Abstract
B‐1 cells are crucially involved in immune defense and regulation of inflammation and autoimmunity. B‐1 cells are predominantly located in the peritoneal and pleural cavities, although body cavity B‐1 cells recirculate systemically under steady‐state conditions. The chemokines CXCL12 and CXCL13 have been identified as the main regulators of peritoneal B‐cell trafficking. In mice deficient for sphingosine‐1‐phosphate receptor 4 (S1PR4), B‐1a and B‐1b cell numbers are reduced in the peritoneal cavity by an unknown mechanism. In this study, we show that S1PR4‐mediated S1P signaling modifies the chemotactic response of peritoneal B cells to CXCL13 and CXCL12 in vitro. In vivo, S1PR4‐mediated S1P signaling affects both immigration into and emigration from the peritoneal cavity. Long‐term reconstitution experiments of scid mice with wt or s1pr 4 –/– peritoneal B cells revealed a distinct distributional pattern in secondary lymphoid organs. As a functional consequence, both plasmatic and mucosal IgM levels, the main product of B‐1a cells, are reduced in mice reconstituted with s1pr 4 –/– peritoneal cells. In summary, our data identify S1PR4 as the second S1P receptor (besides S1PR1), which is critically involved in the regulation of peritoneal B‐1 cell function.
Keywords: chemotaxis, CXCL13, CXCL12, peritoneal B‐1 cells, sphingosine‐1‐phosphate
Sphingosine‐1‐phosphate (S1P) is implicated in the regulation of peritoneal B‐cell trafficking. Here we show that the effects of S1P on peritoneal B‐1 cell migration are mediated by the receptor S1PR4 via direct chemotactic action as well as via upregulation of the expression level of chemokine receptors CXCR4 and CXCR5.
Introduction
The immune system of the peritoneal cavity is an important component of the host's defense against infectious pathogens entering the host organism via breaches in the intestinal barrier, or against ascending infections via the female genital tract, as well as being involved in the development of peritoneal tumor spread and pathologies due to ectopic tissues (i.e. endometrioses) [1, 2]. The peritoneal cavity contains several hematopoietic immune cells, with peritoneal B cells representing the quantitatively most important population, followed in decreasing numbers by macrophages, CD4+ and CD8+ T cells, and neutrophils [3, 4]. Peritoneal B cells comprise a heterogeneous B‐cell population consisting of three major subtypes: B‐1a, B‐1b, and B‐2 cells [5, 6, 7, 8, 9]. Peritoneal B‐cell populations are characterized by a distinct phenotype, biological function, B‐cell receptor (BCR) repertoire, and metabolism [10, 11, 12]. While peritoneal B‐1b and B‐2 cell populations depend on constant inflow from bone marrow B‐cell precursors, B‐1a cells are predominantly maintained by self‐renewal throughout life [13]. Distinct functional properties exist between B‐1 and B‐2 cells: in contrast to B‐2 cells, B‐1 cells do not proliferate well in response to BCR‐mediated stimulation but are efficiently activated by BCR‐independent innate immune signals ([14], reviewed in [15]). In response to BCR‐independent stimuli such as LPS, IL‐5, and IL‐10, peritoneal B cells migrate to the spleen or mucosal sites and predominantly differentiate into IgM (B‐1a cells) or IgA (B‐1b cells) secreting cells (reviewed in [16]). B‐1b cells can accumulate as long‐lived memory B cells in the peritoneal cavity and secrete antigen‐specific IgM against TI‐1 antigens [17]. While peritoneal B‐1a cells located in the peritoneal cavity secrete only small amounts of natural IgM, both the spleen and bone marrow contain B‐1a cell populations that spontaneously produce numerous circulating natural IgM antibodies. Thus, B‐1 cells contribute to the early neutralization of invading pathogens and enhance the later pathogen‐specific B‐2 cell response [16]. B‐1 cells also have an immunoregulatory function by influencing T‐cell‐dependent antibody production by B‐2 cells by their ability to produce high amounts of IL‐10 and by scavenging apoptotic cells (reviewed in [10]). In contrast, the population of B‐2 cells, comprising follicular B cells and marginal zone B cells, is responsible for the T‐cell‐dependent adaptive B‐cell response and for the T‐cell‐dependent and independent B‐cell responses to blood‐born antigens, respectively (reviewed in [18]). Although B‐1 cells constitute the main B‐cell population in the coelomic cavities of the body (peritoneal and pleural cavities) [13], all peritoneal B‐cell subpopulations circulate from the peritoneal cavity through distant tissues and back to the peritoneal micromilieu under steady‐state conditions and B‐1 cells can be found in other tissues in lower numbers [19, 20]. The CXC chemokines CXCL12 and CXCL13 have been identified as the main regulators of peritoneal trafficking of B cells [21, 22, 23]. In a previous study, we found reduced numbers of B‐1a and B‐1b cells in the peritoneal cavity of mice deficient for sphingosine‐1‐phosphate receptor (S1P) type 4 ( s1pr 4 –/–) [24]. S1P is a bioactive sphingolipid that acts as an intra‐ and extracellular messenger and mediates a wide variety of biological cell functions (reviewed in [25, 26]), the majority of which, especially in the immune system, is regulated by its interaction with five membrane‐bound G‐protein–coupled receptors (S1PR1–S1PR5) [27]. S1P binding to its membrane receptors regulates the trafficking of various immune cells and their positioning within the compartments of secondary lymphoid organs, often also by modifying chemokine‐induced migration processes [28, 29]. S1PR1 and S1PR4 are differentially expressed in peritoneal B‐cell subpopulations [24, 30]. S1PR1 expression is downregulated upon TLR4 stimulation [30]. No expression of the remaining S1P receptors S1PR2, S1PR3 and S1PR5 has been shown in peritoneal B cells [24].
In this manuscript, we describe the mechanisms responsible for the reduced peritoneal B‐1 cell number in s1pr 4 –/– mice, as reported previously [24]. To this end, we characterized the impact of S1PR4‐mediated signaling on the migration, proliferation, and viability of peritoneal B cells in vitro and in vivo. Using adoptive transfer models, we demonstrated S1PR4‐mediated modulation of peritoneal B‐cell trafficking, immunoglobulin, and cytokine secretion. Our findings suggest an important role of S1PR4‐mediated signaling in the regulation of peritoneal B‐cell biology.
Materials and methods
Mice
Female C57BL/6J mice (wt) were purchased from Charles River. Female B6.Cg‐Prkdcscid/SzJ mice on a C57BL/6J background (scid) were purchased from The Jackson Laboratory. All mice were kept in the animal facility for at least 2 weeks before the initiation of experiments to allow them to adapt to local conditions. The scid mice were kept under sterile conditions using flow boxes with separate air supplies. S1pr4 −/− mice on a C57BL/6J background were initially created in the Martin Lipps Lab at the Max‐Delbrück‐Centrum für Molekulare Medizin Berlin‐Buch, Germany [31]. The animals used for the experiments reported herein were bred under specific pathogen‐free conditions in the animal facility of the Universitätsmedizin Greifswald, Germany (Zentrale Service‐ und Forschungseinrichtung für Versuchstierkunde; ZSFV). Mice aged between 10 and 14 weeks were used for all experiments. All animal care practices and experimental procedures were performed in accordance with the German Animal Protection Law (TierSchuG) and controlled by the veterinary government authority (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg‐Vorpommern; LALLF‐MV; Project identification code: 7221.3‐1.1‐044/19).
Isolation of peritoneal cells
Cells of the murine peritoneal cavity were obtained by peritoneal lavage, in which 10 mL of ice‐cold PBS supplemented with 0.5% fatty acid‐free BSA (Sigma‐Aldrich) was injected intraperitoneally. After gently moving the mice, the lavage fluid was aspirated and was centrifuged at 120 × g for 10 min. The supernatant was discarded and the cell pellet was used for further analysis and experiments. Absolute cell numbers were determined using BD TruCount tubes (BD Biosciences).
Flow cytometry
Nonspecific antibody binding was blocked with an anti‐FcII/III antibody (anti‐CD16/32; BD Pharmingen). The following antibodies and conjugates were used: anti‐CD5‐PE/Cy7 (clone 53–7.3; Biolegend), anti‐CD19‐eFluor 660 (clone 1D3; eBioscience, Thermo Fisher Scientific), anti‐CD23‐PE (clone B3B4; eBioscience), anti‐CD3‐FITC (clone 145‐2c11; Biolegend), anti‐CD11b‐APC‐eFluor 780 (clone M1/70; eBioscience), anti‐CXCR4‐eFluor 450 (clone 2B11; eBioscience), and anti‐CXCR5‐BV650 (clone L138D7; Biolegend). For adoptive transfer, viability, and proliferation assays, cells were stained with CellTrace CFSE (Life Technologies) or Tag‐it Violet (Biolegend) according to the manufacturer's instructions. All cells were stained with 7‐AAD Viability Staining Solution (Biolegend) before dead cell exclusion analysis. Stained cells were analyzed using a BD LSR II Flow Cytometer (BD Biosciences) and evaluated with FlowJo software (Version 10, LLC). B‐cell subpopulations were identified using the following antibody panels: B–1a cells: CD19+ CD5+ CD23−; B–1b cells: CD19+ CD5− CD23−; and B–2 cells: CD19+ CD5− CD23+. The gating strategy is shown in Supporting Information Fig. S1.
Transwell migration assays
Following the isolation of peritoneal cells, samples were resuspended in migration medium (RPMI‐1640 + GlutaMAX‐I [Gibco, Thermo Fisher Scientific], 0.5% fatty acid‐free BSA [Sigma‐Aldrich], 25 mM HEPES [Biochrom], 100 U/mL penicillin with 100 µg/mL streptomycin [Gibco]) at a final concentration of 5 × 106 cells/mL. The lower chambers of a 24‐well plate were filled with 600 µL migration medium containing appropriate concentrations of chemoattractants. Subsequently, the upper chambers of a transwell insert (5‐µm pore size; Corning Costar) were inserted into the previously filled wells and loaded with 100 µL of the cell suspension containing 5 × 106 cells/mL. Three wells without inserts were filled with the same cell suspension as controls. Following incubation for 4 h at 37°C under 5% CO2, the numbers of B‐1a, B‐1b, and B‐2 cells migrating to the lower chamber were analyzed after staining with the aforementioned antibody panels by FACS using BD TruCount tubes (BD Biosciences). Migration was calculated as follows: (cell number of the lower chambers with insert/cell number loaded into the lower chamber without insert) × 100. For each mouse, the mean of three technical replicates was used for further statistical analysis.
The S1PR4‐specific antagonist CYM50358 (Hycultec) was solubilized according to the recommendations of the manufacturer and used to demonstrate the S1PR4‐specific effects of S1P at the concentrations specified in the results section.
CXCR4 and CXCR5 expression in vitro
The S1PR4‐specific agonist CYM50308 (Sigma‐Aldrich, Sigma‐Aldrich) and the S1PR4‐specific antagonist CYM50358 (Hycultec) were solubilized according to the recommendations of the manufacturer. 2.5 × 105 peritoneal cells were incubated in the presence of the agonist/antagonist for 36 h at 37°C under 5% CO2. Cells were re‐stimulated with the appropriate amount of agonist/antagonist after 24 h. After 36 h, cells were harvested and analyzed by flow cytometry.
Adoptive cell transfer
Cells isolated from the PerC of wt and s1pr4 −/− mice were adjusted to the same concentration and stained with CellTrace CFSE (Life Technologies) or Tag‐it Violet (Biolegend) according to the manufacturer's instructions. After half of the experiments had been conducted, the staining of the genotypes was inversed to exclude the influence of the labeling substances on the experimental outcome. Per mouse, 2.5 × 106 labeled wt and s1pr4 −/− cells were mixed and administered intraperitoneally or intravenously. The organs were harvested after 12 or 48 h, and the total number of transferred cells from each population and genotype were determined by flow cytometry for each tissue (Supporting Information Fig. S2). Total numbers were calculated using BD TruCount tubes (BD Biosciences).
Fluorescence microscopy of splenic tissue sections
Harvested spleens were embedded in TissueTek (Sakura Finetek Europe B.V.) and snap‐frozen in isopentane cooled by liquid nitrogen. Cryostat sections (3 µm) were dried for 24 h and then fixed in acetone at −20°C for 10 min. Nonspecific binding sites were blocked with PBS + 10% fetal calf serum for 30 min and biotin‐binding sites were blocked using the Dako Biotin Blocking System (Dako North America Inc.) according to the manufacturer's instructions. Sections were stained with anti‐CD4‐BV421 (clone: GK1.5), anti‐B220‐FITC (clone: RA3‐6B2), and anti‐IgD‐biotin (clone: MD78Z) at 4°C for 16 h. The sections were then incubated with rhodamine‐conjugated streptavidin (Jackson ImmunoResearch Laboratories Inc.) at room temperature for 1 h and cell nuclei were stained with Draq5 (Biolegend). Stained sections were stored at 4°C for at least 24 h before analysis. All images were acquired on the same day at constant exposure time and magnification.
Immunoglobulin and cytokine analysis
The concentrations of immunoglobulins and cytokines were determined using the LEGENDplex multi‐analyte flow assay kit (Biolegend) according to the manufacturer's instructions (immunoglobulins: Legendplex MU immunoglobulin isotyping panel (6‐plex) w/ VbP, Cat: 740493; cytokines: Legendplex Mouse B‐cell Panel (13‐plex) w/FP, Cat: 740818). Blood plasma was obtained by centrifugation of EDTA‐anticoagulated blood samples. For gastrointestinal lavage fluid (GILF), the small intestine of mice was explanted, washed twice in ice‐cold PBS, and flushed with gastrointestinal lavage buffer containing 25 mM NaCl, 10 mM Na2SO4, 10 mM KCl, 20 mM NaHCO3, 50 mM EDTA, 162 mg/mL polyethylene glycol 3350, 1 mM phenylmethanesulfonyl fluoride, and 0.1 mg/mL Soybean trypsin inhibitor dissolved in distilled water [32, 33]. The fluid was then centrifuged twice at 13,000 rpm and 4°C for 10 min. Blood plasma and GILF were stored at –80°C until further usage.
ELISPOT
Freshly isolated cells from the spleen and bone marrow were used to determine the number of IgM‐secreting cells using the ImmunoSpot Mouse IgM Single‐Color ELISPOT assay kit (CTL‐Europe GmbH) according to the recommendations of the manufacturer. Briefly, appropriate cells numbers in three serial dilutions (spleen: 1.6 × 105; 5.0 × 104; 1.6 × 104; bone marrow: 5.0 × 105; 1.6 × 105; 5.5 × 104) were transferred to the previously coated 96‐well PVDF filter plates provided in the kit. Cells were incubated in the assay medium for 8 h at 37°C and 5% CO2. Plates were then washed and incubated with anti‐mouse IgM detection solution for 2 h in the dark at room temperature. Plates were then dried, treated with tertiary solution, and finally developed by adding CTL‐TrueBlue substrate solution according to the protocol of the manufacturer. IgM‐secreting B cells were enumerated using a Mabtech IRIS2 ELISPOT reader (Mabtech AB) with filtering for spot size using the Mabtech Apex Software (Mabtech AB).
Assessment of proliferation and viability
CellTrace CFSE (Life Technologies) was used for in vitro and in vivo measurement of cell proliferation. For in vitro experiments, B cells were incubated with various concentrations of LPS to induce cell division. After incubation for 4 h at 37°C and 5% CO2, the reduction in cellular CFSE concentration was measured using the mean fluorescence intensity via flow cytometry. For coculture experiments, spleen cells were incubated at a 1:1 ratio with peritoneal cells of the same donor animal. Proliferation was assessed after incubation for 24 h at 37°C and 5% CO2. Cell proliferation was measured using 7‐aminoactinomycin D (7‐AAD; BioLegend) uptake via flow cytometry. For in vivo experiments, the mean 7‐AAD fluorescence intensity was measured by flow cytometry 48 h after intraperitoneal transfer in B6.Cg‐Prkdcscid/SzJ (scid) mice.
Results
Differential migration of peritoneal B‐cell subpopulations to combined chemokine‐S1P gradients in vitro
Recently, the impact of S1P‐mediated signaling on the peritoneal egress of B cells under inflammatory conditions has been demonstrated [30]. The role of CXCL13, another chemotactic molecule, in peritoneal trafficking has also been described [22]. However, interactions between S1P‐ and CXCL13‐mediated signaling have not yet been studied. Therefore, the migration of peritoneal B cells to combined gradients of S1P and CXCL13 was assessed in vitro. Both B‐1a and B‐1b cells showed dose‐dependent chemotaxis toward S1P gradients, which peaked for both cell populations at 10 nM S1P (2.22% versus 1.72% for B‐1a and B‐1b cells, respectively). In contrast, no chemotactic response to S1P gradients was observed in B‐2 cells (Fig. 1A). Using a combination of S1P gradients with a constant CXCL13 gradient, the chemotactic response of all B‐cell populations was significantly increased compared with that of S1P gradients alone. Again, both B‐1 cell populations showed the highest migration rates at 10 nM S1P in a combined gradient with CXCL13 (5.57% versus 4.96% for B‐1a and B‐1b cells, respectively). Although B‐2 cells showed a chemotactic response to the CXCL13 gradient, no dose‐dependent increase in migration was observed following the addition of S1P gradients. No relevant effect of S1P was measured at higher CXCL13 concentrations (Supporting Information Fig. S3).
Figure 1.
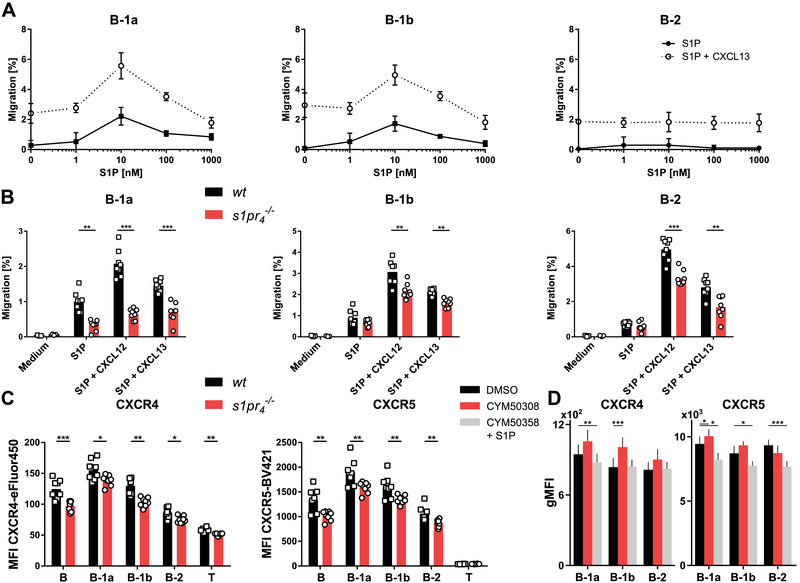
Chemotactic response of s1pr 4 –/– peritoneal B cells in vitro (A) Peritoneal B cells were isolated from the PerC of wt and s1pr 4 –/– mice and seeded in the upper chamber of a Transwell migration assay. The lower chambers were filled with different concentrations of S1P alone (solid line) or with 100 ng/mL CXCL13 (dotted line). After incubation, the number of cells that migrated to the lower chamber was quantified using flow cytometry. Migration was computed as the percentage of cells from the upper well. Values represent the mean ± SD of n = 3 per concentration. The results are representative of three independent experiments. (B) Transwell migration of peritoneal B cells to medium, 10 nM S1P, 10 nM S1P + 200 ng/mL CXCL12, and 10 nM S1P + 100 ng/mL CXCL13. Data are shown as individual values obtained from n = 8 donors per group. (C) Expression of CXCR4 and CXCR5 on peritoneal cell populations quantified by flow cytometry (n = 8). (D) Expression of CXCR4 and CXCR5 on peritoneal B cells that were stimulated with the selective S1PR4 agonist CYM50308 or selective S1PR4 antagonist CYM50358 in the presence of S1P. Bars represent the mean values + SD. *p < 0.05; **p < 0.01; ***p < 0.001 as computed by Student's t‐test.
S1PR4 mediates the modifying effect of S1P on chemokine‐induced chemotaxis in peritoneal B cells
S1PR4 has been shown to be one of the main membrane receptors mediating the effect of S1P on the egress of peritoneal B‐cell populations from the peritoneal cavity under inflammatory conditions [30]. In this study, we used peritoneal B cells from an S1PR4 deficient knock‐out mouse strain ( s1pr 4 –/–) to evaluate the impact of S1PR4 expression on their chemotactic response to S1P in vitro. S1PR4 deficient B‐1a cells showed a significantly reduced chemotactic response to the S1P gradient compared with wt cells (1.00% versus 0.32% for wt and s1pr 4 –/– cells, respectively; Fig. 1B). In contrast, we observed no significant difference in the chemotactic migration of B‐1b or B‐2 cells to S1P gradients. Since various S1PR are expressed on peritoneal B cells we used the S1PR4‐specific antagonist CYM50358 to show the implication of S1PR4‐mediated S1P signaling in the observed migrational response. The results corroborating the data obtained in s1pr 4 –/– cells in vitro are shown in Supporting Information Fig. S4. In the presence of combined gradients of S1P and CXCL13, all s1pr 4 –/– peritoneal B‐cell populations showed reduced chemotactic migration compared with wt cells. Similarly, all s1pr 4 –/– peritoneal B cells displayed a reduced chemotactic response toward combined gradients of S1P and CXCL12, another chemokine implicated in peritoneal cell trafficking [21]. In conclusion, S1PR4‐mediated S1P signaling can modify the migratory behavior of peritoneal B‐cell populations induced by chemokines involved in peritoneal cell trafficking.
S1PR4 deficiency reduces CXCR4 and CXCR5 surface expression on peritoneal lymphocytes
Given the effect of S1P signaling on CXCL13‐induced chemotaxis, we next sought to determine whether S1PR4‐mediated signaling affects the expression of the receptors for CXCL13 and CXCL12, CXCR5, and CXCR4, respectively. Flow cytometric analysis of CXCR4 and CXCR5 on peritoneal B cells revealed decreased expression levels in all peritoneal s1pr 4 –/– B‐cell populations compared with their wt counterparts (Fig. 1C). To further corroborate these data obtained in the s1pr 4 –/– background, we used the S1PR4‐specific agonist CYM50308 and the S1PR4‐specific antagonist CYM50358 to assess the impact of S1PR4‐mediated signaling on CXCR4 and CXCR5 expression on peritoneal B cells of wt mice. In peritoneal B‐1a cells, both CXCR4 and CXCR5 expression was decreased in the presence of S1PR4‐specific antagonist CYM50358 (Fig. 1D). In the presence of the S1PR4‐specific agonist CYM50308 both CXCR4 and CXCR5 expression increased, but only the increase of CXCR5 expression reached statistical significance. B‐1b and B‐2 cells also showed differential expression profiles after incubation with S1PR4‐specific agonist and antagonist. The decrease of CXCR4 expression on peritoneal B‐1a cells upon incubation with the S1PR4‐specific antagonist CYM50358 in the presence of S1P was concentration‐dependent (Supporting Information Fig. S5).
Emigration of peritoneal B cells from the abdominal cavity is affected by S1PR4 deficiency
The regulation of peritoneal B‐cell trafficking is a complex process and the exact mechanisms governing both egress from and immigration into the peritoneal cavity remain incompletely understood. After demonstrating that S1P signaling via S1PR4 contributes to the chemotactic response of peritoneal B cells in a subpopulation‐specific manner in vitro, we sought to investigate the contribution of S1PR4–mediated S1P signaling to peritoneal B‐cell trafficking in vivo in a model of adoptive cell transfer. To this end, we examined the migration of wt and s1pr 4 –/– peritoneal B cells after intraperitoneal adoptive transfer in scid recipient mice. After 12 h, there were significantly more s1pr 4 –/– B‐1a cells than wt counterparts in the peritoneal lavage fluid (Fig. 2). At this time point, the majority of B cells remained in the peritoneal environment and only low cell numbers could be detected in the other tissues screened. After 48 h, the wt B‐cell numbers of all peritoneal B‐cell subsets were significantly lower than those of s1pr 4 –/– genotype in the PerC. The omentum majus (omentum) is a major access and exit route for peritoneal cells [22, 34, 35]. Increased numbers of wt B‐1a cells were recovered from the omentum after 48 h compared with s1pr 4 –/– B‐1a cells, while the differences in recovered B‐1b and B‐2 cells did not reach statistical significance. In the mesenterial lymph nodes (mLN), the spleen, and the inguinal lymph nodes (iLN), the cell numbers of recovered wt peritoneal B‐cell subpopulations were increased compared with the s1pr 4 –/– genotype 48 h after intraperitoneal transfer. These data suggest that after 48 h, s1pr 4 –/– peritoneal B cells showed reduced peritoneal egress and migration to secondary lymphoid organs comp wt B cells.
Figure 2.
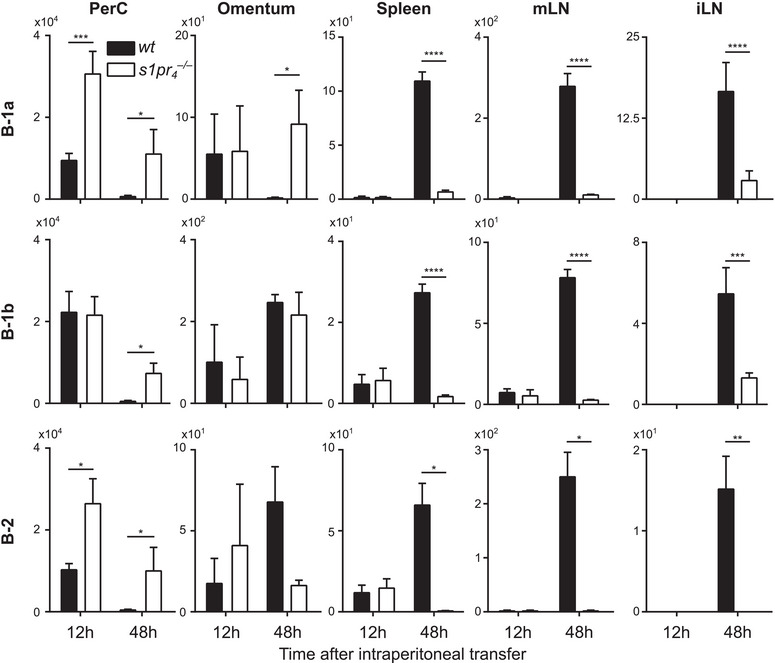
Migrational behavior of peritoneal B cells after intraperitoneal transfer. A mixture of differentially labeled wt and s1pr 4 –/– peritoneal cells was transferred into the PerC of scid mice. Twelve and 48 h after transfer, cells from the peritoneal lavage (PerC), omentum majus (omentum), spleen, mesenterial lymph nodes (mLN), and inguinal lymph nodes (iLN) were quantified by flow cytometry. Bars represent the mean values + SD of n = 10 recipients. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 as computed by one‐way ANOVA with Dunnett's multiple comparison post hoc test.
S1PR4 deficiency inhibits peritoneal B‐cell homing to the peritoneal cavity
The net number of intraperitoneal cells does not only depend on peritoneal egress but also on the intensity of the cellular immigration into the peritoneal cavity. In this process, the omentum has been described as the main route of entry [23]. Twelve hours after intravenous transfer, peritoneal B cells showed a very heterogeneous distribution among the various tissues examined (Fig. 3). In the PerC, a significantly lower number of s1pr 4 –/– B‐1a cells were recovered compared with wt B‐1a cells after 12 h. After 48 h, the number of s1pr 4 –/– B‐1a cells remained clearly inferior to that of wt B‐1a cells in the PerC. Moreover, s1pr4 –/– B‐1a cells showed reduced numbers in the omentum, mLN, and spleen 12 and 48 h after transfer. In contrast, the number of s1pr 4 –/– B‐1a cells was increased compared with wt B‐1a cells in iLN after both twelve and 48 h after intravenous transfer. This distribution of transferred B‐1a cells in recipient mice indicates reduced immigration of s1pr 4 –/– cells through the omentum and into the PerC. In contrast, s1pr 4 –/– B‐1b and B‐2 cells showed no preference for peripheral lymph nodes or those draining the gut (e.g. mLN). Twelve hours after transfer, wt, and s1pr 4 –/– B‐2 cell numbers were similar in the PerC, while the s1pr 4 –/– B‐1b cell number was superior to the number of their wt counterparts. In contrast, at the 48 h time point, similar numbers of wt and s1pr 4 –/– B‐1b, but higher numbers of s1pr 4 –/– B‐2 cells were present in this compartment. S1PR4‐deficient B‐1b cells showed increased migration to the omentum and reduced migration to the spleen compared with wt B‐1b cells. Increased numbers of wt B‐2 cells were observed in the omentum after 48 h and in the spleen after 12 and 48 h. In the mLN, s1pr 4 –/– B‐2 cell numbers were slightly increased compared with wt B‐2 cells 48 h after transfer. In summary, our data suggest that s1pr 4 –/– B‐1a cells in particular were hindered in their attempt to access the peritoneal environment, instead appearing to be preferentially directed to the peripheral lymph nodes (e.g. iLN), with increasing numbers at later time points. Compared with B‐1a cells, S1PR4 deficiency had a reduced effect on the peritoneal immigration of both B‐1b and B‐2 cells.
Figure 3.
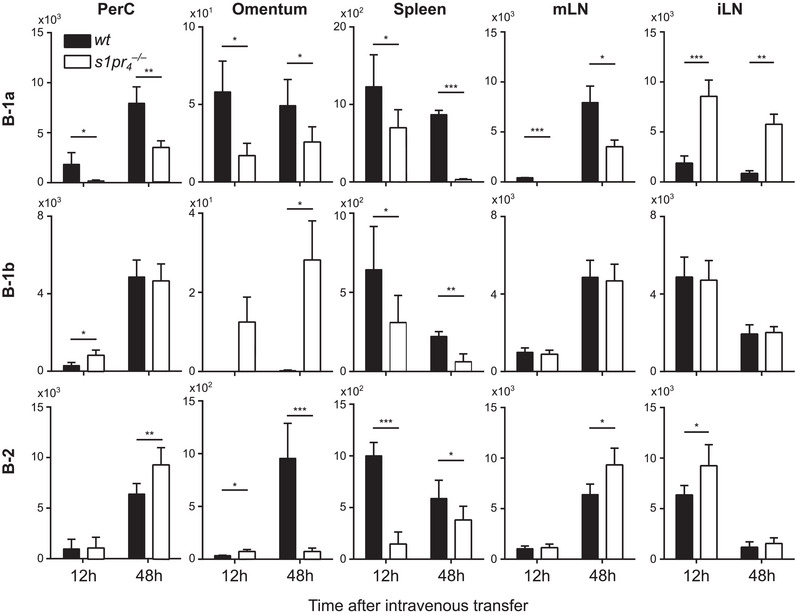
Migrational behavior of peritoneal B cells after intravenous transfer. A mixture of differentially labeled wt and s1pr 4 –/– peritoneal cells was transferred together into the tail vein of scid mice. Twelve and 48 h after transfer, cells from the peritoneal lavage (PerC), omentum majus (omentum), spleen, mesenterial lymph nodes (mLN), and inguinal lymph nodes (iLN) were quantified using flow cytometry. Bars represent the mean values + SD of n = 16 recipients. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 as computed by one‐way ANOVA with Dunnett's multiple comparison post hoc test.
Long‐term reconstitution of peritoneal B‐cell populations after adoptive intraperitoneal transfer in scid mice
The long‐term effects of impaired peritoneal trafficking of s1pr 4 –/– peritoneal B cells were studied 14 days after intraperitoneal transfer into scid mice. In peritoneal lavage, B‐1a and B‐1b cells were more abundant in mice that received wt cells than those that received s1pr 4 –/– cells (Fig. 4A). In the spleen, equivalent numbers of cells with a B‐1a and B‐1b phenotype were found, while B‐2 cells were found at comparable numbers in the PerC and spleen of recipients of wt and s1pr 4 –/– cells. However, upon microscopical analysis of splenic sections, differences between transplants from both genotypes were found. The spleens of recipients of s1pr 4 –/– cells showed only loose and small clusters of B and T cells, while those from recipients of wt cells revealed larger lymphocyte aggregates that resembled primitive follicles (Fig. 4B). These cell conglomerates must have arisen after the second day of cell transfer since no comparable structures were found twelve and forty‐eight hours after adoptive cell transfer (Supporting Information Fig. S6).
Figure 4.
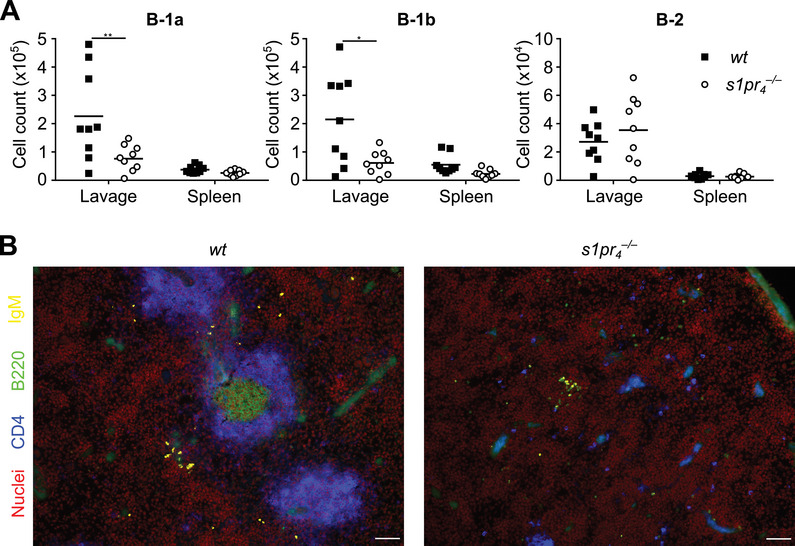
Reconstitution of B‐cell populations in scid mice. Peritoneal cells from wt and s1pr 4 –/– mice were separately transferred into the PerC of scid mice. Fourteen days after transfer, organs were harvested and the splenic architecture and cell numbers in the spleen and PerC were analyzed in n = 9 animals per group. (A) The cell numbers in the peritoneal lavage fluid (lavage) and spleen of recipients were quantified using flow cytometry. (B) Sections of the recipient spleens were stained with Draq5 (nuclei, red), CD4‐BV421 (blue), B220‐FITC (green), and IgD‐rhodamine (yellow); Scale bars: 50 µm. Bars represent the mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 as computed by Mann–Whitney U‐test.
Mucosal and systemic IgM levels and systemic IL‐10 levels are affected by S1PR4 deficiency in peritoneal B cells
To evaluate the impact of S1PR4 deficiency on immunoglobulin production, the levels of natural antibodies (IgA, IgM) and IgG species were measured in plasma and GILF 14 days after adoptive intraperitoneal transfer of peritoneal cells. The results showed no significant difference in IgA levels in GILF and plasma between the two genotypes (Fig 5A). In contrast, the recipients of s1pr 4 –/– cells showed lower levels of IgM in both GILF and plasma. As B‐1 cells in the peritoneal cavity produce only small amounts of IgM but migrate to other lymphatic organs where they significantly increase antibody production and secretion, we measured the number of IgM‐secreting cells in the spleen and the bone marrow [15, 54]. In both organs, significantly lower numbers of IgM‐secreting cells were found in s1pr 4 –/– animals compared with wt controls (Fig. 5C). As IL‐10 secretion is the basis for the immunomodulatory role of peritoneal B‐1 cells and has been shown to affect peritoneal B‐cell migration, we also assessed the levels of IL‐10 in the reconstituted mice [36, 37]. The results showed that the IL‐10 levels were significantly lower in mice reconstituted with s1pr 4 –/– peritoneal cells than in those reconstituted with wt cells (Fig. 5B).
Figure 5.
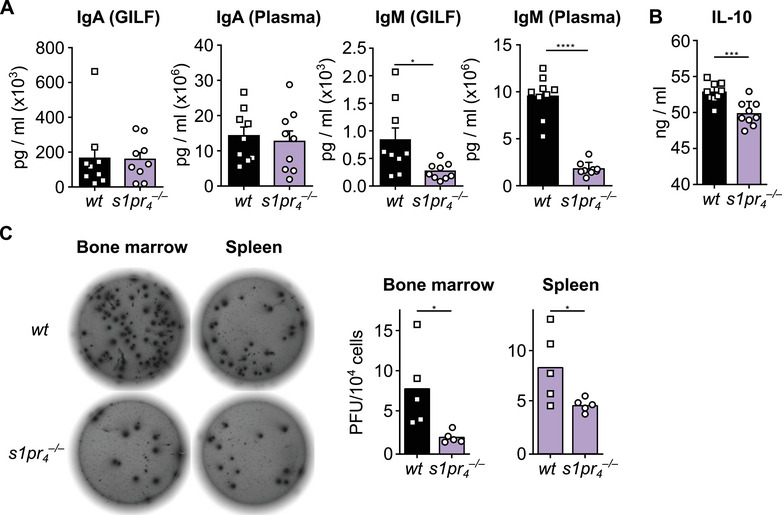
Impact of S1PR4 deficiency on mucosal and systemic IgA and IgM levels and systemic IL‐10 levels. (A) Peritoneal cells from wt and s1pr 4 –/– mice were separately transferred into the PerC of scid mice. Fourteen days after transfer, organs were harvested and the splenic architecture and cell numbers in the spleen and PerC were analyzed in n = 9 animals per group. Quantification of IgM and IgA in the gastrointestinal lavage fluid (GILF) and blood plasma (plasma) of recipient mice, as measured by cytometric bead assay. (B) Quantification of IL‐10 from the blood plasma of recipient scid mice after intraperitoneal transfer of peritoneal cells as described above. (C) The number of IgM‐producing cells in the spleen and the bone marrow in naïve wt and s1pr 4 –/– mice were determined by ELISPOT (n = 5) *p < 0.05; ***p < 0.001; ****p < 0.0001 as computed by Mann–Whitney U‐test.
Proliferation and viability of peritoneal B cells are unaffected by S1PR4 deficiency
Throughout this study, the extent of migration was deduced from changes in cell numbers in various tissues; however, this only reflects migratory processes under the assumption that there is no difference in proliferation and viability between the cells of both genotypes. Therefore, to prove the validity of this assumption, in vitro peritoneal B‐cell proliferation was induced using different concentrations of LPS (Fig. 6A). The results showed no significant difference in cell divisions in wt and s1pr 4 –/– peritoneal B cells. While B‐1a cells showed high proliferation in response to LPS, the response of B‐1b cells was modest. Moreover, no proliferative response of B‐2 cells could be detected in response to LPS. Several authors have described the dependence of B‐1 B‐cell population persistence on the splenic environment [38, 39]. Therefore, we next employed a co‐culture model of peritoneal and splenic cells to assess differences in the proliferative response between the genotypes under these conditions (Fig. 6B). The results showed no significant difference between wt and s1pr 4 –/– peritoneal B cells after culture of peritoneal cells only (PC) or in combination with splenic cells (PC+SC). Next, the viability of peritoneal B‐cell subsets was assessed in vitro and in vivo after 48 h. The results showed no significant difference in the in vitro viability of B‐1a, B‐1b, or B‐2 cells (Fig. 6C). Similarly, no significant difference in the viability in transferred PerC‐resident peritoneal B‐cell subsets was detected in the model of adoptive transfer to scid mice (Fig. 6D). Therefore, it seems reasonable to assume that the observed changes in cell numbers in various organs reflect the migration of the relevant cell populations and are not due to differences in their proliferative capacity or viability.
Figure 6.
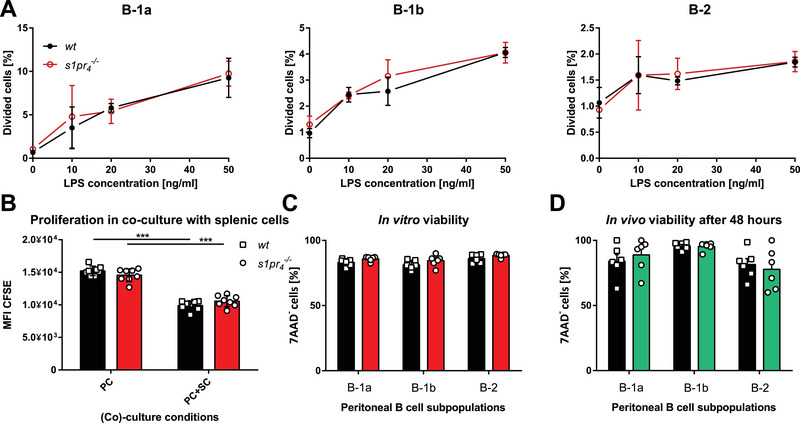
Proliferation and viability of peritoneal B cells from wt and s1pr 4 –/– mice. (A) Peritoneal B cells of wt and s1pr 4 –/– mice were stimulated with various concentrations of LPS to stimulate their proliferation in vitro. The percentages of dividing cells were measured by the decline in CFSE by flow cytometry (n = 3). (B) Peritoneal cells alone (PC) or in co‐incubation with splenic cells (PC+SC) from wt and s1pr 4 –/– mice were cultured ex vivo to assess proliferation (n = 8). (C) The in vitro viability of wt and s1pr 4 –/– B cells was assessed using flow cytometry after 24 h in cell culture medium (n = 6). (D) The in vivo viability of wt and s1pr 4 –/– B cells was assessed 48 h after their transfer into scid mice using 7‐AAD in flow cytometry. Bars represent the mean ± SD. ***p < 0.001 as computed by one‐way ANOVA with Dunnett's multiple comparison post hoc test.
Discussion
Mice deficient for S1PR4 possess significantly reduced peritoneal B‐1a and B‐1b cell numbers compared with wt animals, while their peritoneal B‐2 cell numbers are similar [24]. The causes leading to this distinctive feature of s1pr 4 –/– animals remain incompletely understood. A role for S1P signaling in peritoneal B‐cell trafficking has been previously implied by Kunisawa et al. Using the nonselective functional antagonist of S1P, FTY720, Kunisawa et al. [40] showed strongly reduced peritoneal B‐1 and B‐2 cell numbers in the peritoneum of FTY720‐treated mice. The exact subtype of S1P receptor mediating this effect has not been identified yet. In this study, we demonstrate that S1PR4‐mediated signaling modulates the chemotactic response of peritoneal B cells to chemokine gradients in a subpopulation‐dependent manner. Our in vitro data clearly show that S1P signaling modifies CXCL13‐induced chemotaxis of peritoneal B cells in a cell subtype‐specific manner. Interestingly, the expression of the receptor for CXCL13, CXCR5, but also the expression of CXCR4, the receptor for CXCL12, which is also critically implicated in the control of peritoneal B‐cell migration, is significantly reduced in s1pr4 −/‐ animals [22, 23, 41]. Using adoptive cell transfer techniques, we show that S1PR4 deficiency results in altered distribution patterns of peritoneal B cells within different immunological compartments, while peritoneal B‐cell proliferation and viability were unaffected by the absence of S1PR4 expression. These data strongly support the hypothesis that the changes in the composition of the peritoneal cell populations observed in s1pr 4 –/– mice are driven by the modulation of peritoneal B‐cell trafficking. As reported by other investigators, S1P gradients had only a relatively weak direct chemotactic effect inducing the migration only of a relatively small fraction of B cells in our experimental setting [42, 43, 44]. This contrasts with the important reduction of peritoneal B‐cell subpopulations seen in the s1pr 4 –/– animals. This discrepancy may be due to an activation‐dependent S1P responsiveness of B cells in various activational states or may be due to the S1P‐induced modification of the action of stronger chemoattractants, that is, CXCL12 or CXCL13. Recently, the first description of an interaction between S1PR4 and CXCR4, the main receptor for CXCL12, has been published [34]. In human CD8+ T lymphocytes, activation of S1PR4 increases the expression of CXCR4. For circulatory B cells, a reduction in CXCR4 expression and CXCL12‐mediated migration has been described after treatment with FTY720 [35]. The observation that CXCR4 and CXCR5 expression is reduced in s1pr 4 –/– animals indicates that S1P‐induced increased expression of these molecules, at least partly, contributes to the increased migration of B‐1a and B‐1b cells to combined gradients of CXCL13 and S1P. It has been previously shown that signaling via other S1P receptors affects the expression levels of chemokine receptors such as CXCR4 [45]. However, this is the first description of the modulating action of S1PR4‐mediated signaling on chemokine receptor expression levels in B lymphocytes. Interestingly, s1pr 4 –/– B‐2 cells also showed reduced chemotaxis toward combined S1P and CXCL12 or CXCL13 gradients compared with their wt counterparts, although a single S1P gradient did not induce significant chemotaxis in peritoneal B‐2 cells. This observation supports the hypothesis that S1P‐mediated modification of CXCL12 and CXCL13 chemotaxis of peritoneal B cells is predominantly mediated by the S1P‐induced alteration of chemokine receptor expression levels.
Having established that S1PR4‐mediated signaling modifies chemokine‐induced chemotaxis in vitro, we addressed the question of whether this function affects the migration of peritoneal B cells, such as emigration from and immigration into the peritoneal cavity in vivo. Our data shows that s1pr 4 –/– peritoneal B cells possess a reduced capacity to exit the peritoneal cavity or to translocate to other peritoneal compartments that are inaccessible for peritoneal lavage. Interestingly, Kunisawa et al. [40] showed increased emigration of peritoneal B cells from the peritoneal cavity by blocking S1P signaling with FTY720, which binds to S1PR1 as well as to S1PR3‐5 and induces internalization of S1PR1 and inactivation of S1PR1‐mediated signaling.
Intravenous transfer experiments of peritoneal B cells revealed distinctive migration patterns of the various peritoneal B‐cell subsets. It has been reported that peritoneal B cells show a preference to return to the peritoneal environment [18]. Consistent with this finding, our results showed early and robust numbers of peritoneal B cells of all subsets in the peritoneal cavity after intravenous transfer. S1PR4‐deficient peritoneal B‐1a cells showed the quantitatively most important impairment of peritoneal immigration compared with wt B‐1a cells. Interestingly, transferred peritoneal B‐1a cells showed preferential migration to peripheral lymph nodes, whereas migration to the mLNs and spleen was reduced compared with wt cells. Considering the observation of reduced peritoneal immigration of intravenously transferred peritoneal B cells in FTY720‐treated animals reported by Kunisawa et al. [40], our results suggest a synergistic action of S1PR1 and S1PR4 in this process.
In conclusion, our data implicate S1PR4‐mediated signaling in emigration from and immigration into the peritoneal cavity. The net result of these actions is strongly reduced peritoneal B‐1a cell numbers in s1pr 4 –/– animals, suggesting that in vivo inhibition of immigration into the peritoneal cavity quantitatively exceeds the inhibition of emigration from the peritoneal cavity [24]. However, alternative mechanisms must also be considered. Pedersen et al. [38] reported that the splenic compartment is required for the maintenance of normal peritoneal B‐1a cell numbers. In their model, splenectomy resulted in reduced peritoneal B‐1a cell numbers in adult animals. Our data reveal reduced migration of peritoneal B‐1a and B‐1b cells to the spleen after both intraperitoneal and intravenous transfer; thus, reduced accessibility of s1pr 4 –/– B‐1 cells to splenic survival niches could impair their population maintenance. Further research is required to elucidate the precise mechanism. When interpreting peritoneal B‐cell numbers in the abdominal cavity and the omentum, potential relocation of peritoneal B cells to other lymphoid structures within the peritoneal cavity, for example, fat‐associated lymphoid clusters located within the fatty tissue of the mesentery and foliate lymphoid aggregates associated with the serous membranes of the peritoneal cavity should be taken into account [46, 47]. Relocation of peritoneal B cells in these organs has been shown to rely on chemokine signaling, for example, CXCL13 and CCL21 [46, 47]. Further research is required to determine the influence of S1PR4‐mediated signaling on the migration of peritoneal B cells to these compartments.
The mechanistic interpretation of our findings is impeded by the lack of data on physiological intraperitoneal S1P concentrations. Peritoneal B‐1 cells depend on chemokine signaling for their emergence and renewal; both CXCL12 and CXCL13 are essential in this process, and their absence leads to a substantial reduction of peritoneal B‐1 cells [41, 48]. Although these chemokines are produced in the peritoneal environment, so far, no such evidence has been described for S1P, which is primarily found in blood and lymph [22, 49]. Further research on peritoneal S1P concentrations in health and disease is necessary to interpret the interaction of analogous or inverse chemokine and S1P gradients.
Peritoneal B cells are the source of natural antibody production and participate significantly in the production of secretory antibodies found in the blood and gut lumen [16, 50, 51]. To assess whether the observed migratory alterations of peritoneal B cells induced by S1PR4 deficiency affect the capacity for systemic and mucosal antibody production, IgA and IgM levels were measured in the plasma and gut. Although mucosal and systemic IgA levels were unaffected by S1PR4 deficiency, both systemic and mucosal IgM levels were reduced in scid mice reconstituted with s1pr 4 –/– peritoneal cells. Peritoneal B‐1 cells do not secrete substantial amounts of IgM, but upon activation, they migrate to the spleen where they significantly increase antibody production and secretion [15, 52]. Although the final numbers of B‐1a and B‐1b cells in the spleen of reconstituted scid mice were similar, our short‐term transfer experiments revealed a significant reduction in B‐1a cell migration in s1pr 4 –/– animals, suggesting that this migratory defect may be implicated in the observed reduction of systemic IgM levels. This conclusion is further supported by our finding of reduced numbers of IgM‐producing cells in the spleen and the bone marrow of s1pr 4 –/– animals compared with wt controls. In the intestine, IgA is the predominant isotype of secretory antibodies, followed by IgM and IgG [53]. S1P signaling has been shown to affect the migration of peritoneal B cells to the intestinal mucosa in a model using FTY720 blockage of S1P signaling [40]. In this experimental setting, mucosal IgA levels were significantly reduced, whereas mucosal IgM levels were normal. In our model of selective impairment of S1PR4‐mediated signaling, mucosal IgM levels were reduced in mice reconstituted with s1pr 4 –/– cells. These observations suggest that S1PR4‐mediated signaling also affects the migration of peritoneal B‐1 cells to the nonorganised lymphatic tissue of the gut. These data clearly show that binding of the common ligand S1P to distinct S1P receptor classes differentially affects the cellular functions of peritoneal B cells.
The secretion of IL‐10 is a further crucial physiological role of peritoneal B‐1 cells, which confers them with an immunoregulatory role [36]. In line with the reduced B‐1 cell number in s1pr 4 –/– mice, IL‐10 levels were significantly reduced in mice reconstituted with s1pr 4 –/– peritoneal cells. However, the reduction in IL‐10 levels adds another level of complexity to the S1P‐CXCL12/13 interaction. IL‐10 has been shown to affect both CXCL12‐ and CXCL13‐induced chemotaxis by its chemokinetic action in peritoneal B cells in vivo [37]. Further exploration of the role of reduced IL‐10 levels in the genesis of the observed differences in the migratory behavior of s1pr 4 –/– peritoneal B cells is required.
Although no quantitative differences were observed in the splenic B‐cell population after long‐term reconstitution of scid mice with peritoneal B cells of s1pr 4 –/– and wt genotypes, the histopathological analysis of the spleen of reconstituted mice revealed structural differences. Mice reconstituted with wt peritoneal cells developed splenic follicles, as seen in scid mice after transfer of splenocytes, while no follicles were observed in scid mice reconstituted with s1pr 4 –/– peritoneal cells. In scid mice, primary B‐cell follicles also form in the absence of follicular dendritic cells, suggesting a defect in the interaction between s1pr 4 –/– B cells and T cells or other splenic cell populations [54]. As follicles are formed by conventional B‐2 cells, this defect is likely to concern s1pr 4 –/– B‐2 cells. This observation is also in line with earlier descriptions that after polymicrobial intraperitoneal stimulation, migration of peritoneal B cells showed a distinctive distributional pattern in secondary lymphoid organs of s1pr 4 –/– animals [55]. Further research is required to establish the exact mechanisms leading to this distinctive structural feature.
In summary, our data indicate that the distinctive quantitative changes in peritoneal B‐cell populations observed in s1pr 4 –/– mice are due to a modification of chemokine‐induced chemotaxis by S1PR4‐mediated S1P signaling. These quantitative differences result in distinctive functional features, including a modification of systemic and mucosal IgM production. To date, S1PR1 has been described as an S1P receptor involved in the S1P‐mediated control of peritoneal B‐cell functions. Our data identify S1PR4 as a further S1P receptor critically involved in the regulation of peritoneal B‐cell function.
Conflict of interest
The authors declare no conflict of interest.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202350882.
Abbreviations
- BCR
B‐cell receptor
- CFSE
carboxyfluorescein succinimidyl ester
- FLAg
foliate lymphoid aggregate
- GILF
gastrointestinal lavage fluid
- LPS
lipopolysaccharide
- mLN
mesenterial lymph node
- PC
peritoneal cells
- PerC
peritoneal cavity
- S1P
SPHINGOSINE‐1‐phosphate
- S1PR4
S1P receptor type 4
- SC
splenic cells
- scid
severe combined immunodeficiency
- SLO
secondary lymphoid organ
- TI
T‐cell independent
- wt
wildtype
Supporting information
Supporting Information
Acknowledgements
The authors are grateful to Antje Janetzko for dedicated technical support.
Open access funding enabled and organized by Projekt DEAL.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Melichar, B. and Freedman, R. S. , Immunology of the peritoneal cavity: relevance for host‐tumor relation. Int. J. Gynecol. Cancer 2002. 12: 3–17. [DOI] [PubMed] [Google Scholar]
- 2. Liu, M. , Silva‐Sanchez, A. , Randall, T. D. and Meza‐Perez, S. , Specialized immune responses in the peritoneal cavity and omentum. J. Leukoc. Biol. 2021. 109: 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enderes, J. , Van Der Linde, J. , Müller, J. , Tran, B. T. , Von Bernstorff, W. , Heidecke, C. D. and Schulze, T. , FTY720‐induced lymphopenia does not aggravate mortality in a murine model of polymicrobial abdominal sepsis. Shock 2017. 47: 385–394. [DOI] [PubMed] [Google Scholar]
- 4. Ray, A. and Dittel, B. N. , Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayakawa, K. , Hardy, R. R. , Stall, A. M. , Herzenberg, L A. and Herzenberg, L. A. , Immunoglobulin‐bearing B cells reconstitute and maintain the murine Ly‐1 B cell lineage. Eur. J. Immunol. 1986. 16: 1313–1316. [DOI] [PubMed] [Google Scholar]
- 6. Sidman, C. L. , Shultz, L. D. , Hardy, R. R. , Hayakawa, K. and Herzenberg, L A. , Production of immunoglobulin isotypes by Ly‐1+ B cells in viable motheaten and normal mice. Science 1986. 232: 1423–1425. [DOI] [PubMed] [Google Scholar]
- 7. Martin, F. and Kearney, J. F. , CD21high IgMhigh splenic B cells enriched in the marginal zone: distinct phenotypes and functions. Curr. Top Microbiol. Immunol. 1999. 246: 45–50; discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 8. Martin, F. and Kearney, J. F. , B1 cells: similarities and differences with other B cell subsets. Curr. Opin. Immunol. 2001. 13: 195–201. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman, W. , Lakkis, F. G. and Chalasani, G. , B cells, antibodies, and more. Clin. J. Am. Soc. Nephrol. 2016. 11: 137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumgarth, N. , The double life of a B‐1 cell: self‐reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011. 11: 34–46. [DOI] [PubMed] [Google Scholar]
- 11. Haas, K. M. , B‐1 lymphocytes in mice and nonhuman primates. Ann. N Y Acad. Sci. 2015. 1362: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prohaska, T. A. , Que, X. , Diehl, C. J. , Hendrikx, S. , Chang, M. W. , Jepsen, K. , Glass, C. K. et al., Massively parallel sequencing of peritoneal and splenic B cell repertoires highlights unique properties of B‐1 cell antibodies. J. Immunol. 2018. 200: 1702–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayakawa, K. , Hardy, R. R. , Herzenberg, L. A. and Herzenberg, L. A. , Progenitors for Ly‐1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 1985. 161: 1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morris, D. L. and Rothstein, T. L. , Abnormal transcription factor induction through the surface immunoglobulin M receptor of B‐1 lymphocytes. J. Exp. Med. 1993. 177: 857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baumgarth, N. , A hard(y) look at B‐1 cell development and function. J. Immunol. 2017. 199: 3387–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baumgarth, N. , Herman, O. C. , Jager, G. C. , Brown, L E. , Herzenberg, L. A. and Chen, J. , B‐1 and B‐2 cell‐derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 2000. 192: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alugupalli, K. R. , Leong, J. M. , Woodland, R. T. , Muramatsu, M. , Honjo, T. and Gerstein, R. M. , B1b lymphocytes confer T cell‐independent long‐lasting immunity. Immunity 2004. 21: 379–390. [DOI] [PubMed] [Google Scholar]
- 18. Wang, Y. , et al., B cell development and maturation. Adv. Exp. Med. Biol. 2020. 1254: 1–22. [DOI] [PubMed] [Google Scholar]
- 19. Berberich, S. , Förster, R. and Pabst, O. , The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood 2007. 109: 4627–4634. [DOI] [PubMed] [Google Scholar]
- 20. Labadi, A. and Balogh, P. , Differential preferences in serosal homing and distribution of peritoneal B‐cell subsets revealed by in situ CFSE labeling. Int. Immunol. 2009. 21: 1047–1056. [DOI] [PubMed] [Google Scholar]
- 21. Foussat, A. , Balabanian, K. , Amara, A. , Bouchet‐Delbos, L. , Durand‐Gasselin, I. , Baleux, F. , Couderc, J. et al., Production of stromal cell‐derived factor 1 by mesothelial cells and effects of this chemokine on peritoneal B lymphocytes. Eur. J. Immunol. 2001. 31: 350–359. [DOI] [PubMed] [Google Scholar]
- 22. Ansel, K. M. , Harris, R. B. and Cyster, J. G. , CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 2002. 16: 67–76. [DOI] [PubMed] [Google Scholar]
- 23. Moon, H. , Lee, J. G. , Shin, S. H. and Kim, T. J. , LPS‐induced migration of peritoneal B‐1 cells is associated with upregulation of CXCR4 and increased migratory sensitivity to CXCL12. J. Korean Med. Sci. 2012. 27: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleinwort, A. , Lührs, F. , Heidecke, C. D. , Lipp, M. and Schulze, T. , S1P signalling differentially affects migration of peritoneal B cell populations in vitro and influences the production of intestinal IgA in vivo. Int. J. Mol. Sci. 2018. 19: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blaho, V. A. and Hla, T. , An update on the biology of sphingosine 1‐phosphate receptors. J. Lipid. Res. 2014. 55: 1596–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hannun, Y. A. and Obeid, L. M. , Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018. 19: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kihara, Y. , Maceyka, M. , Spiegel, S. and Chun, J. , Lysophospholipid receptor nomenclature review: IUPHAR review 8. Br. J. Pharmacol. 2014. 171: 3575–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cinamon, G. , Matloubian, M. , Lesneski, M. J. , Xu, Y. , Low, C. , Lu, T. , Proia, R. L. et al., Sphingosine 1‐phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 2004. 5: 713–720. [DOI] [PubMed] [Google Scholar]
- 29. Kabashima, K. , Haynes, N. M. , Xu, Y. , Nutt, S. L. , Allende, M. L. , Proia, R. L. and Cyster, J. G. , Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 2006. 203: 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riese, J. , Gromann, A. , Lührs, F. , Kleinwort, A. and Schulze, T. , Sphingosine‐1‐phosphate receptor type 4 (S1P4) is differentially regulated in peritoneal B1 B cells upon TLR4 stimulation and facilitates the egress of peritoneal B1a B cells and subsequent accumulation of splenic IRA B cells under inflammatory conditions. Int. J. Mol. Sci. 2021. 22: 3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golfier, S. , et al., Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine‐1‐phosphate receptor S1P4. FASEB J. 2010. 24: 4701–4710. [DOI] [PubMed] [Google Scholar]
- 32. Davis, G. R. , Santa Ana, C. A. , Morawski, S. G. and Fordtran, J S. , Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980. 78(5 Pt 1): 991–995. [PubMed] [Google Scholar]
- 33. Elson, C. O. , Ealding, W. and Lefkowitz, J. , A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J. Immunol. Methods 1984. 67: 101–108. [DOI] [PubMed] [Google Scholar]
- 34. Ito, T. , Ishikawa, S. , Sato, T. , Akadegawa, K. , Yurino, H. , Kitabatake, M. , Hontsu, S. et al., Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J. Immunol. 2004. 172: 3628–3634. [DOI] [PubMed] [Google Scholar]
- 35. Berberich, S. , DäHne, S. , Schippers, A. , Peters, T. , MüLler, W. , Kremmer, E. , FöRster, R. et al., Differential molecular and anatomical basis for B cell migration into the peritoneal cavity and omental milky spots. J. Immunol. 2008. 180: 2196–2203. [DOI] [PubMed] [Google Scholar]
- 36. O'garra, A. , Chang, R. , Go, N. , Hastings, R. , Haughton, G. and Howard, M. , Ly‐1 B (B‐1) cells are the main source of B cell‐derived interleukin 10. Eur. J. Immunol. 1992. 22: 711–717. [DOI] [PubMed] [Google Scholar]
- 37. Balabanian, K. , Foussat, A. , Bouchet‐Delbos, L. , Couderc, J. , Krzysiek, R. , Amara, A. , Baleux, F. et al., Interleukin‐10 modulates the sensitivity of peritoneal B lymphocytes to chemokines with opposite effects on stromal cell‐derived factor‐1 and B‐lymphocyte chemoattractant. Blood 2002. 99: 427–436. [DOI] [PubMed] [Google Scholar]
- 38. Kyaw, T. , Tay, C. , Krishnamurthi, S. , Kanellakis, P. , Agrotis, A. , Tipping, P. , Bobik, A. et al., B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 2011. 109: 830–840. [DOI] [PubMed] [Google Scholar]
- 39. Pedersen, G. K. , Li, X. , Khoenkhoen, S. , Ádori, M. , Beutler, B. and Karlsson Hedestam, G. B. , B‐1a cell development in splenectomized neonatal mice. Front. Immunol. 2018. 9: 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kunisawa, J. , Kurashima, Y. , Gohda, M. , Higuchi, M. , Ishikawa, I. , Miura, F. , Ogahara, I. et al., Sphingosine 1‐phosphate regulates peritoneal B‐cell trafficking for subsequent intestinal IgA production. Blood 2007. 109: 3749–3756. [DOI] [PubMed] [Google Scholar]
- 41. Nie, Y. , Waite, J. , Brewer, F. , Sunshine, M. J. , Littman, D R. and Zou, Y. R. , The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J. Exp. Med. 2004. 200: 1145–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Donovan, E. E. , Pelanda, R. and Torres, R. M. , S1P3 confers differential S1P‐induced migration by autoreactive and non‐autoreactive immature B cells and is required for normal B‐cell development. Eur. J. Immunol. 2010. 40: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sic, H. , Kraus, H. , Madl, J. , Flittner, K. A. , Von Münchow, A. L. , Pieper, K. , Rizzi, M. et al., Sphingosine‐1‐phosphate receptors control B‐cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J. Allergy Clin. Immunol. 2014. 134: 420–428.e15. [DOI] [PubMed] [Google Scholar]
- 44. Schulze, T. , Golfier, S. , Tabeling, C. , Räbel, K. , Gräler, M. H. , Witzenrath, M. and Lipp, M. , Sphingosine‐1‐phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17‐cell differentiation in a murine model. FASEB J. 2011. 25: 4024–4036. [DOI] [PubMed] [Google Scholar]
- 45. Ryser, M. F. , Ugarte, F. , Lehmann, R. , Bornhäuser, M. and Brenner, S. , S1P(1) overexpression stimulates S1P‐dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits SDF‐1/CXCR4‐dependent migration and in vivo homing. Mol. Immunol. 2008. 46: 166–171. [DOI] [PubMed] [Google Scholar]
- 46. Jia, X. , Gábris, F. , Jacobsen, Ó. , Bedics, G. , Botz, B. , Helyes, Z. , Kellermayer, Z. et al., Foliate lymphoid aggregates as novel forms of serous lymphocyte entry sites of peritoneal B cells and high‐grade B cell lymphomas. J. Immunol. 2020. 204: 23–36. [DOI] [PubMed] [Google Scholar]
- 47. Jackson‐Jones, L. H. , Duncan, S. M. , Magalhaes, M. S. , Campbell, S. M. , Maizels, R. M. , Mcsorley, H. J. , Allen, J. E. et al., Fat‐associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat. Commun. 2016. 7: 12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Höpken, U. E. , Achtman, A. H. , Krüger, K. and Lipp, M. , Distinct and overlapping roles of CXCR5 and CCR7 in B‐1 cell homing and early immunity against bacterial pathogens. J. Leukoc. Biol. 2004. 76: 709–718. [DOI] [PubMed] [Google Scholar]
- 49. Coulomb L, Herminé, A. , et al., Stromal cell‐derived factor 1 (SDF‐1) and antenatal human B cell lymphopoiesis: expression of SDF‐1 by mesothelial cells and biliary ductal plate epithelial cells. Proceedings of the National Academy of Sciences 1999. 96: 8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kroese, F. G. M. , Butcher, E. C. , Stall, A. M. , Lalor, P A. , Adams, S. and Herzenberg, L. A. , Many of the IgA producing plasma cells in murine gut are derived from self‐replenishing precursors in the peritoneal cavity. Int. Immunol. 1989. 1: 75–84. [DOI] [PubMed] [Google Scholar]
- 51. Fagarasan, S. and Honjo, T. , Intestinal IgA synthesis: regulation of front‐line body defences. Nat. Rev. Immunol. 2003. 3: 63–72. [DOI] [PubMed] [Google Scholar]
- 52. Kawahara, T. , Ohdan, H. , Zhao, G. , Yang, Y. G. and Sykes, M. , Peritoneal cavity B cells are precursors of splenic IgM natural antibody‐producing cells. J. Immunol. 2003. 171: 5406–5414. [DOI] [PubMed] [Google Scholar]
- 53. Brown, W. R. , Relationships between immunoglobulins and the intestinal epithelium. Gastroenterology 1978. 75: 129–138. [PubMed] [Google Scholar]
- 54. Gonzalez, M. , Mackay, F. , Browning, J. L. , Kosco‐Vilbois, M. H. and Noelle, R. J. , The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 1998. 187: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riese, J. , Hähnel, C. , Menz, J. , Hannemann, M. , Khabipov, A. , Lührs, F. and Schulze, T. , S1PR4 deficiency results in reduced germinal center formation but only marginally affects antibody production. Front. Immunol. 2022. 13: 1053490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


