Key Teaching Points.
-
•
Left bundle branch area pacing (LBBAP) is typically guided by the right anterior oblique (RAO) 30° view, but due to individual differences in the angle of the ventricular septum, this standard Working View does not guarantee success, particularly for less experienced operators. Establishing a Working View that considers the patient's unique ventricular septal angle can facilitate a more operator- and patient-independent approach, potentially increasing the procedure's reproducibility.
-
•
The individualized RAO (i-RAO) method uses the RAO angle perpendicular to the ventricular septum's angle as the Working View. This allows the delivery sheath's tip to form a perfect circle and the lead to be placed perpendicularly to the septum.
-
•
The i-RAO method simplifies the procedure, shortens the time, and eliminates the need for computed tomography, potentially reducing the number of attempts by reaching the left ventricular subendocardium via the shortest distance.
Introduction
Left bundle branch area pacing (LBBAP) has been gaining rapid attention for its effectiveness in recent years. According to the MELOS study,1 a high success rate for the procedure has been reported; however, these data are limited to experienced operators, and the results of operators with less experience have not been fully examined. It has also been shown that there is a learning curve for the initial cases, suggesting that the establishment of implant techniques that do not depend on the operator’s experience is necessary for a widespread adoption.
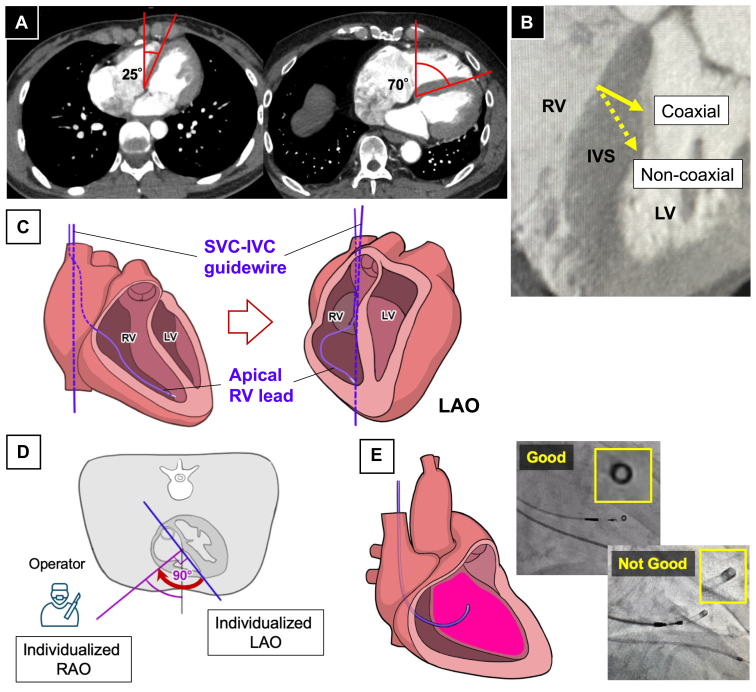
In LBBAP, several implantation techniques to determine the lead position include a method advancing 1–1.5 cm in the apical direction using the His bundle as a landmark,2 a method using the tricuspid valve annulus as a landmark under fluoroscopic guidance,3 and a method that divides the ventricular shadow into nine parts.4 However, all these methods assume that the procedure is performed in the right anterior oblique (RAO) 30° view. However, there are variations in the rotation of the patient’s heart (Figure 1A), and the techniques that fix the working view in the RAO 30° view can have a low reproducibility depending on the patient. This can be a contributing factor to unsuccessful outcomes, especially for operators with less experience.
Figure 1.
A: There is variability in the degree of the cardiac rotation. B: Compared with the coaxial solid line, the noncoaxial dashed line illustrates a longer distance to the left ventricular endocardium. C: Images for determining the iLAO angle using a fluoroscopic image. D: The iLAO view is the angle between the blue and midline and the iRAO view, defined by the midline, and purple line, is the blue line rotated 90° counterclockwise. E: Fluoroscopic image with the delivery catheter positioned on the ventricular septum. The goal is to position the tip of the catheter to form a perfect circle. The upper figure achieves a nearly perfect circle of 1.02. The lower figure does not form a perfect circle, and its roundness is 0.65. iLAO = individualized left anterior oblique; iRAO = individualized right anterior oblique; IVS = interventricular septum; LV = left ventricular; RV = right ventricular.
As reported in the MELOS study,1 many failed LBBAP cases are due to the inability to perform a deep screw-in into the ventricular septum. Although this can be influenced by myocardial characteristics such as fibrosis, the direction in which the lead is advanced can also play a role. As shown in Figure 1B, when the lead is placed coaxially with respect to the ventricular septum, the distance required to reach the left ventricular endocardium is shorter compared with a non-coaxial placement. Therefore, placing the lead coaxially may lead to an improved success rate. In this report, we examined a method to determine the working view, taking into account the individual septa.
Methods
The most convenient method for confirming the angle of an individual ventricular septum is contrast-enhanced computed tomography (CT). However, contrast-enhanced CT is not practical for all cases preoperatively because of concerns about renal function impairment, contrast allergies, and cost. In this report, we used the individualized left anterior oblique (iLAO) method, which was previously reported by Squara and colleagues5 to determine the angle of the septum. This method defines the iLAO through three steps. First, after successful venous access, a standard 0.35-mm guidewire is inserted from the superior vena cava (SVC) into the inferior vena cava (IVC) and is referred to as the SVC-IVC guidewire. Next, the right ventricular (RV) lead is inserted through the tricuspid valve into the RV apex. Finally, as shown in Figure 1C, the LAO angle is progressively adjusted in 5° increments until the SVC-IVC guidewire perfectly overlaps with the tip of the RV lead. The LAO angle at this point is defined as the iLAO. Subsequent reports have indicated that the iLAO correlates with the angle formed by the line connecting the membranous septum to the apical part of the ventricular septum on CT.6
However, it has been reported that more than half of the patients exceed an LAO 60° view with the iLAO, which can be an obstacle to the procedure when using the iLAO as the working view. Therefore, we defined the angle rotated 90° counterclockwise from the iLAO as the individualized right anterior oblique (iRAO), and we used this view as the working view for the implantation (Figure 1D).
Since the iRAO represents the angle at which the operator faces the ventricular septum, adjusting the delivery catheter so that the tip of the catheter forms a perfect circle (circle sign) becomes a landmark indicating that it is theoretically touching the septum perpendicularly (Figure 1E). We named this method of screwing the iRAO method.
We used the roundness [(4 × Area) / (π × Major Axis2)] as an objective measure to indicate how close the circle sign was to a perfect circle. This measure indicated that values closer to 1 corresponded to a shape that was more nearly a perfect circle. We will introduce two cases with significantly different heart rotations using the iRAO method.
Case 1
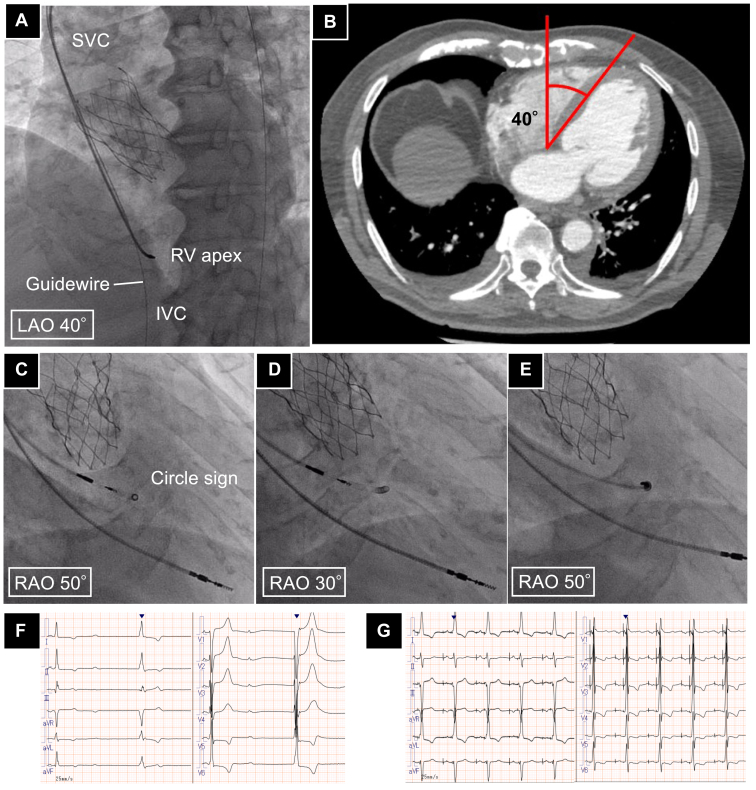
An 81-year-old male patient with a history of a transcatheter aortic valve implantation for severe aortic stenosis presented to our hospital with syncope and complete atrioventricular block. A twelve electrocardiogram revealed a junctional rhythm at 40 bpm, prompting a permanent pacemaker implantation for LBBAP. The patient underwent a puncture from the left subclavian vein, and firstly, an atrial lead (CapSureFix, Medtronic, Minneapolis, MN) was placed in the right ventricular apex as a landmark to determine the iLAO. As a result, the iLAO in this case was determined to be LAO 40° (Figure 2A), and from there, RAO 50° was decided as the iRAO by rotating 90° counterclockwise. The iLAO matched the angle of the septum measured with contrast-enhanced CT before the transcatheter aortic valve implantation operation (Figure 2B). It took 1 minute and 43 seconds to determine the iRAO from the placement of the reference ventricular lead. Subsequently, a C315HIS delivery catheter (Medtronic, Minneapolis, MN) was inserted into the right ventricular outflow tract, and the catheter was adjusted so that the tip formed a circle sign (Figure 2C). At that time, the roundness was 0.98. Advancing the lead in that location and performing pace mapping exhibited a (+/–) in lead II, (–) in lead III, and a ‘w’ pattern in lead V1. In an RAO 30° view, the catheter tip was confirmed not to have formed a perfect circle (roundness 0.46; Figure 2D). While maintaining the circle sign, the 3830 lead (Medtronic, Minneapolis, MN) was screwed in coaxially with the catheter (Figure 2E). Next, we proceeded while verifying the lead depth in LAO 20° and performed continuous unipolar pacing, which exhibited a V6 R wave peak time (RWPT) of 76 ms and V6-V1 interpeak interval of 43 ms, suggesting left bundle capture. The threshold was 0.5 V/0.4 ms, and the lead placement in the left bundle branch area was successful with a single screw procedure. The QRS duration during bipolar pacing improved from 144 ms preoperatively (Figure 2F) to 134 ms postoperatively (Figure 2G). Three months after the procedure, the thresholds and QRS width have remained stable without any significant change.
Figure 2.
A: The angle of the ventricular septum measured with computed tomography is 40°. B: Fluoroscopic image used to determine the iLAO view. The guidewire dropped from the superior vena cava into the inferior vena cava and the ventricular lead placed at the right ventricular apex was aligned by rotating it to an LAO position. In this case, the iLAO is 40°. C: Fluoroscopic image of the iRAO view for this case, with the catheter tip positioned to form a perfect circle, achieving a roundness of 0.98. D: Fluoroscopic image in the standard RAO view, showing the loss of circularity at the catheter tip as compared with the iRAO view, with a roundness of 0.46. E: Fluoroscopic image showing the lead coaxially screwed in. F: Preoperative 12-lead ECG. G: Postoperative 12-lead ECG. ECG = electrocardiogram; iLAO; individualized left anterior oblique; iRAO = individualized right anterior oblique; IVS = interventricular septum; LV = left ventricle; RV = right ventricle; SVC = superior vena cava.
Case 2
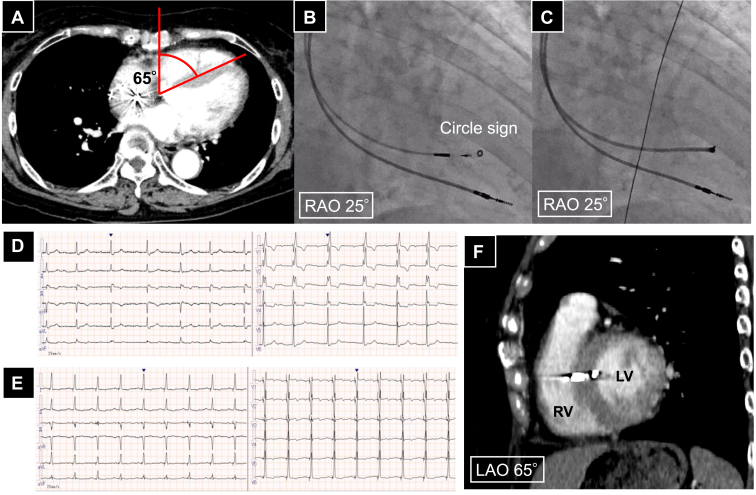
A 72-year-old female patient presented to our hospital with syncope, exhibiting complete right bundle branch block, a heart rate of 53 bpm, and a prolonged PR interval of 351 ms. Holter monitoring revealed intermittent complete atrioventricular block. Consequently, a permanent pacemaker was implanted, and LBBAP was performed. The iLAO was measured using the same method as the previous case and was 65° and the iRAO was 25°, and it took 1 minute and 58 seconds. Right atrial and right ventricular angiography were performed through a C315HIS catheter, and the lead placement position was determined with the tip of the tricuspid valve annulus as a landmark. The catheter was adjusted to create a circle sign (roundness 0.90; Figure 3B), and the 3830 lead was screwed in coaxially (Figure 3C). Upon advancing the lead and performing unipolar pacing, a V6 R wave peak time of 65 ms and V6-V1 interpeak interval of 46 ms were observed, suggesting left bundle branch capture. The procedure was successful on the first attempt. The QRS duration during bipolar pacing improved from 146 ms preoperatively (Figure 3D) to 124 ms postoperatively (Figure 3E). Contrast-enhanced CT for a different reason postoperatively confirmed that the lead was placed orthogonally to the ventricular septum at LAO 65° (Figure 3F).
Figure 3.
A: The angle of the ventricular septum measured with CT is 65°. B: Fluoroscopic image of the iRAO view for this case, with the catheter tip positioned to form a perfect circle, achieving a circularity of 0.90. C: Fluoroscopic image showing the lead coaxially screwed in. D: Preoperative 12-lead ECG. E: Postoperative 12-lead ECG. F: Postoperative contrast-enhanced CT image in the individualized LAO view in this case, showing the ventricular lead placed perpendicular to the ventricular septum. CT = computed tomography; ECG = electrocardiogram; LAO = left anterior oblique; LV = left ventricle; RAO = right anterior oblique; RV = right ventricle.
Discussion
We experienced successful LBBAP with a single screw insertion in two patients with markedly different cardiac rotations using a similar approach. There were two merits of this technique. The first was that it could be performed with a similar approach regardless of the heart’s rotation or the operator. Patients who are good candidates for LBBAP often include younger patients with a standing heart or patients with a horizontal rotation heart owing to heart failure. One of the reasons for unsuccessful LBBAP is that the lead tip is not directed toward the left ventricle. Experienced operators can assess this from the angle of the lead and the pacing waveform, but it can be difficult for operators with less experience. The iRAO method can create a view that is orthogonal to the septum based on the angle of the individual septum, making it theoretically possible to touch the septum perpendicularly by forming a perfect circle with the tip of the catheter. Advancing the lead coaxially with the catheter ensures that the lead is reliably directed toward the left ventricle, which could serve as a useful marker for less experienced operators. The second merit was the potential to correctly interpret the electrophysiological information by being aware of the heart’s rotation. As mentioned in the EHRA consensus statement,7 determining whether LBBAP is being performed can be difficult based on a single parameter, and a comprehensive judgment is necessary. This is also because the rotation of the patient’s heart varies in each case. The V6 RWPT is the simplest method to use, as it evaluates the electrical excitation time to the lateral LV. However, in patients with a significant clockwise rotation of the heart, the V6 lead does not provide true lateral information, and the V6 RWPT may be overestimated. The iRAO method allows for an evaluation of the heart’s rotation, enabling more accurate electrophysiologic assessments.
On the other hand, there were several limitations to the iRAO method. The first was that it depended on the placement position of a reference lead in the right ventricular apex. To obtain the correct iLAO, the reference lead must be correctly placed toward the right ventricular apex, but sometimes it is placed on the lateral free wall. This can be noticed when the lead tip does not face the septum as with an LAO rotation, but it requires careful attention. If a contrast-enhanced CT scan is available, it can also serve as an alternative method to measure the iLAO. The second was that it did not consider the cranial-caudal direction. Considering this aspect could enable a technique that would be more independent of the patient’s anatomy, but it would be a trade-off with the complexity of the procedure. This point requires further investigation. The third was that this was just a case report. Further study is needed, and comparisons with cases fixed in an RAO 30° view should be conducted in the future.
Conclusion
The iRAO method has the potential to enable LBBAP implantations to be performed reproducibly, regardless of the patient’s heart rotation or the operator’s experience.
Disclosures
The authors have no conflicts to disclose.
Acknowledgments
We are grateful to John Martin for his linguistic assistance.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
KO devised the study’s design and concepts. KO analyzed and interpreted the data. KO and KY drafted the manuscript. DK, YM, TN, and KS critically revised the manuscript for its important intellectual content.
Patient Consent
All patients provided written informed consent.
References
- 1.Jastrzebski M., Kielbasa G., Cano O., et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;43:4161–4173. doi: 10.1093/eurheartj/ehac445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang W., Chen X., Su L., Wu S., Xia X., Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791–1796. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Liu X., Niu H.X., Gu M., et al. Contrast-enhanced image-guided lead deployment for left bundle branch pacing. Heart Rhythm. 2021;18:1318–1325. doi: 10.1016/j.hrthm.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Wang Z., Zu L., et al. Simplifying physiological left bundle branch area pacing using a new nine-partition method. Can J Cardiol. 2021;37:329–338. doi: 10.1016/j.cjca.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Squara F., Scarlatti D., Riccini P., Garret G., Moceri P., Ferrari E. Individualized left anterior oblique projection: a highly reliable patient-tailored fluoroscopy criterion for right ventricular lead positioning. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.006107. [DOI] [PubMed] [Google Scholar]
- 6.Squara F., Fourrier E., Diascorn Y., et al. Cardiac anatomical axes by CT scan and confirmation of the accuracy of fluoroscopic individualized left anterior oblique projection for right ventricular lead implantation. J Interv Card Electrophysiol. 2021;60:213–219. doi: 10.1007/s10840-020-00729-7. [DOI] [PubMed] [Google Scholar]
- 7.Burri H., Jastrzebski M., Cano O., et al. EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS) Europace. 2023;25:1208–1236. doi: 10.1093/europace/euad043. [DOI] [PMC free article] [PubMed] [Google Scholar]