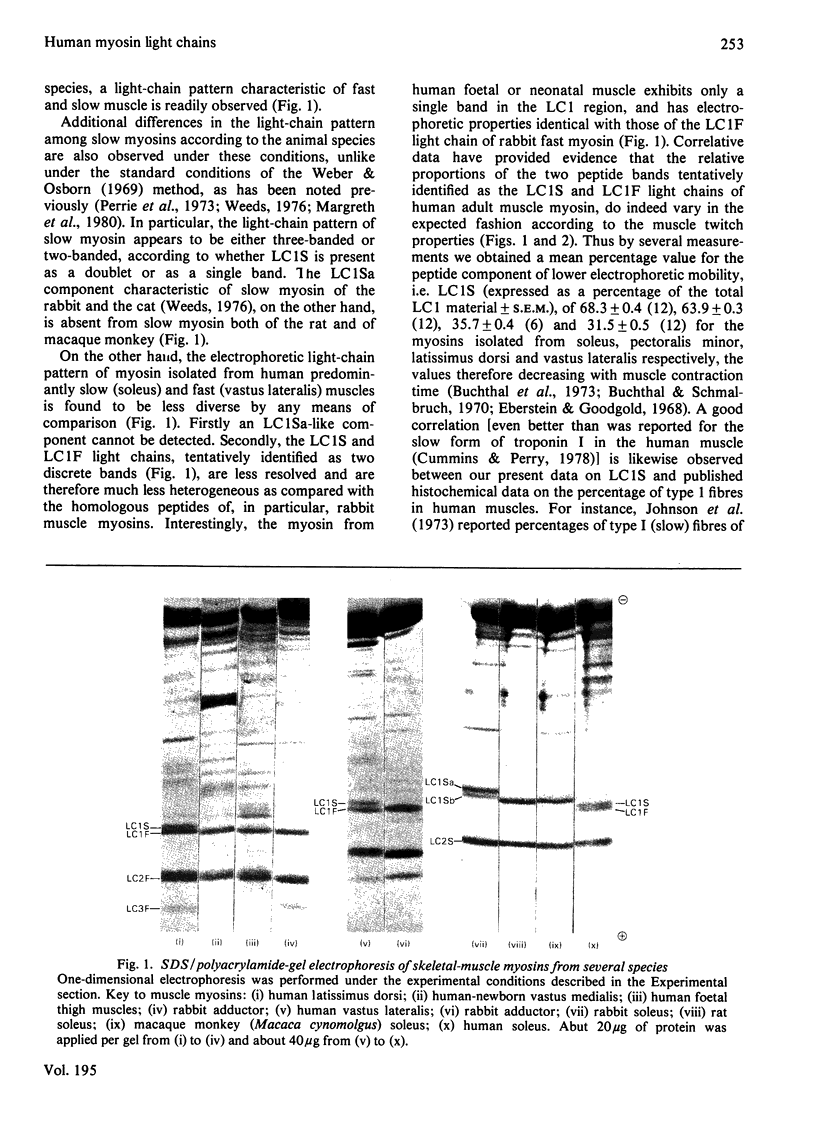
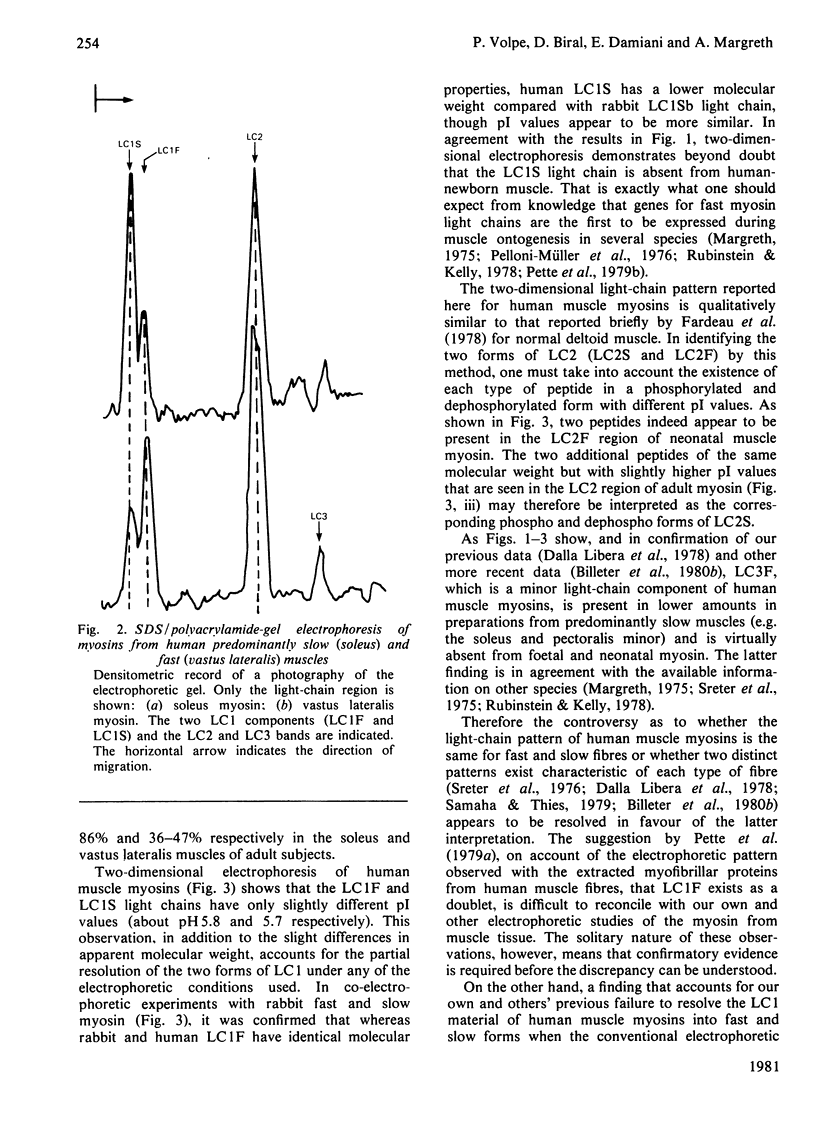
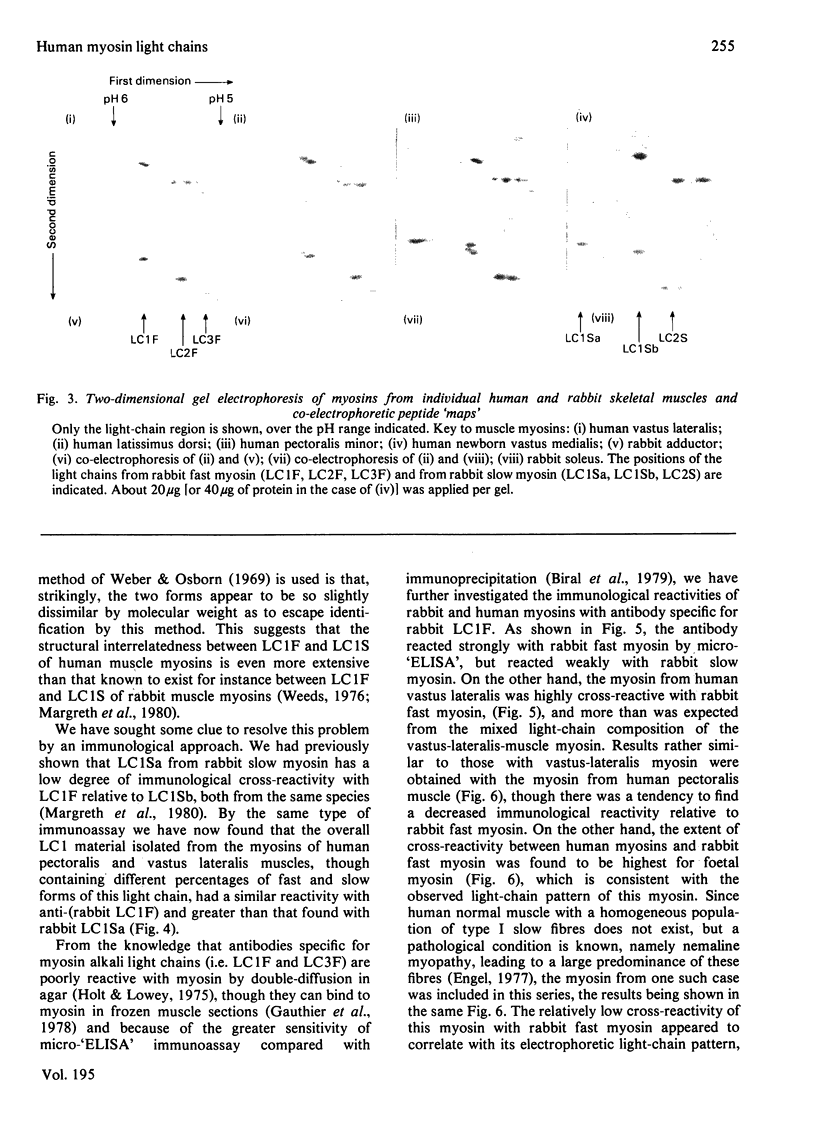
Abstract
Isolated myosins from human predominantly fast and slow muscles, human neonatal and foetal muscle were examined for light chain composition by one- and two-dimensional electrophoresis. The LC1F, LC2F and LC3F light chains were identical with their counterparts from rabbit fast myosin. Human LC1S was identified by correlative criteria as a single component having a molecular weight slightly lower than, but an electric charge similar to, that of rabbit LC1Sb. Consequently, human LC1S appears to be much less heterogeneous relative to LC1F than is the case with other mammalian species. A high immunological cross-reactivity was likewise observed, with antibody specific to rabbit LC1F, between the isolated myosins from several human mixed muscles and rabbit fast myosin, though reactivity was highest with foetal myosin (having a pure-fast-light-chain pattern).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailin G. Myosin and actomyosin from human skeletal muscle. Biochim Biophys Acta. 1976 Nov 9;449(2):310–326. doi: 10.1016/0005-2728(76)90143-2. [DOI] [PubMed] [Google Scholar]
- Billeter R., Weber H., Lutz H., Howald H., Eppenberger H. M., Jenny E. Myosin types in human skeletal muscle fibers. Histochemistry. 1980;65(3):249–259. doi: 10.1007/BF00493174. [DOI] [PubMed] [Google Scholar]
- Biral D., Dalla Libera L., Franceschi C., Margreth A. Microplate enzyme-linked immunosorbent assay in the study of the structural relationship between myosin light chains. J Immunol Methods. 1979;31(1-2):93–100. doi: 10.1016/0022-1759(79)90289-8. [DOI] [PubMed] [Google Scholar]
- Buchthal F., Dahl K., Rosenfalck P. Rise time of the spike potential in fast and slowly contracting muscle of man. Acta Physiol Scand. 1973 Feb;87(2):261–269. doi: 10.1111/j.1748-1716.1973.tb05389.x. [DOI] [PubMed] [Google Scholar]
- Buchthal F., Schmalbruch H. Contraction times and fibre types in intact human muscle. Acta Physiol Scand. 1970 Aug;79(4):435–452. doi: 10.1111/j.1748-1716.1970.tb04744.x. [DOI] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Troponin I from human skeletal and cardiac muscles. Biochem J. 1978 Apr 1;171(1):251–259. doi: 10.1042/bj1710251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberstein A., Goodgold J. Slow and fast twitch fibers in human skeletal muscle. Am J Physiol. 1968 Sep;215(3):535–541. doi: 10.1152/ajplegacy.1968.215.3.535. [DOI] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Hobbs A. W. Fast and slow myosin in developing muscle fibres. Nature. 1978 Jul 6;274(5666):25–29. doi: 10.1038/274025a0. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Lowey S. An immunological approach to the role of the low molecular weight subunits in myosin. II. Interaction of myosin and its subfragments with antibodies to the light chains. Biochemistry. 1975 Oct 21;14(21):4609–4620. doi: 10.1021/bi00692a008. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Lowey S. Distribution of alkali light chains in myosin: isolation of isoenzymes. Biochemistry. 1977 Oct 4;16(20):4398–4402. doi: 10.1021/bi00639a011. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973 Jan;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libera L. D., Margreth A., Mussini I., Cerri C., Scarlato G. Myosin polymorphism in human skeletal muscles. Muscle Nerve. 1978 Jul-Aug;1(4):280–291. doi: 10.1002/mus.880010404. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelloni-muller G., Ermini M., Jenny E. Myosin light chains of developing fast and slow rabbit skeletal muscle. FEBS Lett. 1976 Aug 1;67(1):68–74. doi: 10.1016/0014-5793(76)80872-1. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D., Henriksson J., Emmerich M. Myofibrillar protein patterns of single fibres from human muscle. FEBS Lett. 1979 Jul 1;103(1):152–155. doi: 10.1016/0014-5793(79)81270-3. [DOI] [PubMed] [Google Scholar]
- Pette D., Vrbová G., Whalen R. C. Independent development of contractile properties and myosin light chains in embryonic chick fast and slow muscle. Pflugers Arch. 1979 Jan 31;378(3):251–257. doi: 10.1007/BF00592743. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978 Feb;62(2):473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Samaha F. J., Thies W. H. Myosin light chains in Duchenne dystrophy and paraplegic muscle. Neurology. 1979 Jan;29(1):122–125. doi: 10.1212/wnl.29.1.122. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., Dreizen P. Canine cardiac myosin with special referrence to pressure overload cardiac hypertrophy. I. Subunit composition. J Biol Chem. 1978 Dec 10;253(23):8648–8658. [PubMed] [Google Scholar]
- Silberstein L., Lowey S. Investigation of immunological relationships among myosin light chains and troponin C. Biochemistry. 1977 Oct 4;16(20):4403–4408. doi: 10.1021/bi00639a012. [DOI] [PubMed] [Google Scholar]
- Sreter F. A., Aström K. E., Romanul F. C., Young R. R., Jones H. R., Jr Characteristics of myosin in nemaline myopathy. J Neurol Sci. 1976 Jan;27(1):99–116. doi: 10.1016/0022-510x(76)90238-0. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G. Light chains from slow-twitch muscle myosin. Eur J Biochem. 1976 Jun 15;66(1):157–173. doi: 10.1111/j.1432-1033.1976.tb10436.x. [DOI] [PubMed] [Google Scholar]
- Weeds A. Myosin: polymorphism and promiscuity. Nature. 1978 Aug 3;274(5670):417–418. doi: 10.1038/274417a0. [DOI] [PubMed] [Google Scholar]