Key Teaching Points.
-
•
Interatrial septum lead implantation with a catheter-based system is an alternative to stylet-based systems in challenging implantation cases.
-
•
Past clinical history such as cardiac surgery, atrial arrhythmia, and cardiomyopathy might indicate potentially challenging cases.
-
•
Preimplantation cardiac anatomical and electrophysiological imaging might be helpful when devising an implantation strategy.
Introduction
The right atrial appendage (RAA) using a stylet-based system is a conventional atrial lead fixation site during the implantation of transvenous cardiac implantable electrical devices. However, implantation in the RAA is occasionally unsuccessful owing to the high pacing threshold or anatomical difficulty, with the interatrial septum (IAS) being an alternative site.1,2 Consequently, IAS implantation using a conventional stylet-based system may occasionally be challenging. Recently, attention has been drawn to right ventricular (RV) lead placement into the interventricular septum (IVS) using a guiding catheter–based system.3,4 The SelectSecure C315 guiding catheter–based system (Medtronic, Minneapolis, MN) has a lineup for IVS and IAS implantation (Supplemental Figure 1). The usefulness of catheter-based IAS implantation has also been reported recently.5 Therefore, this study presents a series of challenging implantation cases in which successful atrial lead implantation into the IAS was achieved using a catheter-based system.
Case report
Case 1
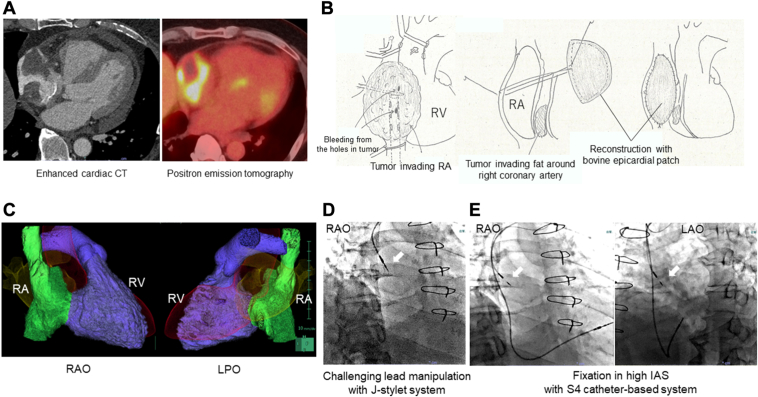
A 52-year-old man with a cardiac tumor was referred to our hospital. Enhanced cardiac computed tomography (CCT) and positron emission tomography results showed that the tumor had invaded the RA free wall (FW) and RAA (Figure 1A). The RAFW and RAA were resected and reconstructed using a bovine pericardial patch to diagnose and reduce the volume of the tumor (Figure 1B). The patient developed symptomatic sick sinus syndrome (SSS) postoperatively and required pacemaker implantation (PMI). The CCT revealed that the reconstructed RA cavity was deformed and narrow, suggesting an unusual atrial lead manipulation (Figure 1C). First, we attempted to implant the screw-in lead (5076 CapSureFix Novus, 58 cm; Medtronic) into the IAS using J-shaped and operator-shaped stylets; however, the lead could not be bent with the stylet owing to a lack of sufficient space (Figure 1D). Consequently, we abandoned the stylet-based implantation method and reused the 5076 lead as the RV lead. Furthermore, we performed a catheter-based IAS implantation using the S4 catheter for the small RA chamber. The S4 catheter tip easily touched the high IAS, and the lead (3830 SelectSecure MRI SureScan, 59 cm; Medtronic) was successfully fixed (Figure 1E). The pacing threshold was 0.5 V with a pulse width (PW) of 0.4 ms and an impedance of 1197 Ω. Chemotherapy was initiated after DDD-PM administration (Azure XT; Medtronic). Seven months after PMI, the pacing threshold was 0.625 V with a PW of 0.4 ms and an impedance of 855 Ω.
Figure 1.
Case 1 with right atrial deformation after surgical resection and reconstruction owing to cardiac tumor. A: Enhanced cardiac computed tomography (CCT) and positron emission tomography (PET) exhibiting a cardiac tumor invading the right atrial (RA) wall. B: RA resection and bovine epicardial patch reconstruction. RV = right ventricle. C: Morphology of the reconstructed RA chamber using enhanced CCT. RAO = right anterior oblique; LPO = left posterior oblique. D: Challenging lead manipulation using the J-stylet system (RAO). White arrow: 5760 leads. E: Successful high interatrial septum (IAS) fixation with the S4 catheter-based system (RAO, left anterior oblique [LAO]). White arrow: 3830 lead.
Case 2
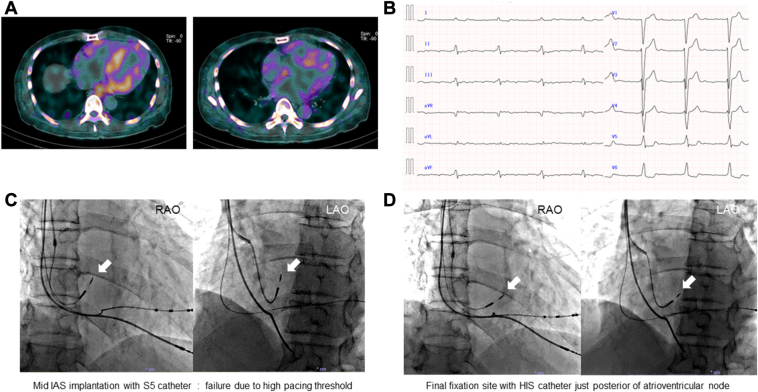
A 65-year-old man with a history of congestive heart failure with reduced left ventricular (LV) ejection fraction, left bundle branch block, LV hypertrophy, and carpal tunnel syndrome was admitted to our hospital. He was diagnosed with wild-type transthyretin amyloid cardiomyopathy. Cardiac 99mtechnecium-hydroxydimethylene diphosphonate (99mTc-HMDP) scintigraphy revealed uptake in the 4 cardiac chambers (Figure 2A). A 12-lead electrocardiogram revealed atrial flutter with low-voltage F wave (Figure 2B). Notably, extensive atrial myocardial damage was expected based on these findings. He developed symptomatic SSS after electrical cardioversion and was scheduled to undergo cardiac resynchronization therapy with pacing (CRT-P). The RV (5076 CapSureFix Novus, 58 cm; Medtronic) and LV (ASQ 4798, 68 cm; Medtronic) leads were implanted without complications. For the atrial lead fixation site, we targeted the IAS, where the voltage is reportedly preserved in the advanced scarred atrium.6 We searched for the mid-IAS with the S5 catheter (Figure 2C); however, the pacing threshold was >2.5 V at a PW of 0.4 ms throughout. Furthermore, we targeted the posterior atrioventricular node; however, the S5 tip did not reach the tricuspid valve annulus (TVA). Using the large-curve HIS catheter, we finally fixed the 3830 lead into the target site, where the pacing threshold was 1.8 V with a PW of 0.4 ms and an impedance of 418 Ω (Figure 2D). The far-field RV potential was small, and tafamidis was initiated after CRT-P (Percepta MRI Quad CRT-P SureScan; Medtronic) implantation. RA contraction severely deteriorated even under atrial pacing (Supplemental Figure 2). One year after PMI, the pacing threshold was 2.0 V with a PW of 0.4 ms and an impedance of 513 Ω.
Figure 2.
Case 2 with atrial myocardial damage owing to wild-type transthyretin amyloid cardiomyopathy. A: Cardiac 99mtechnetium-hydroxydimethylene diphosphonate scintigraphy. B: Twelve-lead electrocardiogram of low F-wave atrial flutter. C: Failure of mid-IAS fixation with the S5 was due to a high pacing threshold (RAO, LAO). White arrow: 3830 lead. D: The final fixation site using the HIS was located posterior to the compact atrioventricular node (RAO, LAO). White arrow: 3830 lead. Abbreviations as in Figure 1.
Case 3
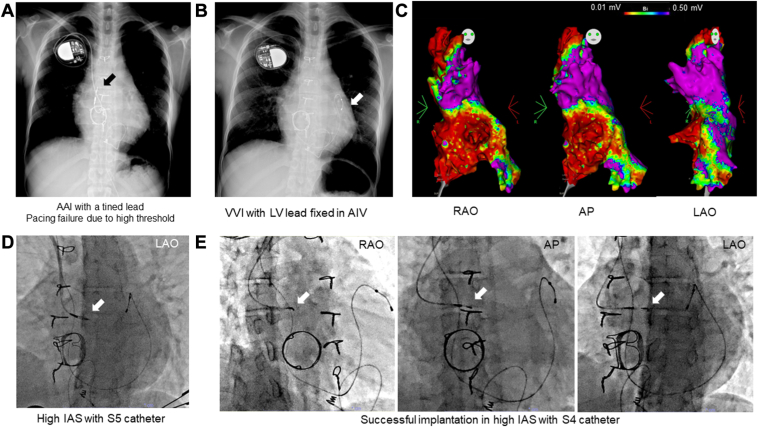
A 35-year-old woman with a history of VVI PMI presented with general fatigue owing to pacing failure. Two months after birth, she underwent ventricular septal defect closure and tricuspid valvuloplasty. At age 21 years, she underwent tricuspid valve replacement with a biological valve (CEP Magna 27 mm; Edwards Life Sciences, Irvine, CA) and maze procedure owing to worsening tricuspid regurgitation and supraventricular tachycardia refractory to multiple catheter ablations. An epicardial RV lead (5071; Medtronic) was implanted owing to symptomatic SSS; however, it did not function because of a high pacing threshold. A few days after the open heart surgery, she underwent transvenous AAI PMI using a tined lead with a stylet-based system; however, the pacing threshold increased immediately after implantation (Figure 3A). A VVI system with an LV lead (4196-78 cm; Medtronic) fixed to the anterior interventricular vein was implanted to avoid RV lead insertion through the prosthetic valve (Figure 3B). There were no appropriate coronary sinus branches other than the anterior interventricular vein (Supplemental Figure 3). The LV pacing threshold gradually increased over 10 years, and capture failure finally occurred. We discussed the possibility of atrial lead implantation because her atrioventricular conduction was normal before de novo PMI. We performed an electrophysiological study to assess the atrial lead implantation site (CARTO3; Biosense Webster, Diamond Bar, CA) 4 days before PMI (Figure 3C). The local voltage and pacing threshold in the high IAS group were acceptable. Therefore, catheter-based implantation was planned considering the extremely counterclockwise rotation and the previous history of unsuccessful stylet-based implantation. We first advanced the 3830 lead in the high IAS with the S5 catheter; however, the pacing threshold was high.
Figure 3.
Case 3 with adult congenital heart disease after unsuccessful atrial lead implantation with the stylet-based system. A: Unsuccessful AAI system with a stylet-based implantation. White arrow: Tined lead in the free wall of the right atrium. B: VVI system with left ventricular (LV) lead fixed to the anterior interventricular vein (AIV). White arrow: LV lead. C: Preimplantation voltage mapping of the RA chamber (right anterior oblique [RAO], anteroposterior [AP], and left anterior oblique [LAO]). Low-voltage area: <0.5 mV of bipolar voltage, scar: <0.01 mV. D: High IAS implantation with the S5 (LAO). White arrow: 3830 lead. E: Final fixation of the high IAS with the S4 (RAO, AP, LAO). White arrow: 3830 lead.
Furthermore, we tried the higher site using the S4 (Figure 3D). The pacing threshold was 1.0 V at 0.4 ms of PW, and the impedance was 817 Ω. To use atrial antitachycardia pacing for SVT, the RA and LV lead were connected to a DDD generator (Azure XT; Medtronic) with the AAI+ (Managed Ventricular Pacing [MVP 2.0]) mode. After 2 months, the pacing threshold was 1.0 V with 0.4 ms of PW and an impedance of 627 Ω.
Discussion
The current study presents 3 successful cases of atrial lead implantation in the IAS using a catheter-based system. Recently, the success rate of catheter-based implantation into the IAS was superior to that of the conventional stylet-based system for routine implantation.5 Our implantation method was as follows: (1) The catheter was inserted into the RV chamber. (2) The catheter was pulled with counterclockwise rotation torque using the right anterior oblique projection. (3) The catheter tip contact toward the IAS wall was confirmed using the left anterior oblique projection. If possible, the perpendicular contact was confirmed by injecting a contrast agent. (4) The tip of the 3830 lead was advanced outside the catheter, and the unipolar parameters were measured. (5) The lead body was rotated clockwise to fix into the IAS. During the rotation maneuver, another operator kept the catheter tip close to the IAS using a counterclockwise torque. (6) After fixation, the catheter tip was retracted until the ring electrode exited the catheter, and the bipolar parameter was measured.
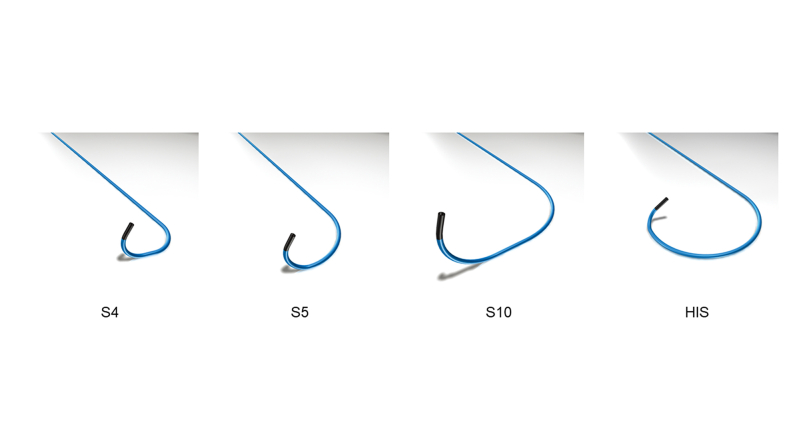
The catheter curve is crucial for delivering the tip toward the target site. A small-curve S4 or S5 is initially selected based on the RA chamber size. When the target site is adjacent to the TVA or the RA chamber is large, a large-curve S10 or HIS is recommended. To grasp the anatomical information, preimplantation imaging (eg, CCT and RA angiography) might be necessary. The tip of the C315 guiding catheter tends to head for the anterior IAS; therefore, the risk of lead perforation of thin fossa ovalis or posterior RA might not be high. However, the impedance change during the rotation of the 3830 lead should be closely monitored. This is because a major impedance drop could indicate helix perforation through the IAS.
In case 1, the implantation site was limited to the IAS because of the RAA resection and patch-reconstructed FW.7,8 The stylet-based lead manipulation was uncontrollable; however, the small-curve S4 catheter tip headed toward the high IAS wall and fixed easily. The backup of the guiding catheter by the SVC lateral wall also helped with the lead fixation. The catheter-based system may fit the deformed and extremely narrow atrium.
In case 2, atrial scarring was expected owing to wild-type transthyretin amyloid cardiomyopathy.9 Patients with cardiac amyloidosis often experience atrial tachyarrhythmia and bradyarrhytmia.10,11 Low-voltage F waves, SSS development, and 99mTc-HMDP uptake in the RA may indicate extensive atrial damage. In a previous report,6 scarring of the RA began at the lateral FW and gradually involved the IAS wall. However, the mid-IAS area adjacent to the compact atrioventricular node remained relatively healthy. The S4 and S5 could not reach the target site; however, the HIS successfully reached the site. A large-curve S10 and HIS, designed for IVS implantation, might be effective for IAS implantation adjacent to the TVA.
Case 3 involved a patient with adult congenital heart disease after repair surgery. The surgeons implanted a 5071 epicardial lead, a large-screw sutureless unipolar non–steroid-eluting lead, causing an immediate pacing threshold rise. A suture-on bipolar steroid-eluting lead might have been more appropriate. We screened the extremely counterclockwise-rotated RA chamber to explore the possibility of atrial lead implantation before the procedure. The stylet-based implantation was previously unsuccessful; however, catheter-based implantation was easily achieved. Therefore, identifying the target site using a preimplantation test should be done in difficult cases.
In this case series, we planned catheter-based implantation because each procedure was considered unusual based on the preimplantation information. For example, a history of surgical repair for adult congenital heart disease, SSS after catheter ablation/maze procedure for long-lasting AF,12 and amyloid cardiomyopathy might indicate potentially challenging cases. Imaging information such as low-voltage atrial excitation in 12-lead electrocardiogram, giant RA, and anatomical anomalies in CCT also help in decision-making. Additional preimplantation electrophysiological study13 is not an over-indication in extremely challenging cases.
We can select a catheter-based system as the bailout tool if we unexpectedly encounter challenging cases during stylet-based implantation. However, the catheter-based system for atrial leads was not available except for the Medtronic system. Therefore, we hope other vendors develop a catheter-based system for conventional screw-in lead or guiding catheters that can be combined with a 3830 lead regardless of the magnetic resonance safety issue. The spread of catheter-based systems would benefit patients requiring RA lead implantation.
Conclusion
This study presented 3 challenging cases of successful atrial lead implantation into the IAS using a catheter-based system. Therefore, catheter-based systems can be an alternative to stylet-based systems.
Disclosures
Medtronic gave T.O. a lecturing fee. Y.S. received scholarship funds from Medtronic. The remaining authors have no conflicts to disclose.
Acknowledgments
We thank Ai Kawamura, MD, PhD, for describing the surgery. We thank the medical engineers for their help with the PMI. The authors thank the radiologists for reconstructing the images. We thank Editage (www.editage.com) for English-language editing.
Funding Sources
This research received no specific grants from public, commercial, or not-for-profit funding agencies.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2024.07.016.
Appendix. Supplementary Data
Echocardiographic four-chamber view under atrial pacing after CRT-P implantation
Supplemental Figure 1.
The lineup of SelectSecure C315 guiding catheter-based system
Supplemental Figure 3.
The venography of coronary sinus during the lead implantation into an anterior interventricular vein (AIV)
References
- 1.Acosta H., Pothula V.R., Rodriguez M., Ramadas S., Castellanos A. Placement of a pacing lead at the inferior portion of the interatrial septum without special tools. Pacing Clin Electrophysiol. 2007;30:S84–S87. doi: 10.1111/j.1540-8159.2007.00612.x. [DOI] [PubMed] [Google Scholar]
- 2.Acosta H., Viafara L.M., Izquierdo D., et al. Atrial lead placement at the lower atrial septum: a potential strategy to reduce unnecessary right ventricular pacing. Europace. 2012;14:1311–1316. doi: 10.1093/europace/eus043. [DOI] [PubMed] [Google Scholar]
- 3.Naruse Y., Sano M., Kurebayashi N., et al. Usefulness of delivery catheter on accurate right ventricular septal pacing: Mt FUJI trial. Europace. 2023;25:1451–1457. doi: 10.1093/europace/euad027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyajima K., Urushida T., Naruse Y., et al. The usefulness of a delivery catheter system for right ventricular "true" septal pacing. Heart Vessels. 2021;36:1056–1063. doi: 10.1007/s00380-021-01780-8. [DOI] [PubMed] [Google Scholar]
- 5.Narumi T., Naruse Y., Miyajima K., et al. Actual conditions of atrial septal lead implantation and the factors related to successful implantation. J Cardiol. 2023;82:371–377. doi: 10.1016/j.jjcc.2023.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Nakazato Y., Nakata Y., Hisaoka T., et al. Clinical and electrophysiological characteristics of atrial standstill. Pacing Clin Electrophysiol. 1995;18:1244–1254. doi: 10.1111/j.1540-8159.1995.tb06964.x. [DOI] [PubMed] [Google Scholar]
- 7.Araki T., Namura M. Right atrial tumor and sick sinus syndrome. Intern Med. 2003;42:450–451. doi: 10.2169/internalmedicine.42.450. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor M., Barbero U., Kramer D.B., et al. Anatomic, histologic, and mechanical features of the right atrium: implications for leadless atrial pacemaker implantation. Europace. 2023;25 doi: 10.1093/europace/euad235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnellan E., Wazni O.M., Saliba W.I., et al. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am J Cardiol. 2020;128:140–146. doi: 10.1016/j.amjcard.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Gray L.W., Duca P.R., Chung E.K. Sick sinus syndrome due to cardiac amyloidosis. Cardiology. 1978;63:212–219. doi: 10.1159/000169900. [DOI] [PubMed] [Google Scholar]
- 11.Pattanshetty D.J., Bhat P.K., Chamberlain W.A., et al. Isolated cardiac involvement in primary amyloidosis: presenting as sick sinus syndrome and heart failure. Tex Heart Inst J. 2013;40:615–618. [PMC free article] [PubMed] [Google Scholar]
- 12.Bokeria L.A., Kulikov A.A. [Incidence of sinus node dysfunction in patients with long-standing, persistent atrial fibrillation who require simultaneous surgical correction of mitral and tricuspid valve defects and the "Maze IIIB" procedure] Kardiologiia. 2017;57:40–48. doi: 10.18087/cardio.2385. [Article in Russian] [DOI] [PubMed] [Google Scholar]
- 13.Bellmann B., Roser M., Muntean B., et al. Atrial standstill in sinus node disease due to extensive atrial fibrosis: impact on dual chamber pacemaker implantation. Europace. 2016;18:238–245. doi: 10.1093/europace/euv098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiographic four-chamber view under atrial pacing after CRT-P implantation