Key Teaching Points.
-
•
Catheter ablation for premature atrial contractions (PACs) originating from epicardial structures is challenging owing to the difficulty in detecting their true origin.
-
•
PACs from epicardial structures may exhibit a widespread breakthrough site, resulting from the extensive connections between the structures and the atria.
-
•
Since the breakthrough site of those PACs may differ from their origin, ablation at a site distant from the breakthrough site may be necessary to eliminate them.
Introduction
Premature atrial contractions (PACs) are often symptomatic and can significantly affect quality of life.1 Moreover, frequent PACs may lead to adverse atrial structural remodeling, thereby increasing the risk of atrial fibrillation.2 While catheter ablation for PACs is feasible and effective for some patients, it becomes challenging when the origin of the PACs is unclear. In this case report, we describe a patient with PACs of indeterminate origin owing to a widespread breakthrough site.
Case report
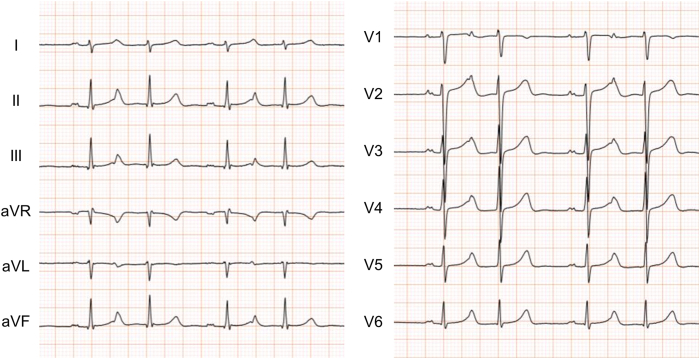
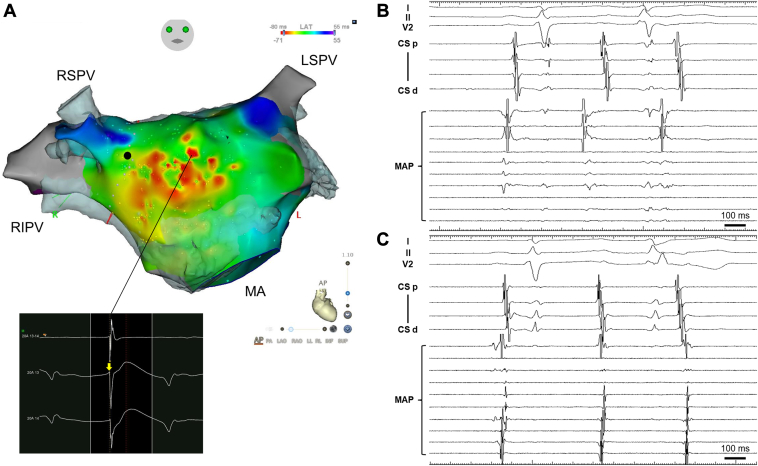
A 38-year-old woman was referred to our institution owing to skipped beats and general fatigue. Atrial bigeminy was recorded on a surface electrocardiogram (Figure 1). Although the detailed morphology of PACs was difficult to discern owing to its overlap with T waves, positive P waves in inferior leads and lead V1 suggested that PACs originated from the left atrium close to the right superior pulmonary vein (PV).3 In a 24-hour Holter electrocardiogram, PACs occurred at a frequency of 30,880 beats per day. Although 1.25 mg of bisoprolol reduced the PAC burden, it had to be discontinued owing to sinus bradycardia. Given the severity of her symptoms, we decided to perform catheter ablation for PACs. The activation map of PACs was created on a high-density mapping system (CARTO 3 mapping system; Biosense-Webster, Inc, Diamond Bar, CA). After transseptal puncture, sequential contact mapping of the left atrium was performed using a multispline mapping catheter (PentaRay; Biosense Webster, Inc). The activation map showed a centrifugal activation with a widespread breakthrough on the anterior wall (Figure 2A). The unipolar signal recorded at the earliest activation site showed an rS pattern. Local potentials recorded in the earliest activation site preceded the distal coronary sinus potential, which served as a reference, by 110 ms (Figure 2B). In contrast, the local potentials recorded in the right pulmonary vein were only 10 ms earlier than the reference (Figure 2C). Where is the origin of the PACs? What is the optimal ablation strategy for this case?
Figure 1.
A surface electrocardiogram during atrial bigeminy.
Figure 2.
Activation map and intracardiac electrograms of premature atrial contractions (PACs). A: The activation map of the left atrium, showing centrifugal activation with widespread breakthrough in the anterior wall. The unipolar signal recorded at the earliest activation site showed an rS pattern (yellow arrow). B, C: Intracardiac electrograms recorded when the mapping catheter (MAP) was positioned in the anterior wall (B) and right superior pulmonary vein (C). CS = coronary sinus; LSPV = left superior pulmonary vein; MA = mitral annulus; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
Discussion
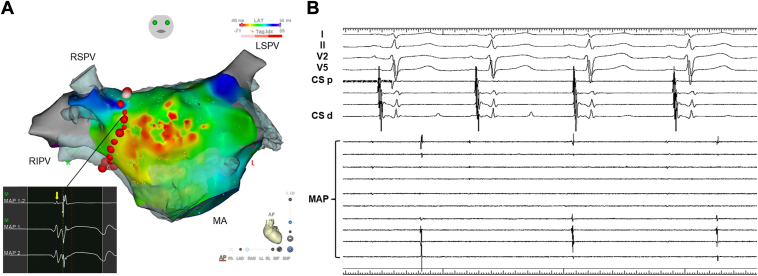
Centrifugal activation with widespread breakthrough suggested that the activation originated from an epicardial structure.4,5 The rS pattern in the unipolar signal recorded at the earliest activation site also suggested an epicardial origin, as the initial r wave indicated that the activation propagated from an epicardial structure to the endocardial surface. Considering the location of the breakthrough site, it was likely that the PACs originated from the Bachmann bundle or its branches.5 Extensive ablation was deemed necessary to eliminate these PACs if we targeted the widespread breakthrough. Therefore, despite the local potentials in the right PV being later than those in the anterior wall, we performed wide antral circumferential ablation (WACA) for the right PV to disconnect the PAC origin from the anterior wall (Figure 3A). Following the completion of right PV isolation, PACs were eliminated, and frequent ectopic beats were observed in the right PV (Figure 3B). These ectopic beats originated from the antrum of the right superior PV. Additional ablation targeting these ectopic beats was performed; however, they were not completely eliminated. To prevent PV stenosis, further ablation was avoided. The patient was discharged 2 days postprocedure without any complications, and no recurrence of PACs has been observed over a 28-month follow-up period.
Figure 3.
Activation map and intracardiac electrograms recorded after wide antral circumferential ablation. A: The activation map with tags indicating ablation sites. A small, blunt prepotential (yellow arrow) was observed in the point corresponding to the proximal segment of the Bachmann bundle. This point is also indicated by a black tag in Figure 2A. B: Intracardiac electrograms with the mapping catheter (MAP) positioned in the right superior pulmonary vein. Abbreviations are as defined in Figure 2.
In our retrospective analysis of the baseline left atrial map, we identified a small, blunt prepotential localized to the area associated with the proximal Bachmann bundle (Figure 3A). Since this prepotential preceded the earliest activation site of the centrifugal activation on the anterior wall, we considered this prepotential to represent the far-field sensing of the PAC origin within the proximal Bachmann bundle. In this case, PACs were eliminated during right PV isolation. This phenomenon can likely be attributed to the disruption of the electrical connection between the PAC origin and the distal Bachmann bundle. Additionally, we observed ectopic beats originating from the antrum of the right superior PV after WACA. The disruption of the preferential conduction pathway toward the distal segment of the Bachmann bundle may have unveiled electrical connections between the proximal segment of the Bachmann bundle and the antrum of the right superior PV. We performed ablation targeting the ectopic beats to prevent the recurrence of PACs if reconnection occurs between the PAC origin and the distal Bachmann bundle. However, the ectopic beats were not eliminated, likely owing to their epicardial origin.
If the prepotential from the epicardial PAC origin had been detected during the procedure, targeting just the site where the prepotential was recorded might have been effective. However, since we were unaware of this prepotential, we opted to perform WACA to disrupt the connection between the PAC origin and the anterior wall. It remains uncertain whether our strategy of isolating the PAC origin in the Bachmann bundle through WACA is applicable to all similar cases, as ablating epicardial structures such as the Bachmann bundle via endocardial ablation poses challenges. However, this case provides critical insights into ablation strategies for PACs originating from epicardial structures like the Bachmann bundle.
Disclosures
Dr Nakatani received research funding from DVx, Medtronic, Biotronik, Japan Lifeline, and Boston Scientific. Other authors have no disclosures.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.He B., Li Y., Huang W., et al. Mapping and ablation of isolated frequent symptomatic premature atrial contractions in patients with structurally normal heart. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.862659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higuchi S., Voskoboinik A., Im S.I., et al. Frequent premature atrial contractions lead to adverse atrial remodeling and atrial fibrillation in a swine model. Circulation. 2024;149:463–474. doi: 10.1161/CIRCULATIONAHA.123.065874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo J.Y., Tai C.T., Tsao H.M., et al. P wave polarities of an arrhythmogenic focus in patients with paroxysmal atrial fibrillation originating from superior vena cava or right superior pulmonary vein. J Cardiovasc Electrophysiol. 2003;14:350–357. doi: 10.1046/j.1540-8167.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 4.Pathik B., Lee G., Sacher F., et al. Epicardial-endocardial breakthrough during stable atrial macroreentry: evidence from ultra-high-resolution 3-dimensional mapping. Heart Rhythm. 2017;14:1200–1207. doi: 10.1016/j.hrthm.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Nakatani Y., Nakashima T., Duchateau J., et al. Characteristics of macroreentrant atrial tachycardias using an anatomical bypass: pseudo-focal atrial tachycardia case series. J Cardiovasc Electrophysiol. 2021;32:2451–2461. doi: 10.1111/jce.15186. [DOI] [PubMed] [Google Scholar]