Abstract
Mucosal‐associated invariant T cells (MAIT) are innate‐like lymphocytes enriched in mucosal organs where they contribute to antimicrobial defense. APECED is an inborn error of immunity characterized by immune dysregulation and chronic mucocutaneous candidiasis. Reduction in the frequency of circulating MAITs has been reported in many inborn errors of immunity, but only in a few of them, the functional competence of MAITs has been assessed. Here, we show in a cohort of 24 patients with APECED, that the proportion of circulating MAITs was reduced compared with healthy age and sex‐matched controls (1.1% vs. 2.6% of CD3+ T cells; p < 0.001) and the MAIT cell immunophenotype was more activated. Functionally the IFN‐γ secretion of patient MAITs after stimulation was comparable to healthy controls. We observed in the patients elevated serum IFN‐γ (46.0 vs. 21.1 pg/mL; p = 0.01) and IL‐18 (42.6 vs. 13.7 pg/mL; p < 0.001) concentrations. Lower MAIT proportion did not associate with the levels of neutralizing anti‐IL‐22 or anti‐IL‐12/23 antibodies but had a clear negative correlation with serum concentrations of IFN‐γ, IL‐18, and protein C‐reactive protein. Our data suggest that reduction of circulating MAITs in patients with APECED correlates with chronic type 1 inflammation but the remaining MAITs are functionally competent.
Keywords: AIRE, Anti‐cytokine antibodies, APECED, APS‐1, IFN‐γ, IL‐18, Inborn errors of immunity, MAIT
We found a reduced frequency of circulating mucosal‐associated invariant T cells (MAITs) in patients with APECED with a clear negative correlation to inflammatory markers CRP, IL‐18, and IFN‐γ. These findings suggest that the decrease in MAITs is secondary to type 1 immune dysregulation present in the disease.
Introduction
Human inborn errors of immunity (IEI) have revealed important pathways governing mucosal immunity. One of these diseases is an inborn immune dysregulation syndrome called autoimmune polyendocrinopathy, candidiasis, and ectodermal dystrophy (APECED) resulting from mutations in the autoimmune regulator (AIRE). AIRE regulates the expression of tissue‐restricted antigens in thymic medullary epithelial cells [1]. Lack of AIRE leads to failure of negative selection and escape of autoreactive T lymphocytes to the periphery. True to its name, APECED is characterized by autoimmunity against especially endocrine organs and mucocutaneous candidiasis [2]. A key feature of the disease is the almost uniform production of neutralizing anti‐cytokine antibodies toward type I interferons that predispose the patients to severe viral infections [3, 4].
A large proportion of APECED patients have also antibodies against Th17 cytokines IL‐17 which are important in mounting effective anti‐fungal immune responses, and patient T cells have a lower production of IL‐22 after microbial stimulation [5, 6]. Hence, predisposition to mucocutaneous candidiasis in APECED has been suggested to be an additional autoimmune manifestation attributed to the anti‐cytokine antibodies causing a defective Th17 response against Candida spp. A recent analysis of oral mucosal immunity both in patients with APECED and in Aire−/− mice challenged this theory, as the authors could not detect a mucosal Th17 defect, but instead demonstrated that the infiltration of IFN‐γ producing CD4+ and CD8+ T cells into the oral mucosa impaired the mucosal integrity and predisposed to oral candidiasis [7]. We have since replicated these findings in samples from genital mucosa [8]. Dysregulated skewing of mucosal effector cells toward type I inflammation, potentially evoked by the absence of an efficient Th17 response, could therefore be a contributing factor in chronic mucocutaneous candidiasis. In all, the underlying cause of the increased susceptibility to Candida spp. infection in APECED remains unresolved and is most likely multifactorial.
Tissue‐resident and innate‐like lymphocytes are key effectors in the mucosal immune system. Mucosal‐associated invariant T cells (MAIT) are innate‐like lymphocytes enriched in the mucosal tissues while in circulation their frequency is 1 to 10% of αβ T cells [9]. MAITs are characterized by expression of the semi‐invariant T‐cell receptor, consisting of Vα7.2 paired with a limited repertoire of Vbeta chains, and high expression of CD161 (KLBR1, killer cell lectin‐like receptor subfamily B, member 1) which is typically expressed by innate lymphocytes, such as natural killer cells. The best‐defined role for the semi‐invariant MAIT TCR specific for MAITs is to recognize microbe‐originating riboflavin (B2 vitamin) metabolites presented in the major histocompatibility complex class 1‐related protein (MR1) [10]. MAITs can also be activated without TCR involvement by inflammatory cytokines alone, especially IL‐18 [11, 12, 13]. MAITs are believed to contribute to antimicrobial defense at mucosal sites by producing cytokines and performing cytotoxic effector functions but their role in autoimmunity is still unclear since both pathogenic and protective roles have been proposed [14]. Preliminary support for MAIT cell involvement in APECED came from a report where a reduced frequency of circulating MAITs was described in a cohort of eight patients [15].
Reduction in the frequency of MAITs is not an uncommon finding in inborn errors of immunity, but apart from a few conditions (such as MR1 deficiency), a direct causal link between the genetic defect and reduction in MAITs has not been established [16]. Here, we carried out a comprehensive immunophenotypic and functional characterization of MAITs in a large Finnish cohort of patients with APECED. We show that a reduction of circulating MAITs in patients with APECED correlated with chronic type 1 inflammation while the remaining MAITs appeared functionally competent.
Results and discussion
Patients with APECED have a decreased proportion of circulating MAITs
We assessed the frequency of circulating MAITs in peripheral blood in 24 patients with APECED at the mean age of 40.4 years (age range 7–70; 13 females). The relative abundance of CD3+Vα7.2+CD161++ MAITs was markedly reduced in patients compared with age and sex‐matched healthy controls (n = 26) (1.1% vs. 2.6% of CD3+ T cells; Mann–Whitney U test, p < 0.001; Fig. 1A and B). Most MAITs are CD8+ and this population is the most well‐studied, while the role of CD8−CD4− double negative (DN) and CD4+ MAITs remains more elusive. DN MAITs have been suggested to be an MAIT subset arising after prolonged TCR‐mediated stimulation [17]. In patients with APECED, we saw a reduction in the CD4−CD8+ MAIT subset compared with controls (83.2 vs. 88.7% of MAITs; p = 0.02; Fig. 1C) with a relative increase in CD4+CD8− cells (5.0 vs. 2.2% of MAITs; p = 0.05; Fig. 1C) and no difference in DN MAIT subset (9.9 vs. 6.9% of MAITs; ns.).
Figure 1.
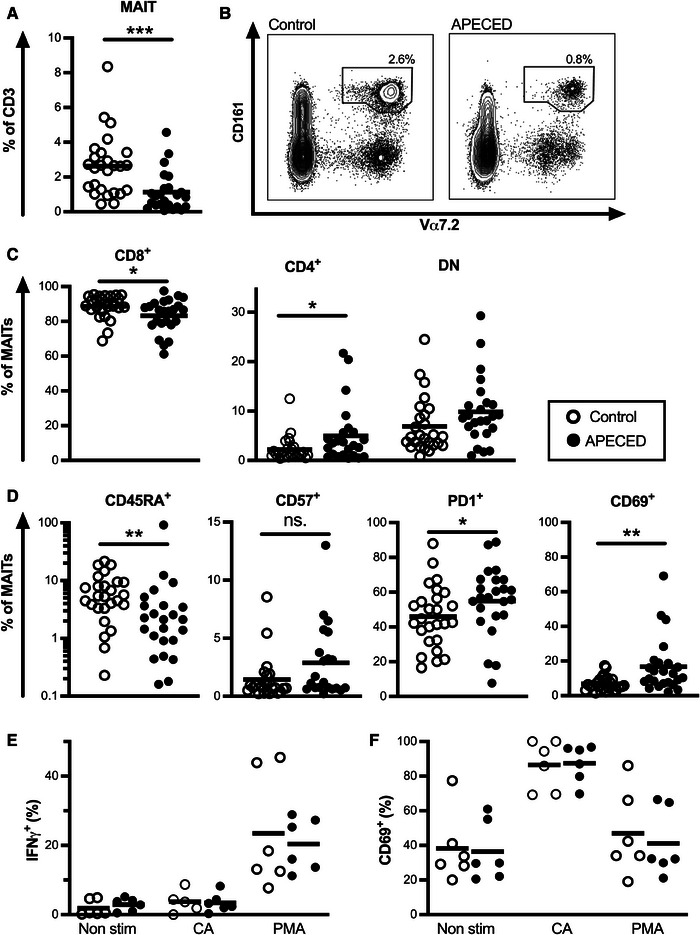
Frequency and function of MAITs in patients with APECED. (A) Frequency of blood CD3+CD161++Vα7.2 MAITs in controls (open circle) and patients with APECED (black circle). (B) A representative flow cytometry plot for MAIT cell frequency of CD3+ T cells for control and a patient is shown. (C) Frequency of CD4−CD8+, CD4+CD8−, and CD4−CD8− double negative (DN) cells of MAITs in controls and patients. (D) Frequency of CD45RA+, CD57+, PD1+, and CD69+ cells of MAITs in controls and patients. (E) Frequency of IFN‐γ+ and (F) CD69+ MAITs without stimulation, after stimulation with C. albicans (CA), and after stimulation with PMA in controls and patients. The gating strategy for (A–D) is shown in Supporting Information Fig. S1A and for (E and F) in Supporting Information Fig. S2. Statistical significance was calculated with Mann–Whitney U‐test; *p < 0.05, **p < 0.01, ***p < 0.001, ns. = non‐significant.
While the TCR Vα7.2+ CD161++ population is enriched for MAITs, it also contains conventional T cells. To confirm our findings, we used MR1 tetramer loaded with 5‐(2‐oxopropylideneamino)‐6‐D‐ribitylaminouracil (5‐OP‐RU) that is specific for the invariant TCR found in the majority of MAITs [18]. A vast majority of CD3+Vα7.2+CD161++ cells stained positive with MR1 tetramer in both patients and healthy controls (94.9 vs. 95.0%, ns.; n = 4+7; Supporting Information Fig. S1C). In CD4+ subset of CD3+Vα7.2+CD161++, there was considerable individual variation in the fraction of cells staining positive for MR1‐5‐OP‐RU tetramer in both patients and controls (range 9–76%; Supporting Information Fig. S1C). The decrease in the CD8+ subset with a reciprocal increase in CD4+ and DN subsets was observed also in the MR1‐5‐OP‐RU tetramer positive MAITs (Supporting Information Fig. S1D). However, using this gating strategy the frequency of CD4+ MAITs was lower in both patients and controls than with Vα7.2+ CD161++ gating reflecting variable contamination of conventional CD4+ T cells to the latter population. This contamination might be proportionally higher in APECED due to a general decrease in circulating MAITs.
MAIT cell depletion has been associated with exhaustion and excessive activation in several chronic viral infections [14]. We therefore next examined MAIT maturation and activation status in APECED patients. In patients, a higher percentage of MAITs were CD45RA‐ memory phenotype than in controls (89.1 vs. 84.6% of MAITs; p = 0.002; Fig. 1D). One patient was a clear outlier with 98% of MAITs being CD45RA+. Expression of CD57, a marker of T cell senescence, was comparable in patients and controls (3.1 vs. 2.4% of MAITs; ns.; Fig. 1D) while expression of exhaustion marker PD‐1 was higher in patients compared with healthy controls (54.9 vs. 46.0; p = 0.04; Fig. 1D). Finally, the patients’ MAITs displayed markedly higher levels of activation and tissue retention marker CD69 (16.7 vs. 6.8%; p = 0.002; Fig. 1D).
Next, we stimulated peripheral blood mononuclear cells (PBMCs) with PMA/ionomycin to evaluate the ability of MAITs to produce cytokines. To evaluate activation via the MR1 — invariant TCR route we used inactivated C. albicans to stimulate MAITs in vitro. PMA stimulation resulted in good IFN‐γ+ production while with C. albicans stimulation IFN‐γ+ production was less pronounced despite robust upregulation of CD69. We did not see any difference in response between patients and controls (n = 6+6) to either of the stimulations (Fig. 1E and F). We also analyzed expression of chemokine receptors CCR2, CCR4, CCR5, CCR6, CCR7, CXCR3, and CXCR5 ex vivo in MAITs (n = 6+6). Almost all MAITs expressed CCR2 and CCR5 in both patients and healthy controls reflecting their highly inflammatory nature, also CCR6 expression was high in both groups (Supporting Information Fig. S3). We detected no difference in the expression of chemokine receptors between the groups.
In conclusion, in a cohort of 24 APECED patients representing approximately one‐third of living Finnish patients with APECED, we found a clear decrease in the fraction of circulating MAITs, corroborating an earlier report with a smaller sample [15]. Furthermore, our extended characterization of MAITs reveals that especially the CD8+ subset was reduced, while the remaining MAIT cells were more often activated and retained their ability to produce IFN‐γ.
Proportion of MAITs does not correlate with anti‐commensal antibodies
MAIT homeostasis is intimately influenced by interactions with commensal microbiota. We have previously shown that patients with APECED have altered gut microbiota characterized by expanded Bacteroidetes and Proteobacteria phyla, which include many microbes encoding the riboflavin pathway capable of activating MAITs [19, 20]. APECED patients also have increased antibody responses against commensal microbes [21], and chronic gut inflammation in APECED could account for increased sequestration of MAITs into the gut mucosa as is observed in inflammatory bowel disease [22]. We next studied whether altered anti‐commensal immunity might be associated with the reduction of MAITs using anti‐Saccharomyces cerevisiae antibodies (ASCA) as a marker. In Crohn's disease, ASCA antibodies are associated with more severe disease [23]. Twelve out of the 21 (57.1%) tested patients were positive for ASCA compared with 4 out of 22 controls (18.2%; Fisher exact t‐test p = 0.01). However, no correlation between ASCA and MAIT frequency was observed, nor did we see any difference in the frequency of MAITs in ASCA‐positive and negative patients (Fig. 2A, Supporting Information Fig. S4A).
Figure 2.
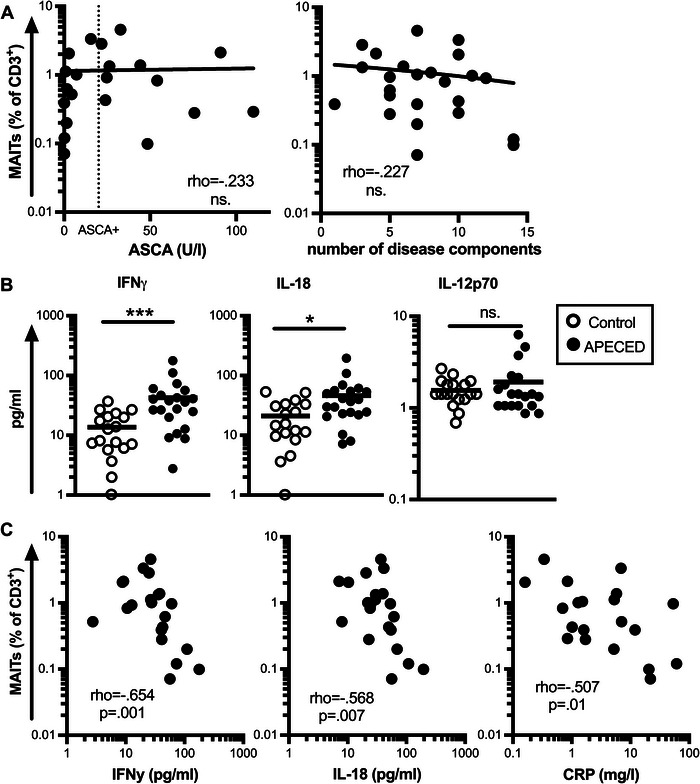
Association of MAIT proportion to serum cytokine concentrations in patients with APECED. (A) Correlation of anti‐Saccharomyces cerevisiae antibodies (ASCA) and number of disease components with the proportion of circulating MAITs in patients with APECED. (B) Serum IFN‐γ, IL‐18, and IL‐12 concentrations in controls (open circle) and patients with APECED (black). (C) Correlation of serum IFN‐γ, IL‐18, and CRP concentrations with the frequency of MAITs in patients with APECED. Statistical significance was calculated with nonparametric Spearman's rho (A, C) and Mann–Whitney U‐test (B); *p < 0.05, ***p < 0.001, ns. = non‐significant.
Frequency of MAITs does not align with the number of disease components
We also tested for possible correlation between the frequency of MAITs and the severity of APECED. There are currently no established scoring criteria or laboratory markers to measure the clinical severity of APECED. Therefore, as a surrogate, we used the number of disease components as a robust proxy, but no correlation was observed (Fig. 2A). Further, the frequency of MAITs had no correlation with patient age (Spearman's rho −.206, ns.).
Reduced proportion of circulating MAITs does not correlate with anti‐cytokine antibody levels
A previous publication examining MAITs in APECED hypothesized that their reduction might be associated with neutralizing anti‐cytokine antibodies as neutralizing IL‐22 antibodies were also found in the patients’ saliva [15]. Neutralizing antibodies against IL‐22 were detected in 23/24 of our patients. With such a high rate of patients displaying neutralizing antibodies, we were unable to make comparison to patients without antibodies, but the titer of neutralizing anti‐IL‐22 antibodies did not correlate with the frequency of MAITs in patients (Supporting Information Fig. S4B). Another possible cause of reduced MAITs might be antibodies against IL‐12/23, reported to cause an MAIT defect in patients with thymoma, a neoplasm of the thymus with somatically reduced expression of AIRE [24]. However, no anti‐IL12/23 antibodies were detected in our cohort of patients with APECED (data not shown).
MAIT proportion shows a clear negative association with serum IFN‐γ, IL‐18, and CRP concentration
MAITs are activated upon stimulation with IL‐18 and IL‐12 [11, 13]. We therefore measured the concentration of these cytokines in the serum using a flow‐cytometry‐based multiplexed assay. The IL‐18 concentration was significantly elevated in patients compared with healthy controls (42.6 vs. 13.7 pg/mL; p < 0.001; Fig. 2B). The concentration of IL‐12 was elevated in three patients but its overall level was comparable in patients and controls (1.7 vs. 1.5 pg/mL; ns.; Fig. 2B). We also found serum IFN‐γ to be increased in patients (46.0 vs. 21.1 pg/mL; p = 0.01) supporting previous findings of a pronounced Th1 skewing in patients’ circulating T lymphocytes and mucosa [7, 8, 15]. Using the acute phase protein C‐reactive protein (CRP), a widely used clinical marker of inflammation, we detected low‐grade inflammation in many of the patients (range 0–61, mean 7.78 mg/L; Fig. 2C). The serum concentrations of IL‐18, IFN‐γ, and CRP all showed a clear negative correlation with the proportion of circulating MAITs (Fig. 2C).
Reduction of circulating MAITs is not an uncommon finding in IEI, but a causal link between the genetic defect and MAIT reduction, except for a few specific genetic defects, has not been established [16]. Depletion of circulating MAITs has also been reported in numerous other conditions ranging from acute bacterial to chronic viral infections, autoimmune disease, cancer, gout, and even repeated injuries [12, 14, 25–30]. What these conditions of greatly varying etiology have in common is inflammation. MAITs do not need a specific antigen for their activation but can also be activated by inflammatory cytokines alone. They have a high expression of IL‐18 receptor and sensitivity for IL‐18 stimulation. In multiple sclerosis and systemic lupus erythematosus, reduced frequency of circulating MAITs correlates with serum IL‐18 concentration [25, 26]. In systemic lupus erythematosus, freshly isolated MAITs were prone to apoptosis [25], and prolonged exposure to IL‐12 and IL‐18 led to their death in vitro [12]. While we did not have a chance to study if IFN‐γ or IL‐18 stimulation directly leads to cell death of MAITs in APECED patients, given the negative correlation we observe in APECED between the frequency of circulating MAITs with serum IL‐18 as well as IFN‐γ concentration, we propose that the reduction in the proportion of circulating MAITs is secondary to their excessive consumption in chronic type 1 inflammation milieu.
Data limitations and perspectives
We could not study the thymic development of MAITs in APECED, but we deem it unlikely that it would be disturbed, as MAIT commitment in the thymus relies on MR1‐dependent recognition of predominantly commensal antigens presented by cortical CD4+CD8+ thymocytes [31]. MAIT commitment thus occurs in the thymic cortex where AIRE is not expressed [32], although indirect effects in the thymic microenvironment caused by AIRE deficiency cannot be ruled out.
While analyses of chemokine receptor expression indicated no difference in MAITs of the patients and controls, without biopsies we cannot exclude the possibility that MAIT reduction in the circulation might reflect their increased homing to the gut or other tissues. Identifying MAITs in formalin‐fixed paraffin‐embedded tissue samples is currently not possible, the development of such a method would advance our understanding of MAITs’ distribution in human tissues.
Conclusion
In conclusion, we establish a strong association between excessive type I inflammation and a reduction in the proportion of circulating MAITs in patients with APECED without observing a link with anti‐cytokine antibodies. A similar phenomenon is likely at play in other IEIs characterized by overt immune activation, which is in many conditions IFN‐γ driven [33]. Although the root causes of excessive inflammation in different conditions may vary, the innate‐like nature of MAITs might render them vulnerable to the detrimental effects of acute or chronic immune dysregulation. For instance, a reduced frequency of MAITs is observed during the acute inflammation phase in Puumala virus and SARS‐CoV2 infection [29, 30]. Interestingly, after a hantavirus infection, the MAIT cell compartment is completely restored, while after severe COVID‐19, MAITs recover with signs of residual functional abnormalities [29, 30]. It remains intriguing to see whether the treatment of chronic type I inflammation in APECED with JAK inhibitors [34] will lead to full restoration of the MAIT compartment and improved mucosal integrity. We also advise caution in interpreting the reduction in MAIT frequency as a “disease‐specific” feature as it is often likely also in other conditions the consequence of excessive overall inflammation.
Materials and methods
Study cohort
The recruitment of patients and collection of clinical data have been previously described [3]. PBMCs were available from 24 patients with APECED with a mean age of 40.4 years (age range 7–70; 13 women). The following clinical components were assessed when calculating the number of disease components: adrenal insufficiency, hypoparathyroidism, candidiasis, alopecia, enamel dysplasia, oral squamous cell carcinoma, keratitis, vitiligo, rash with fever, hypogonadism, type I diabetes, diarrhea, obstipation, gastritis, celiac disease, malabsorption, hepatitis, exocrine pancreatic insufficiency, asplenia, nephritis, hypothyroidism, growth hormone deficiency. The control group consisted of 26 healthy volunteers (mean age 38.2 years, age range 10–66, 15 women).
The study was conducted according to the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of Helsinki University Hospital. All study participants or their guardians (for subjects aged <18 years) gave informed written consent.
Flow cytometry
PBMCs were isolated using Ficoll‐Paque (GE Lifesciences) gradient centrifugation. The cells were frozen fresh after the isolation with CTL‐Cryo ABC (CTL) freezing kit and stored at −140°C before analysis. Fresh or thawed cells were incubated for 30 min at +4°C with antibodies and with live/dead fixable green dead cell stain (at dilution of 1:500; ThermoFisher) that were diluted in Brilliant Stain Buffer (BD Biosciences) for staining of surface antigens. For detection of transcription factors, the cells were permeabilized after surface staining with FoxP3 transcription factor staining set (eBioscience) and of intracellular cytokines with fixation/permeabilization solution kit (BD Bioscience) as instructed by the manufacturer. The data was acquired using LSR Fortessa (BD Biosciences) and analyzed with FlowJo (BD Biosciences, LLC). The gating was mostly done using biological negative populations. The antibodies used in the study are shown in Supporting Information Table S1. The optimal concentration for antibodies was titrated with live PBMCs.
For analysis of chemokine receptor expression thawed cells were stained with Fixable Viability Stain 440UV (at dilution of 1:1000, BD Bioscience) for 10 min at room temperature. A cocktail containing antibodies for chemokines receptors and MR1 tetramer in a mix of Brilliant Stain Buffer (BD Bioscience) and True‐Stain monocyte blocker (BioLegend) with Human TruStain FcX (BioLegend) was added and incubated first at room temperature for 10 min, followed by at 37°C for 40 min. After washing, antibodies for surface antigens were added for staining at 4°C for 6 h. Permeabilization was performed as mentioned above. Intracellular staining was carried out at 4°C for 16 h. The data was acquired using FACSDiscover S8 cell sorter (BD Biosciences).
MAIT stimulation
C. albicans were fixed with CellFIX (BD Biosciences) and added to the cell culture in a concentration of 3 × 106 CFU and an unconjugated anti‐CD28 antibody was added after 1 h of culture. Cells were incubated for 18 h and Brefeldin A (BD Biosciences) was added for the last 6 h. For PMA stimulation a commercial PMA/ionomycin preparation was used (Leukocyte Activation Cocktail, with BD GolgiPlug, BD Biosciences) for 6 h.
Cytokine, CRP, and anti‐Saccharomyces cerevisiae antibody quantification
Cytokine 25‐Plex Human ProcartaPlex Panel 1B (Thermo Fischer) was performed from serum samples stored at −80°C. The results were acquired with the Bio‐Plex 200 system (Biorad)
CRP was measured from all patients and hsCRP was measured from all but four patients (n = 20) in a clinically validated laboratory at HUS Diagnostic Center (Helsinki, Finland). These data were combined for the correlation analysis. The four patients from whom we did not have hsCRP measurement available all had CRP under the detection rate (<3) with the less sensitive method and were excluded from the correlation analysis.
Antibodies against S. cerevisiae were evaluated with anti‐Saccharomyces cerevisiae antibodies IgG test kit (Bio‐Rad) and the cutoff for positive samples was set at 15 U/mL as instructed by the manufacturer. Absorbances were read using the iEMS reader MF (Labsystems).
Anti‐cytokine antibody measurements
The anti‐cytokine antibodies were measured using a radioligand binding assay as previously described [3].
Statistical analysis
The statistical analysis was performed using SPSS version 23 (IBM). Nonparametric Mann–Whitney U‐test and Spearman's rank correlation coefficient were used with the limit for statistical significance set to 0.05.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Author contributions
All authors contributed to the study's conception and design. Sample collections were performed by Saila Laakso and Outi Mäkitie, and experimental preparation and data analysis were performed by Iivo Hetemäki, Joona Sarkkinen, Huai Hui Wong, Nelli Heikkilä, Simo Miettinen, and Mikko Mäyränpää. The first draft of the manuscript was written by Iivo Hetemäki, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval statement
The study was conducted according to the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of Helsinki University Hospital.
Patient consent statement
All study participants or their guardians (for subjects aged <18 years) gave informed written consent.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.202451189.
Abbreviations
- APECED
autoimmune polyendocrinopathy candidiasis ectodermal dystrophy
- AIRE
autoimmune regulator
- CRP
protein C‐reactive protein
- DN
double negative
- IEI
inborn errors of immunity
- MAIT
mucosal‐associated invariant T cell
- 5‐OP‐RU
5‐(2‐oxopropylideneamino)‐6‐d‐ribitylaminouracil
Supporting information
Supporting Information
Supporting Information
Acknowledgements
The authors thank Eveliina Holappa, Nea Boman, Åsa Hallgren, and Tamas Bazsinka for their technical assistance. The MR1 tetramer technology used in the study was developed jointly by Dr. James McCluskey, Dr. Jamie Rossjohn, and Dr. David Fairlie, and the material was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne.
This work was funded by Helsinki University research funds, the Helsinki University Doctoral Programme in Biomedicine, Helsinki University Hospital, Emil Aaltonen Foundation, Instrumentarium Science Foundation, Sigrid Jusélius Foundation, Novo Nordisk Foundation, Foundation for Pediatric Research, Finnish Medical Foundation and EJPRD JTC2019 & Academy of Finland (308912 and 308913).
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request due to privacy restrictions.
References
- 1. Anderson, M. S. and Su, M. A. , AIRE expands: new roles in immune tolerance and beyond. Nat. Rev. Immunol. 2016. 16: 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferré, E. M. N. , Schmitt, M. M. and Lionakis, M. S. , Autoimmune polyendocrinopathy‐candidiasis‐ectodermal dystrophy. Front Pediatr. 2021. 9: 723532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hetemäki, I. , Laakso, S. , Välimaa, H. , Kleino, I. , Kekäläinen, E. , Mäkitie, O. and Arstila, T. P. , Patients with autoimmune polyendocrine syndrome type 1 have an increased susceptibility to severe herpesvirus infections. Clin. Immunol. 2021. 231: 108851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bastard, P. , Orlova, E. , Sozaeva, L. , Lévy, R. , James, A. , Schmitt, M. M. , Ochoa, S. et al., Preexisting autoantibodies to type I IFNs underlie critical COVID‐19 pneumonia in patients with APS‐1. J. Exp. Med. 2021. 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Puel, A. , Döffinger, R. , Natividad, A. , Chrabieh, M. , Barcenas‐Morales, G. , Picard, C. , Cobat, A. et al., Autoantibodies against IL‐17A, IL‐17F, and IL‐22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010. 207: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kisand, K. , Bøe Wolff, A. S. , Podkrajšek, K. T. , Tserel, L. , Link, M. , Kisand, K. V. , Ersvaer, E. et al., Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17‐associated cytokines. J. Exp. Med. 2010. 207: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Break, T. J. , Oikonomou, V. , Dutzan, N. , Desai, J. V. , Swidergall, M. , Freiwald, T. , Chauss, D. et al., Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 2021. 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hetemäki, I. , Saari, V. , Yohannes, D. A. , Holopainen, E. , Holster, T. , Jokiranta, S. , Mäyränpää, M. I. et al., Increased type 1 inflammation in gynecological cervicovaginal samples in patients with APS‐1. J. Allergy Clin. Immunol. 2024. 10.1016/j.jaci.2024.02.012. [DOI] [PubMed] [Google Scholar]
- 9. Legoux, F. , Salou, M. and Lantz, O. , MAIT cell development and functions: the microbial connection. Immunity 2020. 53: 710–723. [DOI] [PubMed] [Google Scholar]
- 10. Awad, W. , Ciacchi, L. , McCluskey, J. , Fairlie, D. P. and Rossjohn, J. , Molecular insights into metabolite antigen recognition by mucosal‐associated invariant T cells. Curr. Opin. Immunol. 2023. 83: 102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Wilgenburg, B. , Scherwitzl, I. , Hutchinson, E. C. , Leng, T. , Kurioka, A. , Kulicke, C. , De Lara, C. et al., MAIT cells are activated during human viral infections. Nat. Commun. 2016. 7: 11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Böttcher, K. , Rombouts, K. , Saffioti, F. , Roccarina, D. , Rosselli, M. , Hall, A. , Luong, T. et al., MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 2018. 68: 172–186. [DOI] [PubMed] [Google Scholar]
- 13. Ussher, J. E. , Bilton, M. , Attwod, E. , Shadwell, J. , Richardson, R. , De Lara, C. , Mettke, E. et al., CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL‐12+IL‐18 in a TCR‐independent manner. Eur. J. Immunol. 2014. 44: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godfrey, D. I. , Koay, H.‐F. , McCluskey, J. and Gherardin, N. A. , The biology and functional importance of MAIT cells. Nat. Immunol. 2019. 20: 1110–1128. [DOI] [PubMed] [Google Scholar]
- 15. Kaleviste, E. , Rühlemann, M. , Kärner, J. , Haljasmägi, L. , Tserel, L. , Org, E. , Trebušak Podkrajšek, K. et al., IL‐22 paucity in APECED is associated with mucosal and microbial alterations in oral cavity. Front. Immunol. 2020. 11: 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howson, L. J. and Bryant, V. L. , Insights into mucosal associated invariant T cell biology from human inborn errors of immunity. Front. Immunol. 2022. 13: 1107609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dias, J. , Boulouis, C. , Gorin, J.‐B. , Van Den Biggelaar, R. , Lal, K. G. , Gibbs, A. , Loh, L. et al., The CD4‐CD8‐ MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc. Natl. Acad. Sci. U. S. A. 2018. 115: E11513–E11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corbett, A. J. , Eckle, S. B. G. , Birkinshaw, R. W. , Liu, L. , Patel, O. , Mahony, J. , Chen, Z. et al., T‐cell activation by transitory neo‐antigens derived from distinct microbial pathways. Nature 2014. 509: 361–365. [DOI] [PubMed] [Google Scholar]
- 19. Hetemäki, I. , Jian, C. , Laakso, S. and Mäkitie, O. , Fecal bacteria implicated in biofilm production are enriched and associate to gastrointestinal symptoms in patients with APECED–a pilot study. Frontiers in 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tastan, C. , Karhan, E. , Zhou, W. , Fleming, E. , Voigt, A. Y. , Yao, X. , Wang, L. et al., Tuning of human MAIT cell activation by commensal bacteria species and MR1‐dependent T‐cell presentation. Mucosal Immunol. 2018. 11: 1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hetemäki, I. , Jarva, H. , Kluger, N. , Baldauf, H.‐M. , Laakso, S. , Bratland, E. , Husebye, E. S. et al., Anticommensal responses are associated with regulatory T cell defect in autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy patients. The Journal of Immunology 2016. 196: 2955–2964. [DOI] [PubMed] [Google Scholar]
- 22. Serriari, N.‐E. , Eoche, M. , Lamotte, L. , Lion, J. , Fumery, M. , Marcelo, P. , Chatelain, D. et al., Innate mucosal‐associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 2014. 176: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prideaux, L. , De Cruz, P. , Ng, S. C. and Kamm, M. A. , Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm. Bowel Dis. 2012. 18: 1340–1355. [DOI] [PubMed] [Google Scholar]
- 24. Gao, Y. , Rae, W. , Ramakrishnan, K. A. , Barcenas‐Morales, G. , Döffinger, R. , Eren, E. , Faust, S. N. et al., Mucosal‐associated invariant T (MAIT) cells are impaired in Th17 associated primary and secondary immunodeficiencies. PLoS One 2016. 11: e0155059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiba, A. , Tamura, N. , Yoshikiyo, K. , Murayama, G. , Kitagaichi, M. , Yamaji, K. , Takasaki, Y. et al., Activation status of mucosal‐associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis Res. Ther. 2017. 19: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willing, A. , Leach, O. A. , Ufer, F. , Attfield, K. E. , Steinbach, K. , Kursawe, N. , Piedavent, M. et al., CD8+ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL‐18 serum levels in multiple sclerosis. Eur. J. Immunol. 2014. 44: 3119–3128. [DOI] [PubMed] [Google Scholar]
- 27. Cho, Y.‐N. , Jeong, H.‐S. , Park, K.‐J. , Kim, H.‐S. , Kim, E.‐H. , Jin, H.‐M. , Jung, H.‐J. et al., Altered distribution and enhanced osteoclastogenesis of mucosal‐associated invariant T cells in gouty arthritis. Rheumatology 2020. 59: 2124–2134. [DOI] [PubMed] [Google Scholar]
- 28. Jo, Y.‐G. , Choi, H.‐J. , Kim, J.‐C. , Cho, Y.‐N. , Kang, J.‐H. , Jin, H.‐M. , Kee, S.‐J. et al., Deficiencies of circulating mucosal‐associated invariant T cells and natural killer T cells in patients with multiple trauma. J. Korean Med. Sci. 2017. 32: 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maleki, K. T. , Tauriainen, J. , García, M. , Kerkman, P. F. , Christ, W. , Dias, J. , Wigren Byström, J. et al., MAIT cell activation is associated with disease severity markers in acute hantavirus infection. Cell Reports Medicine 2021. 2: 100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kammann, T. , Gorin, J.‐B. , Parrot, T. , Gao, Y. , Ponzetta, A. , Emgård, J. , Maleki, K. T. et al., Dynamic MAIT cell recovery after severe COVID‐19 is transient with signs of heterogeneous functional anomalies. J. Immunol. (2023). 10.4049/jimmunol.2300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Legoux, F. , Bellet, D. , Daviaud, C. , El Morr, Y. , Darbois, A. , Niort, K. , Procopio, E. et al., Microbial metabolites control the thymic development of mucosal‐associated invariant T cells. Science 2019. 366: 494–499. [DOI] [PubMed] [Google Scholar]
- 32. Heino, M. , Peterson, P. , Kudoh, J. , Nagamine, K. , Lagerstedt, A. , Ovod, V. , Ranki, A. et al., Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem. Biophys. Res. Commun. 1999. 257: 821–825. [DOI] [PubMed] [Google Scholar]
- 33. Unger, S. , Seidl, M. , Van Schouwenburg, P. , Rakhmanov, M. , Bulashevska, A. , Frede, N. , Grimbacher, B. et al., The TH1 phenotype of follicular helper T cells indicates an IFN‐γ‐associated immune dysregulation in patients with CD21low common variable immunodeficiency. J. Allergy Clin. Immunol. 2018. 141: 730–740. [DOI] [PubMed] [Google Scholar]
- 34. National Institute of Allergy and Infectious Diseases , Evaluate the Efficacy and Safety of Ruxolitinib on Hair Regrowth in Patients With Autoimmune Polyendocrinopathy Candidiasis Ectodermal Dystrophy (APECED)‐Associated Alopecia Areata. Clinical Trial 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request due to privacy restrictions.


