Key Teaching Points.
-
•
Cardiac resynchronization therapy (CRT) in patients with the persistent left superior vena cava (PLSVC) is feasible through various techniques and tools, including the use of different hooked catheters, sub-selectors, and snaring methods to deliver the coronary sinus lead.
-
•
The alpha loop technique can be utilized for placing the right ventricular lead through the PLSVC.
-
•
When traditional CRT implantation becomes difficult owing to anatomical constraints, conduction system pacing emerges as a viable alternative for achieving efficient cardiac resynchronization.
Introduction
Persistent left superior vena cava (PLSVC) is a rare congenital anomaly occurring in approximately 0.3%–0.5% of the general population.1 This anatomical variation is characterized by the sustained presence of the embryonic left superior cardinal vein throughout embryonic development, resulting in drainage into the right atrium (RA) via dilated coronary sinus (CS). It is usually a benign malformation and is often detected incidentally during invasive procedure or diagnostic imaging studies.2 In the context of cardiac implantable electronic devices, especially cardiac resynchronization therapy (CRT) device implantation encountering this entity, difficulties emerge in appropriately positioning leads, especially the right ventricular (RV) lead and the CS lead.3 Here we describe 3 cases of successful cardiac resynchronization in the presence of PLSVC that we encountered in the last year and the options that we opted for to achieve successful device implantation in every case. We present technical challenges encountered during device implantation and our approach to delineate the specific challenges faced, the diverse technical strategies applied, and the ultimate outcomes of each procedure.
Case report
Case 1
The first case involved a 64-year-old man with a diagnosed case of idiopathic dilated cardiomyopathy (DCMP) who presented with symptoms of recurrent heart failure, New York Heart Association (NYHA) class III, despite being on guideline-directed medical therapy (GDMT). The 12-lead electrocardiograms showed left bundle branch block (LBBB) with a QRS duration of 160 ms. Echocardiography revealed left ventricular (LV) end-diastolic volume of 344 mL, and the LV end-systolic volume was 268 mL with LV ejection fraction (LVEF) of 22%. Consequently, the decision was made to proceed with the implantation of a CRT-defibrillator (CRT-D) after stabilization of the patient. A bilateral venogram illustrated the coexistence of both the right superior vena cava (SVC) and PLSVC, without the presence of an interconnecting vein (Figure 1A and 1B, Supplemental Videos 1 and 2). The selective angiogram of the CS through the PLSVC revealed that the CS is dilated and the origin of the lateral vein exhibits an almost hairpin bend angle as it takes off from the CS (Figure 1C). Opting for implantation from the right side through the right SVC might look like a more straightforward approach in this case, but on the contrary, implanting defibrillators on the right side is associated with higher defibrillation thresholds compared to the left side, possibly because the defibrillating field is not as well distributed relative to the heart muscle.4 Therefore, a decision was made to prioritize the enhanced functionality of the implantable cardioverter-defibrillator (ICD) by opting for a left-side approach through the PLSVC.
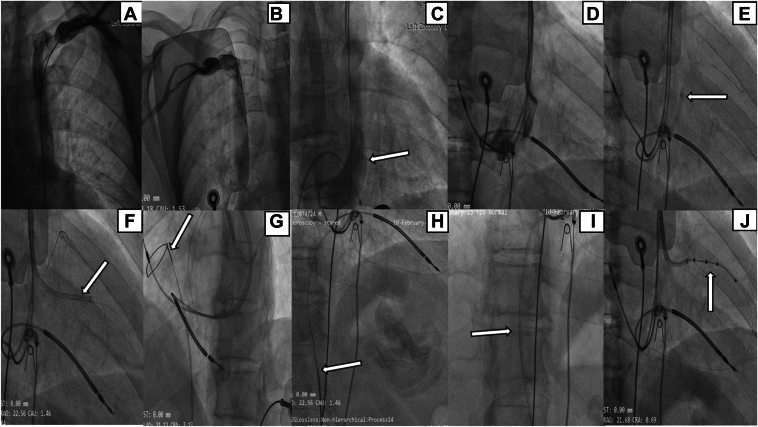
Figure 1.
Cardiac resynchronization therapy-defibrillator implantation procedure for case 1. A, B: Bilateral venograms depict the coexistence of both the right superior vena cava and persistent left superior vena cava, without the presence of an interconnecting vein. C: Dilated coronary sinus (CS) with the lateral vein originating at a reverse angle with the CS (arrow). D: Multiple unsuccessful attempts are made to engage the lateral vein using various catheters. E: Successful engagement of the lateral vein is achieved using an EBU guiding catheter (Medtronic, Minneapolis, MN) (arrow). F: A sub-selector (arrow) is employed to navigate the lead through the bend. G, H: The wire is snared through femoral vein (arrow). I: A coronary balloon (arrow) is inflated within the guide catheter to secure the wire and stabilize the system. J: Successful delivery of the quadripolar CS lead into the lateral vein.
In pursuit of implanting the CS lead, numerous endeavors were undertaken to engage the lateral vein through the PLSVC. As there was a superior take-off and sharp hairpin bend of the lateral vein, we accordingly tried using the Judkins right (JR) guiding catheter (Medtronic, Minneapolis, MN), internal mammary guiding catheter (Medtronic), and hockey stick guiding catheter (Boston Scientific, Marlborough, MA) (Figure1D) to reach near the origin of the lateral vein and then using a 0.014” hydrophilic guidewire (HI-TORQUE WHISPER ES Guide Wire; Abbott Vascular, Abbott Park, IL) to cross this bend, but we were unsuccessful in reaching near to and crossing the hairpin bend. Following several attempts, successful engagement of the lateral vein was achieved using a 6F extra back-up (EBU; Medtronic) guide catheter 3.5 (Figure 1E, Supplemental Video 3). The pivotal factor was the well-suited curvature of the EBU, aligning seamlessly with the superior origin of the lateral vein. A whisper wire was carefully advanced into the lateral vein, navigating its course deep into the RA through the pericardium. In anticipation of the challenge associated with delivering the CS lead through a reverse angle, a sub-selector (St Jude Medical CPS Aim SL inner catheter; Abbott) was used to negotiate and navigate the lead through the bend (Figure 1F). Despite the improved support provided by the deep whisper wire positioned in the RA and sub-selector sheath for the delivery of the CS lead (St Jude Medical Quartet 1458Q; Abbott), navigating the lead proved difficult and challenging owing to the presence of a reverse angle and hairpin bend. Therefore, the only viable option remaining was to enhance support by creating a venous-venous loop by snaring the whisper wire positioned in the RA through femoral vein.
We successfully snared the wire through femoral vein using a 10 mm endovascular snare (Merit Medical, South Jordan, UT) placed in the 6F JR guiding catheter (Figure 1G and 1H, Supplemental Video 4). While snaring the wire, we encountered a minor setback as the wire slipped midway from the snare. Fortunately, a significant portion of the wire was still inside the guiding catheter; hence, we inflated a 2.5 × 15 mm coronary angioplasty balloon (Sprinter; Medtronic) through the femoral vein, positioned near the tip of the guiding catheter at 14 atm to provide the desired support needed to deliver the CS lead (Figure 1I, Supplemental Video 5). Finally, we were able to successfully deliver the quadripolar CS lead into the lateral vein with acceptable electrical parameters (pacing threshold, 1.5 V @ 0.5 ms; pacing impedance, 760 Ω) (Figure 1J).
Subsequently the alpha-loop technique was used for the placement of the RV lead. We used a combination of J-shaped and U-shaped stylets, engaging in persistent manipulation and making ongoing adjustments to the stylet angle. Ultimately, we accomplished successful single-coil ICD lead (St Jude Medical Durata 7122Q 58 cm; Abbott) placement (Figure 1J), establishing a secure apical position with favorable electrical parameters (sensed R, 10.2 mV; pacing threshold, 0.9 V @ 0.4 ms; pacing impedance, 520 Ω). Thereafter, an active fixation RA lead (St Jude Medical Tendril STS, 52 cm; Abbott) was effortlessly positioned in the RA appendage. The procedural time was 188 minutes and fluoroscopy time was 66 minutes.
Case 2
The second patient was a 52-year-old diabetic woman with a diagnosed case of DCMP with severe LV dysfunction (LV end-diastolic function 318 mL, LV end-systolic function 240 mL, and LVEF 24%). The patient was symptomatic despite being on GDMT for heart failure (NYHA functional class III). Electrocardiogram revealed LBBB with a wide QRS complex (QRS width of 170 ms). She underwent a previous attempt of CRT device implantation at another tertiary care hospital. However, the procedure was abandoned owing to the presence of PLSVC and she was referred to our center for a second opinion. Given our prior experience in dealing with similar cases, we chose to review this particular case very carefully. We thoroughly discussed the potential for success and the associated risks and benefits, as well as the technical challenges, with the patient before proceeding with the procedure. In anticipation of potential technical challenges, we chose to do levo-phase coronary angiograms, bilateral venograms (Figure 2A and 2B), and a selective angiogram of the PLSVC (Figure 2C) for a detailed mapping of the CS and its tributaries. This comprehensive approach aimed to provide a thorough understanding of the anatomical considerations, assisting us in making well-informed decisions regarding the management of this specific case.
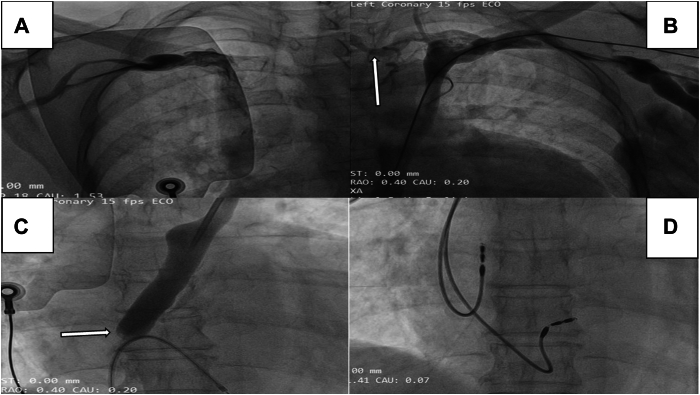
Figure 2.
Cardiac resynchronization procedure for case 2. A, B: Bilateral venogram revealing the presence of persistent left superior vena cava (PLSVC) and right superior vena cava connected by an interconnecting vein (arrow). C: Selective angiogram of the coronary sinus (CS) via the PLSVC reveals CS dilation and minimal contrast efflux, indicating severe stenosis of the CS ostium (arrow). D: Dual-chamber pacemaker with left bundle branch area pacing.
The selective angiogram of the CS through the PLSVC demonstrated that the CS appeared dilated, and there was negligible efflux of contrast from the CS, suggesting the presence of severely stenotic CS ostium (Figure 2C, Supplemental Video 6). There was reversal of contrast flow from the CS to the PLSVC draining into left subclavian vein (SCV). Eventually, the contrast drained into the right SVC through an interconnecting vein (Supplemental Video 7). We encountered an exceedingly rare condition known as CS ostial stenosis (CSOS) with PLSVC. In the presence of CSOS and acute angulation of the lateral vein from the CS, it was exceedingly difficult to negotiate the CS lead from either the right or left side. Moreover, the interconnecting vein was very tortuous (Figure 2B), thus increasing the length of the path if we opted to go through it. Such tortuosity of the interconnecting vein will become a hurdle in manipulating the hardware through it. In view of these limitations, we opted for conduction system pacing (CSP) as an alternative strategy from the right side. If we had chosen to implant the CRT device from the right side, we would have encountered a major obstacle in the form of a severely stenosed CS ostium. Overcoming this hurdle would have required us to navigate through it, necessitating the use of larger balloons to dilate it sequentially. Managing the acute angle of the lateral vein would have added to the complexity. Consequently, this approach would have not only prolonged the procedure time considerably but also increased the volume of contrast required.
The patient received a dual-chamber pacemaker, and the left bundle branch area was targeted for the implantation of the lead (Figure 2D). The stylet-driven extendable-helix lead Solia S60 (Biotronik, Berlin, Germany) was engaged at the septum of the RV through a specific designed sheath (Biotronik Selectra 3D) and subsequently guided to the left conduction system by rotating the lead approximately 6–7 times with guidance from fluoroscopy in the right anterior oblique view. The pacing threshold was recorded at 0.7 V with a pulse width of 0.4 ms; the R wave amplitude measured at 12 mV and pacing impedance, 580 Ω. The paced QRS duration exhibited a reduction with left bundle branch area pacing (LBBAP) at 124 ms and the LV activation time, defined as the time from stimulus to the peak of the R wave in the lateral precordial leads (V4–V6), was measured at 60 ms. Following these steps, we successfully positioned an active fixation RA lead (Biotronik Solia S53) into the RA appendage (Figure 2D).
Case 3
The patient was a 62-year-old man with a diagnosed case of DCMP, severe LV systolic dysfunction (LVEF = 25%), LBBB (QRS width = 160 ms), and NYHA class III symptoms despite being on GDMT. Consequently, it was decided to go for CRT-D implantation. Under the guidance of fluoroscopy, an attempt was made to puncture the left axillary vein, but the J-tip guide wire failed to advance into the right SVC. Subsequently, bilateral venography was performed, revealing the presence of an isolated PLSVC (Figure 3A, Supplemental Video 8). With an isolated PLSVC, neither a right-sided approach for CRT implantation nor CSP would be feasible. The sole viable option to resynchronize the heart is by implanting a CRT via the PLSVC, recognizing that it will pose a greater challenge for the placement of the RV lead. The difficulty stemmed from the sharp angle created by the convergence of the left SCV and the PLSVC. Additionally, the highly acute angle between the CS and the tricuspid valve–imposed limitations on the lead’s navigational flexibility.
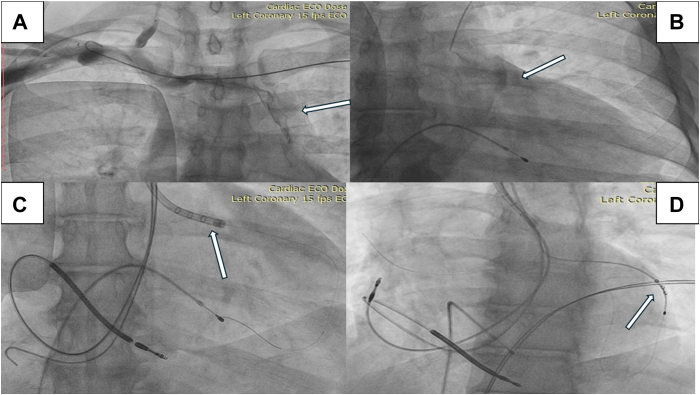
Figure 3.
Cardiac resynchronization therapy-defibrillator implantation procedure for case 3. A: Right subclavian venogram revealing the presence of an isolated persistent left superior vena cava (arrow). B: Selective angiogram of the coronary sinus (CS) showing CS dilation with appropriate origin of the lateral vein (arrow). C: Successful engagement of a lateral vein using an outer delivery sheath (arrow). D: A quadripolar CS lead is positioned (arrow).
A single-coil ICD lead (St Jude Medical Durata 7122Q 58 cm; Abbott) into the RA was facilitated using a straight stylet. Successful advancement to the RV apex was achieved through multiple attempts of manipulation, involving reshaping the stylet curves several times. Favorable electrical parameters were observed, including a pacing threshold of 0.9 V @ 0.5 ms, a pacing impedance of 630 Ω, and sensed R wave at 10.1 mV. Through the PLSVC, CS angiography disclosed an appropriate lateral vein (Figure 3B). A successful engagement of a lateral vein of the CS was achieved using an outer delivery sheath (St Jude Medical CPS 115 slittable outer guide catheter; Abbott) (Figure 3C) in conjunction with an acute angle sub-selector sheath (St Jude Medical 90° CPS Aim SL inner catheter; Abbott). Subsequently, a quadripolar CS lead (St Jude Medical Quartet 1458Q; Abbott) was positioned (Figure 3D, Supplemental Video 9), yielding acceptable lead parameters (pacing threshold, 1.3 V @ 0.5 ms; pacing impedance, 800 Ω). Thereafter, an active fixation RA lead (St Jude Medical Tendril STS, 52 cm; Abbott) was positioned in the RA appendage. The procedural time was 140 minutes and the fluoroscopic time was 44 minutes.
Discussion
In this case series, we present 3 noteworthy cases involving individuals with a PLSVC, wherein successful cardiac resynchronization was achieved. All 3 cases in our study met the criteria for a Class I recommendation for CRT implantation according to the current guidelines.5 The cases presented in our case series exemplify the diverse strategies employed to attain successful cardiac resynchronization procedures. Each case required a tailored approach, showcasing the importance of individualized strategies in the context of unique anatomical challenges. In 2 of the reported cases (cases 1 and 3), successful implantation of CRT-D was achieved. However, in case 2, owing to anatomical challenges rendering the CS and its tributaries inaccessible, an alternative approach was taken. In this instance, LBBAP was pursued to optimize ventricular resynchronization.
According to Schummer’s classification of SVC variations,6 type I represents the normal variant, while type II is characterized by the presence of only PLSVC, termed isolated PLSVC. In types IIIa and IIIb, both left and right SVC exist, with the former having a interconnecting vein between them and the latter lacking this bridging vein. This case series illustrates the entire range of abnormal variants of the SVC that we encountered. Case 1 was classified as type IIIb while cases 2 and 3 were distinguished as type IIIa and type II category, respectively.
Positioning the LV lead poses a notable challenge, particularly in cases where the CS is significantly dilated, compounded by the acute or at times 180-degree turn of branches arising from the CS. In such scenarios, assistance is often sought through specialized tools such as angled sub-selectors or hook catheters. In our first case, we employed an EBU catheter to successfully access the target lateral vein, leveraging the well-suited curvature of the EBU to harmoniously align with its superior origin. Subsequently, we heightened support with a sub-selector to aid in the smooth delivery of the CS lead. In this case, we successfully enhanced the support for the CS lead by allowing a snaring of the 0.014" wire that was initially placed in the RA through the CS. This strategic approach proves especially beneficial when faced with challenges such as the reverse angle of the target vein’s origin and the presence of a band in its course. Use of snaring not only adds maneuverability but also provides crucial support, contributing to the successful navigation of anatomically complex scenarios during the CS lead delivery.
In the second case, we dealt with the extremely rare entity CSOS and PLSVC. Our attempts to access the posterolateral vein or the lateral vein of the CS from the PLSVC were hindered by 2 primary factors. Firstly, the ostium of the CS was severely stenotic, compounded by the tortuosity of the interconnecting vein. Even attempting the procedure from the right side would have posed difficulties Secondly, the origins of the lateral vein or posterolateral vein exhibited a reversed angle in relation to the PLSVC, further complicating the scenario. Opting for CRT-D implantation from either side would have needlessly prolonged the procedural time and increased the risk of contrast-induced complications. Given the constrained possibilities for CRT implantation, we opted for an alternative approach and decided to proceed with CSP as a bail-out strategy.
Another significant challenge that we encountered was the delivery of the ICD lead into the RV, notably owing to the acute angle between the CS and the tricuspid valve. The complexity was further heightened by the inherent heaviness of the ICD lead profile, making the manipulation of this robust lead a notable and intricate aspect of the procedure. Our procedural approach commenced with the use of a straight stylet to guide the lead into the RA. Subsequently, we tailored variations in stylet curves (pigtail, J, or L type) to accommodate the specific angle and anatomy, which naturally varies slightly from patient to patient. A pivotal aspect of our strategy involved manipulating the stylet, rotating it to orient the lead tip toward the tricuspid valve. With a slight withdrawal, we successfully crossed the tricuspid valve, fixing the stylet in place to facilitate the advancement of the lead (Alpha loop technique).
We advocate for a routine subclavian venogram at the outset of the procedure. In cases where there is suspicion of PLSVC, we recommend performing bilateral venography. This comprehensive imaging approach is instrumental in enhancing the delineation of venous anatomy, providing valuable insights that contribute to informed decision-making throughout the course of the procedure. The implantation of CRT in PLSVC demands an experienced operator and is associated with longer procedural times. Despite the inherent difficulty in lead implantation through the PLSVC, our experience with 2 cases demonstrates that this challenge does not preclude the successful implantation of CRT via the PLSVC. Our strategy encompassed using inner sub-selectors and hooked catheters to effectively engage the target vein. In instances where additional support is required, we advocate the snaring method to enhance wire support, facilitating improved delivery of the CS lead. This holistic approach allows a more adaptable and successful implantation process in cases of PLSVC. When CS cannulation proves unfeasible, CSP emerges as a practical alternative, exemplified in our second case (CSOS with PLSVC). This method provides an alternative avenue for achieving effective cardiac resynchronization in scenarios where traditional CS lead placement poses challenges or is not viable. In our case series, the implantation procedures were executed without encountering any complications. Subsequently, throughout the follow-up period, all lead parameters remained within acceptable and stable ranges.
Conclusion
PLSVC can pose technical challenges during CRT implantation. However, as operator experience grows along with diverse maneuvers and use of appropriate tools, successful CRT implantation becomes achievable in these scenarios. In situations where anatomy makes traditional CRT implantation unfeasible, CSP stands out as a practical alternative for achieving effective cardiac resynchronization.
Disclosures
There are no conflicts of interest to report.
Acknowledgments
Funding Sources
There are no sources of funding to declare.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2024.07.009.
Appendix. Supplementary Data
A left subclavian venogram shows the presence PLSVC without any interconnecting vein to right SVC.
A right subclavian venogram shows the right SVC is draining through its normal course.
Successful engagement of the lateral vein achieved using a 6 Fr extra back-up catheter.
The wire is successfully captured through the femoral vein with a 10 mm endovascular snare positioned in the 6Fr JR guiding catheter.
A coronary angioplasty balloon is advanced through the femoral vein and inflated near the tip of the guiding catheter to provide the necessary support for delivering the CS lead.
The selective angiogram of the CS via the PLSVC shows a dilated CS with minimal contrast efflux, indicating a severely stenotic CS ostium.
Contrast reverses from the CS to the persistent left PLSVC, then drains via the left SCV into the right SVC through an interconnecting vein.
Bilateral venography reveals the presence of an isolated PLSVC.
A quadripolar CS lead is positioned in the lateral vein of the CS.
References
- 1.Azizova A., Onder O., Arslan S., Ardali S., Hazirolan T. Persistent left superior vena cava: clinical importance and differential diagnoses. Insights Imaging. 2020;11:110. doi: 10.1186/s13244-020-00906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dave V., Sesham K., Mehra S., Roy T.S., Ahuja M.S. Persistent left superior vena cava: an anatomical variation. Med J Armed Forces India. 2022;78:S277–S281. doi: 10.1016/j.mjafi.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bera D., Bachani N., Pawar P., Rathi C., Ramalingam V., Lokhandwala Y. Cardiac resynchronization therapy device implantation in a patient with persistent left superior vena cava without communicating innominate vein - should we proceed from the same side? - A dilemma revisited. Indian Pacing Electrophysiol J. 2018;18:112–114. doi: 10.1016/j.ipej.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman P.A., Rasmussen M.J., Grice S., Trusty J., Glikson M., Stanton M.S. Defibrillation thresholds are increased by right-sided implantation of totally transvenous implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 1999;22:1186–1192. doi: 10.1111/j.1540-8159.1999.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 5.Glikson M., Nielsen J.C., Kronborg M.B., et al. ESC Scientific Document Group 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 6.Schummer W., Schummer C., Fröber R. Persistent left superior vena cava and central venous catheter position: clinical impact illustrated by four cases. Surg Radiol Anat. 2003;25:315–321. doi: 10.1007/s00276-003-0138-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A left subclavian venogram shows the presence PLSVC without any interconnecting vein to right SVC.
A right subclavian venogram shows the right SVC is draining through its normal course.
Successful engagement of the lateral vein achieved using a 6 Fr extra back-up catheter.
The wire is successfully captured through the femoral vein with a 10 mm endovascular snare positioned in the 6Fr JR guiding catheter.
A coronary angioplasty balloon is advanced through the femoral vein and inflated near the tip of the guiding catheter to provide the necessary support for delivering the CS lead.
The selective angiogram of the CS via the PLSVC shows a dilated CS with minimal contrast efflux, indicating a severely stenotic CS ostium.
Contrast reverses from the CS to the persistent left PLSVC, then drains via the left SCV into the right SVC through an interconnecting vein.
Bilateral venography reveals the presence of an isolated PLSVC.
A quadripolar CS lead is positioned in the lateral vein of the CS.