Key Teaching Points.
-
•
Catheter ablation for atrial fibrillation is an effective rhythm control strategy for patients with long QT syndrome, although redo procedures may be required.
-
•
If an ablation strategy is chosen, periprocedural anesthesia and sympathomimetic, antiemetic, and other QT prolonging agents should be used cautiously.
-
•
Patients with long QT syndrome had normal left atrial voltages during electroanatomic mapping without significant areas of fractionated atrial electrograms arguing against atrial myopathy driving atrial fibrillation.
Introduction
Management of atrial fibrillation (AF) in patients with long QT syndrome (LQTS) is complicated by the QT-prolonging effects of commonly used antiarrhythmic drugs (AADs). In addition, the role of catheter ablation in this population remains unclear.1,2 Herein, we highlight that invasive catheter ablation is an effective rhythm control management strategy in LQTS and describe our institutional experience of 5 patients who underwent this procedure. The index ablation procedure was defined as the first AF ablation procedure performed at our institution. LQTS was diagnosed if the patient had a Schwartz LQTS score ≥3.5 and/or had an unequivocal pathogenic mutation in one of the LQTS genes, and/or had QTc ≥500 milliseconds on repeated 12-lead electrocardiogram (ECG) in the absence of a secondary cause for QT prolongation, in accordance with the 2013 Heart Rhythm Society Expert Consensus Statement.3 All ECGs using standard lead placement were obtained using a MAC 5500 HD (GE Healthcare, Chicago, IL), recorded at 25 mm/s with a gain of 1 mV/10 mm, and stored in the ECG system (MUSE, GE Healthcare). Automated ECG measurements were validated independently by means of manual measurement when needed. Manual QT measurements were measured as the average of 3 consecutive beats in lead II or V5, with the end of the T wave defined as the intersection of a tangent to the steepest slope of the terminal limb of the T wave and the isoelectric baseline.
Case reports
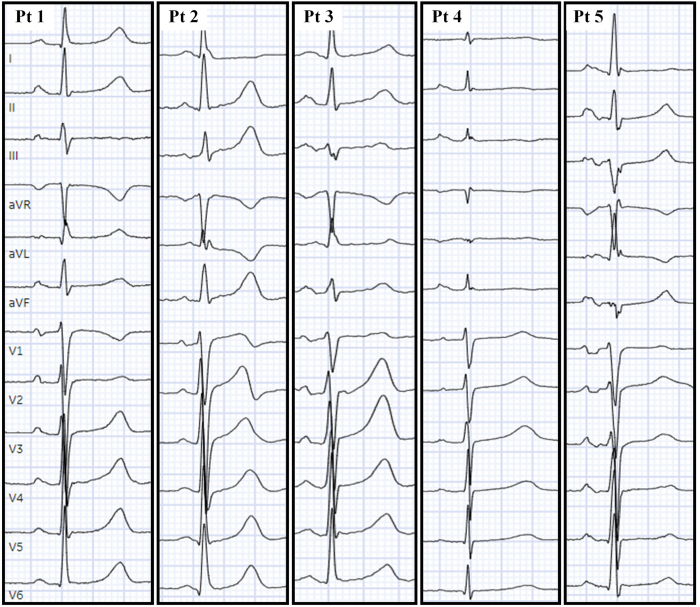
All patients are summarized in Table 1 and the 12-lead ECGs in sinus rhythm are displayed in Figure 1.
Table 1.
Patient details
| Patient no./ sex/age (y) | LQTS variant and ACMG classification | Clinical characteristics | Time from AF diagnosis to index ablation (y) | TTE before index ablation (LAVI, mL/m2; LVEF, %; RVSP, mmHg) | Mean LAP at time of transseptal puncture (mm Hg) | Total no. of AF ablation procedures | Time from index ablation to last follow-up (y) | Free from AF at 12 mo | Case resolution at last follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1/female/72 | p.Pro448GInfs∗18-KCNQ1 Pathogenic |
BMI 20 HLD iCM HTN HLD T2DM No NMD |
0.3 | LAVI: 41 LVEF: 60 RA: normal RV: normal RVSP: 32 Bileaflet MVP Moderate MR |
6 | 1 | 1.6 | Yes |
Symptom free Maintained on nadolol 60 mg once daily |
| 2/male/48 | p.Ile235Asn-KCNQ1 Pathogenic |
BMI 28 HTN No NMD |
7.8 | LAVI: 54 LVEF: 60 RA: enlarged RV: normal RVSP: 37 Trivial MR |
11 | 2 | 8.9 | Yes |
Symptom free No AAD |
| 3/male/53 | p.Tyr111Cys-KCNQ1 Pathogenic |
BMI 28 HTN HLD T2DM No NMD |
9.6 | LAVI: 37 LVEF: 56 RA: normal RV: normal RVSP: 21 Trivial MR Trivial TR |
10 | 1 | 1.3 | Yes |
Symptom free Maintained on nadolol 20 mg twice per day |
| 4/male/61 | p.Arg555Cys-KCNQ1 Pathogenic |
BMI 26 No NMD |
1.2 | LAVI: 35 LVEF: 64 RA: normal RV: normal RVSP: 25 Trivial MR |
8 | 1 | 6.6 | Yes | Symptom free Maintained on nadolol 20 mg twice per day |
| 5/male/57 | p.Arg858His-CACNA1C Pathogenic |
BMI 37 NICM No NMD |
5.7 | LAVI: 45 LVEF: 40 RA: enlarged RV: mild-moderate Enlarged RVSP: 24 Mild MR |
19 | 3 | 1.7 | Yes | Symptom free No AAD |
AAD = antiarrhythmic drug; ACMG = American College of Medical Genetics and Genomics; AF = atrial fibrillation; BMI = body mass index; EF = ejection fraction; HLD = hyperlipidemia; HTN = hypertension; iCM = ischemic cardiomyopathy; LAP = left atrium pressure; LAVI = left atrium volume index; LQTS = long QT syndrome; LV = left ventricle; MR = mitral regurgitation; MVP = mitral valve prolapse; NMD = neuromuscular disease; RA = right atrium; RV = right ventricle; RVSP = right ventricular systolic pressure; T2DM = type 2 diabetes mellitus; TTE = transthoracic echocardiogram.
Figure 1.
The 12-lead electrocardiograms in sinus rhythm are illustrated with evidence of interatrial conduction delay in patient 5. Pt = patient.
Patient 1
Patient 1 was a 72-year-old woman with coronary artery disease and microvascular dysfunction who developed QT prolongation with ranolazine. This raised concern for LQTS, and subsequent genetic testing unearthed a pathogenic variant in KCNQ1-encoded Kv7.1 (p.Pro448GInfs∗18-KCNQ1). She was otherwise asymptomatic from an LQT1 perspective, but developed symptomatic paroxysmal AF 3.5 years after her LQTS diagnosis. She was maintained on metoprolol tartrate 50 mg twice per day because of chronic angina. Due to underlying structural heart disease precluding a class I AAD, she underwent catheter ablation. During electroanatomic mapping, her left atrial voltage was normal and she underwent pulmonary vein isolation (PVI) using radiofrequency ablation. She developed symptomatic atrial tachycardia 6 months after her procedure and underwent repeat ablation, during which her PVs were noted to have chronic isolation. Isoproterenol was used to identify extrapulmonary triggers and 3 atrial tachycardia foci were targeted from the left atrial appendage, coronary sinus, and posterior right atrium. On sheath removal, the patient had an R-on-T phenomenon with a short-coupled premature ventricular complex degenerating into ventricular fibrillation requiring defibrillation. It was felt that isoproterenol, which was not used in the index procedure, induced ischemia. She was transitioned to nadolol 40 mg once daily on discharge. During follow-up, she had ongoing dyspnea, which was concerning for angina, and suffered a witnessed ventricular fibrillation out-of-hospital cardiac arrest 43 days after her ablation, which required 2 defibrillation shocks before return of spontaneous circulation was achieved. Her coronary angiogram showed 80% mid left anterior descending artery and 99% distal left circumflex artery occlusions, for which she received drug-eluting stents. It was felt her cardiac arrests were a combination of coronary artery disease and LQT1 and, therefore, not a completely reversible cause. Given this, a secondary prevention dual-chamber implantable cardioverter defibrillator was implanted. After her 8-day hospitalization, she recovered her baseline health and was followed up for 1 year after her second ablation, with no recurrent episodes of atrial arrhythmias noted on interrogations of the implantable cardioverter defibrillator and is maintained on nadolol 60 mg once daily.
Patient 2
A 48-year-old man with asymptomatic LQT1 secondary to p.Ile235Asn-KCNQ1, diagnosed as part of LQTS family screening, developed paroxysmal AF at age 31 years, requiring multiple direct current (DC) cardioversions. He did not tolerate nadolol and developed recurrent, symptomatic episodes of AF despite trials of mexiletine 300 mg 3 times per day and flecainide 100 mg twice per day. A catheter ablation strategy was pursued, and he had normal left atrial (LA) voltage during mapping and underwent cryoballoon PVI. He was not on any AADs post procedure and remained symptom free for 3 years before developing AF recurrence. During repeat ablation, the left PVs had reconnected and were re-isolated using radiofrequency ablation. He developed recurrence 1 year after his second procedure and required DC cardioversion, but has remained symptom free for the past year and remains off any AADs and β-blockers.
Patient 3
A 53-year-old man with asymptomatic LQT1 secondary to p.Tyr111Cys-KCNQ1, diagnosed as part of LQTS family screening and treated with nadolol 20 mg twice per day, developed paroxysmal AF at age 39 years. He continued to develop symptomatic paroxysmal AF episodes requiring multiple DC cardioversions, despite β-blocker therapy. Due to his LQTS, no AADs were trialed and, after shared decision making, a catheter ablation rhythm control approach was chosen. During mapping, LA voltage was normal and radiofrequency PVI was performed. He has remained symptom and arrhythmia free as of his last follow-up 1.3 years post procedure.
Patient 4
A 61-year-old man developed paroxysmal AF at age 53 years, requiring multiple DC cardioversions. A rhythm control strategy of flecainide 50 mg twice per day was chosen, but he continued to develop symptomatic, paroxysmal AF episodes. After shared decision making, a catheter ablation rhythm control strategy was chosen. During mapping, LA voltage was normal and he underwent radiofrequency PVI. Post procedure, his AADs were discontinued. He developed symptomatic recurrence 4 years after ablation and resumed flecainide 50 mg twice per day. On heart rhythm follow-up 6.5 years after ablation for increasingly frequent recurrence, suspicion for LQTS was raised because of QT prolongation and characteristic T-wave morphology on ECG. This unearthed a pathogenic p.Arg555Cys-KCNQ1 genetic variant and nadolol 20 mg was added to his regimen. He remained otherwise asymptomatic from an LQT1 perspective and has since had excellent control of AF.
Patient 5
A 57-year-old man with asymptomatic LQT8 secondary to p.Arg858His-CACNA1C, diagnosed as part of LQTS family screening, developed symptomatic paroxysmal AF at age 49 years. He underwent 2 prior catheter ablations at an outside hospital before being referred to our institution. During the first ablation procedure, PVI was performed and he remained symptom free for 18 months before developing recurrent AF requiring multiple cardioversions. During his second procedure, 1 of the 4 PVs was reconnected and posterior wall isolation was performed. He continued to experience symptomatic AF episodes and, after shared decision making, a repeat catheter ablation approach was chosen at our institution. The PVs and posterior wall were found to be chronically isolated and a mitral isthmus line, superior vena cava isolation, and cavotricuspid isthmus ablation were performed. He was not maintained on any AADs due to his LQT8 and has remained arrhythmia and symptom free as of his last follow-up 1.7 years post procedure.
Discussion
In our series, patients 1 and 4 were index cases of LQTS, prompting cascade family screening and the remaining patients’ genetic variants were uncovered through cascade family genetic screening. Albeit rare, AF can occur in patients with LQTS with an estimated prevalence of 2%.2 Focusing on patients with LQTS treated with AF ablation in our study, we found patients with LQTS developed AF at a young age (ie, 49 years; interquartile range, 42–54 years), were commonly LQT1, had normal LA voltage during mapping, and catheter ablation was a reasonable rhythm control strategy. Although it has been reported previously that the QTc can increase significantly post PVI,4 we did not observe this phenomenon in these 5 patients (mean ± SD QTc pre-PVI: 479 ± 21 versus PVI post PVI: 479 ± 23; P = .99). If an ablation strategy is chosen, periprocedural anesthesia, sympathomimetics, antiemetics, and other QT-prolonging agents should be used cautiously and QT should be monitored. The mechanism of AF in LQTS remains unclear, although it has been postulated that abnormalities in atrial myocyte refractoriness leading to prolonged atrial action potentials and effective refractory period durations render the atria susceptible to early afterdepolarizations, triggering atrial arrhythmias referred to as “atrial torsades de pointes.”5 In our study, patients had normal LA voltage during electroanatomic mapping without significant areas of fractionated atrial electrograms, but most patients (4 of 5) had abnormal P waves during sinus rhythm, suggestive of atrial myopathy driving AF (Figure 1).
Conclusion
Catheter ablation of AF in patients with LQTS is effective and safe, although redo procedures may be required.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Enriquez A., Antzelevitch C., Bismah V., Baranchuk A. Atrial fibrillation in inherited cardiac channelopathies: from mechanisms to management. Heart Rhythm. 2016;13:1878–1884. doi: 10.1016/j.hrthm.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Johnson J.N., Tester D.J., Perry J., Salisbury B.A., Reed C.R., Ackerman M.J. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm. 2008;5:704–709. doi: 10.1016/j.hrthm.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priori S.G., Wilde A.A., Horie M., et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen D.D., Akoum N., Hourmozdi J., et al. Catheter ablation of atrial fibrillation results in significant QTc prolongation in the postoperative period. Heart Rhythm O2. 2021;2:500–510. doi: 10.1016/j.hroo.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchhof P., Eckardt L., Franz M.R., et al. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1027–1033. doi: 10.1046/j.1540-8167.2003.03165.x. [DOI] [PubMed] [Google Scholar]