Abstract
Background
Digital adherence technologies (DATs) with associated differentiated care are potential tools to improve tuberculosis (TB) treatment outcomes and reduce associated costs for both patients and healthcare providers. However, the balance between epidemiological and economic benefits remains unclear. Here, we used data from the ASCENT trial to estimate the potential long-term epidemiological and economic impact of DAT interventions in Ethiopia.
Methods
We developed a compartmental transmission model for TB, calibrated to Ethiopia and parameterised with patient and provider costs. We compared the epidemiological and economic impact of two DAT interventions, a digital pillbox and medication labels, to the current standard of care, assuming each was introduced at scale in 2023. We projected long-term TB incidence, mortality and costs to 2035 and conducted a threshold analysis to identify the maximum possible epidemiological impact of a DAT intervention by assuming 100% treatment completion for patients on DAT.
Findings
We estimated small and uncertain epidemiological benefits of the pillbox intervention compared with the standard of care in Ethiopia, with a difference of −0.4% (95% uncertainty interval (UI) −1.1%; +2.0%) incident TB episodes and −0.7% (95% UI −2.2%; +3.6%) TB deaths. However, our analysis also found large total provider and patient cost savings (US$163 (95% UI US$118; US$211) and US$3 (95%UI: US$1; US$5), respectively, over 2023–2035), translating to a 50.2% (95% UI 35.9%; 65.2%) reduction in total cost of treatment. Results were similar for the medication label intervention. The maximum possible epidemiological impact a theoretical DAT intervention could achieve over the same timescale would be a 3% (95% UI 1.4%; 5.5%) reduction in incident TB and an 8.2% (95% UI 4.4%; 12.8%) reduction in TB deaths.
Interpretation
DAT interventions, while showing limited epidemiological impact, could substantially reduce TB treatment costs for both patients and the healthcare provider.
Keywords: Tuberculosis, Health economics, Mathematical modelling
WHAT IS ALREADY KNOWN ON THIS TOPIC
Recent empirical epidemiological evidence on digital adherence technologies (DATs) is contradictory, with different studies and settings showing a mixture of both positive impact and no impact of DAT interventions on tuberculosis (TB) treatment outcomes.
WHAT THIS STUDY ADDS
This is the first modelling study to use direct trial results to estimate long-term epidemiological and economic outcomes. We used results from the ASCENT trial and modelled two DAT interventions in Ethiopia.
Results showed limited epidemiological impact of DATs in Ethiopia, but substantial reductions in TB treatment costs for both patients and the healthcare provider.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Estimated savings over a 12-year time frame indicate that DATs could have important budgetary implications from the provider side. In 2023 alone, these savings would have been equivalent to ~10%–11% of Ethiopia’s annual TB budget.
Introduction
Tuberculosis (TB) is the 13th leading cause of death worldwide, and world’s top infectious killer, despite being largely treatable.1 Effective first-line therapy for TB is widely available,2 however, standard treatment is 6 months and often requires that patients make frequent visits to the healthcare facility for supervised treatment. This can make supporting treatment adherence both challenging and costly, especially in resource-constrained settings.3,6
Ethiopia is 1 of the 30 high TB burden countries, with an estimated incidence of 126 TB episodes per 100 000 in 2022.7 Ethiopia is also one of only seven countries to have reached the first milestone of the WHO End TB Strategy, with a reduction of 20% in estimated TB incidence and 35% reduction in TB deaths in 2020 compared with 2015.8 However, it is unknown if future End TB targets, of 90% and 95% reductions in incidence and deaths, respectively, and no people with TB facing catastrophic costs (ie, costs due to a TB disease episode of >20% of the annual household income), will be met. Key indicators for monitoring implementation of the End TB Strategy at global and national levels include TB treatment coverage and treatment success rates, with recommended target levels of at least 90% for both. Most recent estimates show that Ethiopia attains a treatment coverage of 73% and a treatment success rate of 86%,7 8 leaving limited room for epidemiological improvement but unclear economic opportunities. Ethiopia’s per capita expenditure has steadily increased in the last two decades (from US$4.50 in 1995/1996 to US$36.40 in 2019/2020), however, this amount is still low compared with the average per capita health expenditure among low-income African countries (US$43).9 Additionally, recent surveys have shown that at least 64.7% of TB patients faced catastrophic costs in 2021 and that this percentage can substantially decrease if interventions targeting the main drivers of catastrophic costs are implemented.10 In the latest National Strategic Plan, the Ministry of Health committed to reduce the families facing catastrophic costs due to TB to below 25%.11
Digital adherence technologies (DATs) include such tools as phone-based technologies, digital pillboxes and video-supported therapy. These, combined with associated differentiated care, are one potential tool to provide more patient-centred treatment support.12 Through improved treatment support, these tools could reduce missed doses,13,15 and as a consequence could potentially improve treatment outcomes,16 therefore, reducing population-level TB incidence and mortality.17 DAT interventions could also reduce the number of visits a patient needs to make to the healthcare facility, reducing costs for both patients and healthcare providers, allowing the latter to focus on those patients requiring additional support.18 They could contribute to the End TB goal of reducing TB-associated catastrophic costs and meet national targets.19
Recent empirical epidemiological evidence, however, is contradictory, with different studies and settings showing a mixture of both positive impact and no impact of DAT interventions on TB treatment outcomes.20 21 Some studies have shown DAT interventions lead to a reduction in risk of on-treatment death, loss to follow-up, or treatment failure,22 23 or an increase in treatment completion and cure rates24 compared with the standard of care (SoC). However, others have shown no statistically significant impact.1525,29 Meanwhile, some economic analyses agree that DAT interventions are likely to reduce catastrophic costs30 and may be a cost-effective tool for TB treatment support.31,34
The Adherence Support Coalition to End TB (ASCENT) project in Ethiopia was a large cluster randomised trial (PACTR202008776694999) which implemented and assessed the effectiveness of two DAT interventions (digital pillboxes and medication labels) compared with the SoC, for drug-susceptible pulmonary TB.35 The participants in the intervention arms were linked to a web-based adherence platform for daily adherence monitoring and differentiated response to participant adherence for those who missed doses.35 36 The primary outcome comprised on-treatment death, loss to follow-up, treatment failure, switch to drug-resistant TB treatment or recurrence 6 months after the end of treatment; secondary outcomes included loss to follow-up. Effectiveness results showed that neither the pillbox (adjusted OR 1.04, 95% CI 0.74 to 1.45) nor the label (adjusted OR 1.14, 95% CI 0.83 to 1.61) interventions reduced the risk of the primary composite outcome. However, a reduction of loss to follow-up (adjusted OR 0.37, 95% CI 0.15 to 0.95) was observed in the label arm.37 The within-trial health economic analysis found that the labels and pillbox arms had a 60%–70% likelihood of being cost-effective when compared against the SoC, at a cost-effectiveness threshold (CET) of up to US$100 per disability-adjusted life-years (DALY) averted—largely driven by the effect neutral finding and human resources time saving.38
Here, we used results from the ASCENT trial to model the long-term epidemiological and economic impact of these DAT interventions, and their prospective contribution to achieving Ethiopia’s End TB Strategy targets.
Methods
We built a dynamic, age-structured, compartmental model for the transmission of Mycobacterium tuberculosis infection in Ethiopia, to study the impact of DAT interventions on TB incidence, mortality and costs. The model’s structure (online supplemental figure 1) was informed by the care cascade in Ethiopia, including compartments for individuals with TB infection (early (Le) and late (Ll)), undiagnosed active TB (D), diagnosed TB undergoing treatment (Dt and Dt*) and on-treatment lost to follow-up (LTFU) (F). The model included transmission of infection to healthy individuals, reinfection of already infected individuals, reinfection of cured individuals, reactivation and relapse. Due to the young age of Ethiopia’s population, two age groups were considered in the model: 0 – 14 years and ≥15 years. The model did not explicitly consider HIV coinfection, nor drug resistance, as individuals with drug-resistant TB were excluded from the ASCENT trial. BCG vaccination in the younger age group was included by assuming a constant coverage of 70%.7
The model was initialised to Ethiopia’s 2010/2011 TB prevalence,39 calibrated to Ethiopia’s estimates of TB incidence and mortality from 2011 to 2019 using WHO Global TB reports40 and parametrised using trial data37 to inform treatment outcomes. The model was coded using R (V.4.1.2) and calibration was performed through the History Matching and Emulation (hmer) package,41 42 which uses Bayes Linear emulation and history matching. Model schematic, description and equations, can be found in online supplemental table 1 and calibration targets (online supplemental table 2).
Costs per patient in Ethiopia by treatment outcome (treatment completed, LTFU or died) are reported in online supplemental table 3. These were estimated as the sum of treatment costs and intervention costs (for pillbox and label scenarios). Treatment costs include staff salaries, hospitalisations and drug regimen costs and were collected during the trial for a subsample of participants, where a full cost breakdown can be found in the within-trial health economic analysis.38 Intervention costs include technology, network and training costs and were instead estimated in a post-trial scenario for Ethiopia as US$16.56 and US$4.12 for pillbox and label, respectively. The same was done for four additional countries for comparison: Tanzania, South Africa, Philippines and Ukraine (see online supplemental file 2) for full intervention cost breakdown for the five countries). Data for these countries were collected during similar trials (but with slightly different designs) under ASCENT.43
DALYs were estimated using modelled incident TB and deaths occurring every year from 2023 to 2035, in order to estimate years of life lost and years of life lived with disability. A 3% yearly discount was applied to both costs and health gains. Probabilistic sensitivity analysis was also performed by running the model 1000 times, estimating means and uncertainty intervals (UI). Results were reported in the form of incremental costs and DALYs averted for each of the two DAT interventions compared with the SoC, estimating incremental cost-effectiveness ratios where appropriate.
We conducted three main analyses for Ethiopia: (1) epidemiological impact, (2) cost-effectiveness analysis and (3) epidemiological threshold analysis.
Epidemiological analysis
We modelled the SoC compared with the ASCENT interventions between 2023 and 2035, estimating long-term projections of TB incidence and deaths. Outcomes were expressed in the form of cumulative and incremental (intervention vs SoC) incidence and mortality from 2023 to 2035. We compared our results to the End TB Strategy targets.
Cost-effectiveness analysis
We estimated incremental costs and DALYs averted for the two DAT interventions against the SoC between 2023 and 2035, and compared against an estimated CET range for Ethiopia.44
Epidemiological thresholds analysis
We varied the intervention treatment outcomes (ie, treatment completion, LTFU, relapse and on-treatment death, setting different poor treatment outcomes to zero) to identify the maximum possible impact an intervention to improve treatment outcomes such as a DAT could achieve. We investigated four different scenarios by considering a hypothetical DAT which (1) minimised relapse; or (2) minimised on-treatment mortality; or (3) minimised LTFU; or (4) all of the above, that is, maximised successful treatment completion.
Patient and public involvement
Patients were involved in the trial component of ASCENT, they were not involved in the development of this modelling analysis. Results have been disseminated to the community advisory board.
Results
We obtained ~700 parameter sets that fitted all targets as a result of the calibration. For each set, we sampled 10 times from the uncertainty around intervention parameters, thus obtaining ~7000 parameter sets for each intervention. These sets were used as inputs for the projection analysis to estimate the epidemiological and economic impact of the interventions. Calibration plots and posteriors are reported in online supplemental figure 2 and table 1.
Epidemiological impact
Results of modelling projections to 2035 are reported in table 1. All three scenarios (SoC, pillbox and labels) led to similar rates of incidence and mortality in 2035, approximately 88 per 100 000 and 16 per 100 000 respectively, suggesting limited to no epidemiological impact of the DAT interventions compared with SoC.
Table 1. Modelling projections of TB incidence, mortality and costs of treatment for standard of care (SoC), pillbox intervention and label intervention. Whole national population, 2023−2035 time frame.
| SoC value (95% uncertainty interval) | Pillbox value (95% uncertainty interval) | Labels value (95% uncertainty interval) | |
|---|---|---|---|
| Epidemiological outcomes | |||
| TB incidence in 2035, per 100 000 persons | 89.30 (47.93; 140.56) |
88.07 (46.71; 138.50) |
86.97 (45.05; 136.94) |
| Difference vs SoC in number of TB episodes over 2023–2035 | −10 963 (−31 629; 56 550) |
−22 104 (−73 566; 20 750) |
|
| Percentage difference vs SoC in TB episodes over 2023–2035 | −0.4% (−1.1%; 2.0%) |
−0.8% (−0.7%; 2.6%) |
|
| TB mortality in 2035 per 100 000 persons | 16.20 (8.47; 24.09) |
15.85 (8.21; 23.62) |
15.88 (8.22; 23.75) |
| Difference vs SoC in number of TB deaths over 2023–2035 | −3665 (−11 353; 18.81) |
−1382 (−14 273; 6.76) |
|
| Percentage difference vs SoC in TB deaths over 2023–2035 | −0.7% (−2.2%; 3.6%) |
−0.3% (−2.7%; 3.2%) |
|
| DALYs averted, million | 0.068 (−1.12; 1.28) |
0.002 (−1.25; 1.30) |
|
| Economic outcomes | |||
| Patient costs, million USD | 12.06 (10.43; 13.57) |
9.20 (8.034; 10.38) |
9.03 (7.76; 10.20) |
| Incremental patient costs, USD | −2.87 (−0.94; −4.85) |
−3.04 (−1.07; −5.06) |
|
| Percentage change in patient costs | −23.8% (−7.8%; −40.2%) |
−25.2% (−8.9%; −41.9%) |
|
| Provider costs, million USD | 317.69 (275.84; 357.56) |
154.89 (135.32; 174.90) |
137.24 (117.81; 154.75) |
| Incremental provider costs, million USD | −162.80 (−118.08; −209.64) |
−180.45 (−134.88; −225.02) |
|
| Percentage change in provider costs | −51.2% (−37.2%; −66.0%) |
−56.8% (−42.5%; −70.8%) |
|
| Total costs, million USD | 329.69 (286.27; 370.24) |
164.07 (143.12; 184.94) |
146.24 (125.78; 164.59) |
| Incremental total costs, million USD | −165.62 (−118.29; −214.87) |
−183.44 (−135.76; −228,66) |
|
| Percentage change in total costs | −50.2% (−35.88%; −65.2%) |
−55.6% (−41.20%; −69.40%) |
|
DALY, disability-adjusted life-year; TB, tuberculosis.
We estimated small and uncertain epidemiological benefits of the pillbox intervention compared with the SoC, with 11 000 (95% UI −57 000; 32 000) incident TB episodes and 3700 (−19 000; 11 000) TB deaths averted, that is, a difference of −0.4% (−1.1%; +2.0%) and −0.7% (−2.2%; +3.6%), respectively. Results were similar for the medication label intervention.
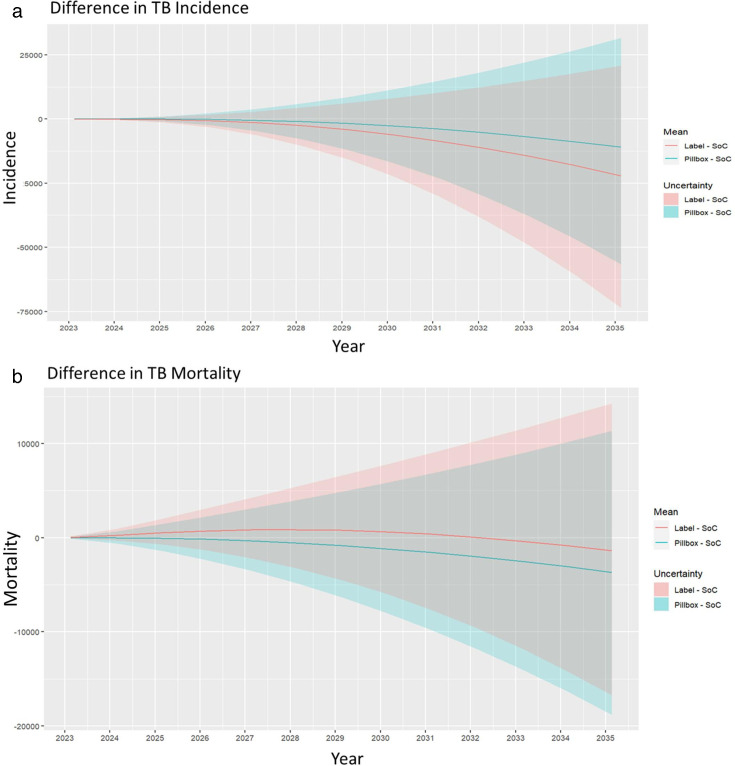
For both interventions, when offered from 2023 to 2035 to all adults with pulmonary TB, 68% of model runs showed a cumulative reduction in TB incidence compared with the SoC (figure 1). Similarly, 82% of runs showed a reduction in mortality for the pillbox intervention compared with the SoC, and 57% of runs for the label intervention (figure 1). However, although fewer cumulative incident TB episodes and deaths can be seen in the intervention arms compared with the SoC, these translate to only a 0.4% and 0.8% reduction in TB episodes for the pillbox and labels interventions, respectively, compared with the SoC. Similarly, only a 0.7% and 0.3% reduction in mortality was seen for the pillbox and labels interventions, respectively, compared with the SoC. Wide UIs suggest that there is no evidence of an epidemiological effect of the interventions.
Figure 1. Difference in cumulative (a) incidence and (b) mortality for the pillbox and label interventions compared with the standard of care (SoC). TB, tuberculosis.
Health economic impact
Results of the cost analysis can be found in table 1. When associating costs to these epidemiological projections, implementing either the pillbox or the label intervention from 2023 to 2035 leads to a cumulative reduction of 25% in patient costs when compared with the SoC. In terms of provider costs, the pillbox intervention is more expensive than the label intervention due to the cost of purchasing the boxes, but both are considerably cheaper than the SoC due to a reduction in human resources requirements, with the pillbox intervention leading to a reduction of 51% of provider costs and the label intervention to a 57% reduction.
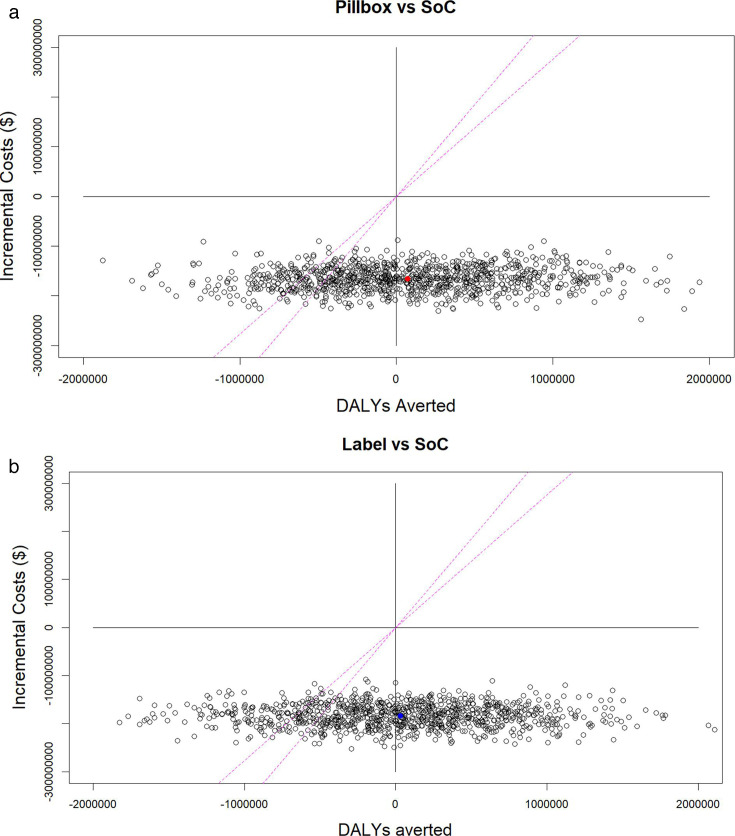
Overall, the pillbox intervention leads to US$165.62 (US$118.29; US$214.87) million (ie, 50.2%) cost savings compared with the SoC, and to 68 000 (−1.12; 1.28 million) DALYs averted. The label intervention leads to US$183.44 (US$135.76; US$228.66) million (ie, 55.6%) cost savings compared with the SoC, and to 2000 (−1.25; 1.30 million) DALYs averted. These results indicate that while the interventions show no improved effectiveness, they are always cost saving. Additionally, when compared with a CET of 27%–36% Gross Domestic Product (GDP) (ie, US$277–US$37044), the probability of the intervention being cost-effective was 79%–85% for the pillbox intervention and 76%–84% for the label intervention (figure 2).
Figure 2. Cost-effectiveness planes showing incremental costs versus disability-adjusted life-years (DALYs) averted of pillbox and label interventions compared with standard of care (SoC) and CET (27%–36% Ethiopian GDP.44 Each dot represents one of the 1000 model runs performed during probabilistic sensitivity analysis, with red and blue dots representing the mean cost per DALY averted for pillbox and label interventions, respectively. CET, cost-effectiveness threshold.
Finally, we performed univariate sensitivity analysis to inform the impact of patient-level adoption of DAT on the interventions’ yearly costs, by considering 20%, or 50%, or 80%, or 100% pillbox or label uptake. Results (reported in online supplemental table 4) show that even a small uptake of either DAT intervention can save costs. Considering 20% of either pillbox of label intervention uptake (with the remaining 80% SoC) can save around ~US$2.76–US$3.03 million in 2023.
Epidemiological thresholds
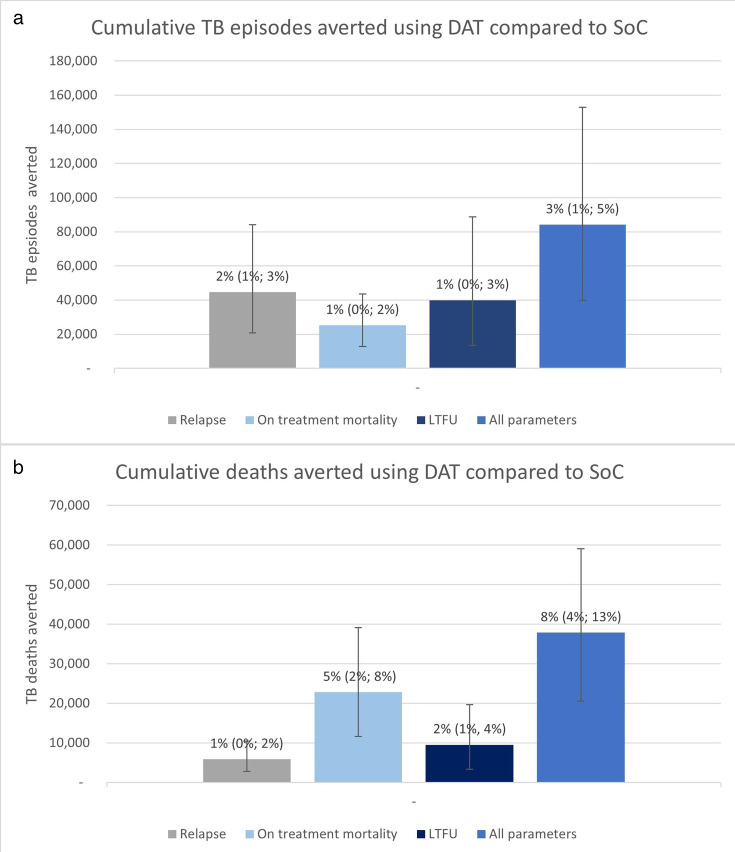
Results of the threshold analysis when considering a hypothetical DAT intervention are shown in figure 3. In this instance, we do not specify whether this is a pillbox-based or label-based intervention, and this could represent other forms of DAT. We found that by 2035 a ‘perfect’ and somewhat unrealistic intervention, which improved treatment success to 100%, was only able to reduce incidence and mortality by 3% (1.4%; 5.5%) and 8.2% (4.4%; 12.8%), respectively, compared with the SoC. This was equivalent to an overall reduction 84 100 (39 700‒153 000) fewer TB episodes and 37 800 (23 300; 67 100) fewer TB deaths by 2035, or a 43.5% (27.9; 64.2) reduction in incident TB episodes and a 54.9% (37.8%; 73.1%) reduction in deaths compared with 2015. Cumulatively by 2035 possible reductions in incidence are driven by reductions in relapse, closely followed by LTFU. In comparison, reductions in overall TB deaths are mainly driven by reductions in death on treatment.
Figure 3. The maximum impact a digital adherence technology (DAT) intervention could have on tuberculosis (TB) incidence and mortality in Ethiopia, showing the cummulative (a) TB episodes and (b) TB deaths averted in four hypothetical intervention scenarios minimising poor treatment outcomes compared with the standard of care (SoC). Percentages indicate the percentage of reduction of incidence and mortality of a ‘perfect’ intervention (ie, an intervention minimising relapse, on-treatment death and loss to follow-up (LTFU)) compared with SoC.
Discussion
We estimated limited evidence of epidemiological benefits of the pillbox intervention compared with the SoC between 2023 and 2035, with 11 000 (95% UI −57 000; 32 000) incident TB episodes and 3700 (−19 000; 11 000) TB deaths averted, that is, a difference of −0.4% (−0.8%; +3.1%) and −0.7% (−1.6%; +5.4%), respectively, demonstrating that DAT interventions will not contribute to reaching the End TB targets of a 90% decrease in incidence and a 95% in mortality compared with 2015. However, large total provider and patient cost savings (US$162 800 000 (US$118 000 000; US$210 643 000) and US$2 900 000 (US$936 000; US$4 851 000), respectively, over 2023–2035) were also observed. This translated to a 50.2% (35.88%; 65.2%) reduction in total cost of treatment (excluding prediagnosis costs). Results were similar for the medication label intervention. When compared with estimated CETs, both interventions were cost-effective in most cases, with a probability of 79%–85% and 76%–84% for the pillbox and label interventions, respectively.
Our economic results require careful interpretation. Differences in costs between the interventions and the SoC are mostly a consequence of fewer visits to the healthcare facility required when using DAT, while from the provider perspective, cost savings are a result of reduced human resource time, as concluded in.38 If this cannot be allocated to different tasks, then this could affect the budget savings. Our results align with the in-trial cost-effectiveness analysis, although showing an increased probability of cost-effectiveness of the interventions. This difference is driven by two main factors; different CETs used, and a small reduction in point estimates of disease burden due to transmission, as a result of the longer time horizon, which affects the amount of costs saved.
The limited epidemiological impact estimated by the model in our main analysis is a direct result of the null impact observed in the trial. However, threshold analysis showed that the maximum possible epidemiological impact a theoretical DAT intervention could achieve over the same timescale would be a 43.5% (27.9%; 64.2%) reduction in incident TB episodes and a 54.9% (37.8%; 73.1%) reduction in deaths compared with 2015; still far from reaching the End TB target of a 90% reduction in TB incidence and 95% reduction in TB deaths in 2035 compared with 2015. This suggests that additional measures focusing on aspects of the TB care cascade beyond treatment (such as large-scale case-finding activities, improved diagnostics, preventive therapy and vaccination) are needed and should be explored in future analysis.
Our study has several limitations. First, due to lack of treatment outcome data by intervention and HIV status, we were not able to model HIV coinfection. Consequently, it is possible that our results might underestimate the cost-savings and thus the cost-effectiveness of the interventions, as they could probably increase the overall success of treatment (as treatment success is lower in people living with HIV).7 Further, if DATs were used differentially based on HIV status, it is possible that the intervention could have had an effect on TB incidence and mortality. Second, we did not model the possible impact of DAT interventions when used for TB preventive treatment or treatment of drug-resistant TB, as these were outside the scope of the trial, which may lead to an underestimation of both effectiveness (due to non-completion being higher for preventing treatment) as well as possible costs saved. Third, we did not consider the impact of the COVID-19 pandemic on Ethiopia’s TB burden here, although given the intervention is focused on treatment this is unlikely to have affected results. Finally, our model is only calibrated to Ethiopia, where treatment success in SoC is already high compared with other settings.
Our study also has several strengths. We used a transmission model, which estimated the long-term epidemiological and economic impact of DAT by studying TB transmission across the whole Ethiopian population. This allowed us to discuss the long-term policy implications of this technology on a country level. Additionally, this is the first modelling study on DAT to use direct trial results and to estimate economic outcomes.
Literature on the modelling of DAT in TB is scarce, and to our knowledge, only two other modelling studies of DAT interventions exist in the literature, neither of which were able to use direct trial results or to estimate economic outcomes. The first modelled several combinations of intervention scenarios, including medication monitoring during TB treatment in China, by assuming an improvement in drug-susceptible TB treatment success from 82% to 90%.45 The second modelled the potential epidemiological impact of DAT interventions in India, showing that such interventions could reduce cumulative incidence and mortality by up to 16% and 15%, respectively, between 2020 and 2030.17 However, this study also made strong assumptions about treatment outcomes, assuming treatment completion increased from 50% in the private sector and 86% in the public sector to 100%. In comparison, our threshold analysis (which took a similar approach) suggested much more limited reductions in incidence and mortality were likely, driven by the already-high rates of treatment success in Ethiopia, which is 87%, closer to the global average of 88%.7 In contrast to these two previous studies, our results, based directly on trial estimates, instead show no epidemiological impact of DAT on TB in Ethiopia, suggesting a possible overestimation in the literature of what DAT could achieve.
Using a transmission model allowed us to look beyond the time restrictions of the trial and study the long-term health economic impact of the interventions. We estimated savings over a 12-year time frame, which could have important budgetary implications from the provider side when taken as a proportion of the total budget, as results indicate that in 2023 alone, total provider cost savings would be equivalent to ~10%–11% of Ethiopia’s annual TB budget.11 From the patient perspective, a significant reduction in patient costs has important implications for the affordability of TB treatment and could contribute to reaching End TB targets aimed at the reduction of catastrophic costs.
In conclusion, DAT interventions, despite showing limited epidemiological impact, could help save substantial TB treatment costs and are, therefore, highly likely to be cost-effective in Ethiopia.
Supplementary material
Footnotes
Funding: This work was funded by Unitaid Adherence Support Coalition to End TB (20193–3-ASCENT). RGW is funded by the Wellcome Trust (218261/Z/19/Z), NIH (1R01AI147321-01, G-202303-69963, R-202309-71190), EDTCP (RIA208D-2505B), UK MRC (CCF17-7779 via SET Bloomsbury), ESRC (ES/P008011/1), BMGF (INV-004737, INV-035506), and the WHO (2020/985800-0).
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Emma Veitch
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: This project received ethics approval under the overall trial protocol from the Institutional Review Board of the Public Emergency and Health Research Directorate at the Health Bureau of Addis Ababa City Administration (AA16238/227) and Oromia Region (BEFO/HBTFH/1–16/10415) of Ethiopia, the Ethics Committee of the London School of Hygiene & Tropical Medicine (19120)) and the WHO Ethical Review Committee (ERC.0003297).
Data availability statement
All data used in this modelling study has been reported in the supplementary documentation and sources have been accordingly cited.
References
- 1.World Health Organisation Tuberculosis: key facts. 2023. https://www.who.int/news-room/fact-sheets/detail/tuberculosis Available.
- 2.Iseman MD. Tuberculosis therapy: past, present and future. Eur Respir J. 2002;20:87S–94s. doi: 10.1183/09031936.02.00309102. [DOI] [PubMed] [Google Scholar]
- 3.Barter DM, Agboola SO, Murray MB, et al. Tuberculosis and poverty: the contribution of patient costs in sub-Saharan Africa--a systematic review. BMC Public Health. 2012;12:1–21.:980. doi: 10.1186/1471-2458-12-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurence YV, Griffiths UK, Vassall A. Costs to Health Services and the Patient of Treating Tuberculosis: A Systematic Literature Review. Pharmacoeconomics. 2015;33:939–55. doi: 10.1007/s40273-015-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nezenega ZS, Perimal-Lewis L, Maeder AJ. Factors Influencing Patient Adherence to Tuberculosis Treatment in Ethiopia: A Literature Review. Int J Environ Res Public Health. 2020;17:5626. doi: 10.3390/ijerph17155626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long Q, Smith H, Zhang T, et al. Patient medical costs for tuberculosis treatment and impact on adherence in China: a systematic review. BMC Public Health. 2011;11:1–9.:393. doi: 10.1186/1471-2458-11-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organisation . World Health Organisation; 2022. Global tuberculosis report 2022.https://apps.who.int/iris/handle/10665/363752 Available. [Google Scholar]
- 8.World Health Organisation . World Health Organization; 2021. Global tuberculosis report 2021.https://apps.who.int/iris/handle/10665/346387 Available. [Google Scholar]
- 9.Ethiopia Ministry of Health Ethiopia national health accounts report 2019-20. 2022
- 10.Deribew AA, Dememew ZG, Alemu KM, et al. TB-related catastrophic costs in Ethiopia. Public Health Action. 2024;14:71–5. doi: 10.5588/pha.24.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministry of Health Ethiopia Tuberculosis and leprosy national strategic plan (tbl-nsp) July 2021 – June 2026. 2020
- 12.Subbaraman R, de Mondesert L, Musiimenta A, et al. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob Health. 2018;3:e001018. doi: 10.1136/bmjgh-2018-001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velen K, Nguyen T-A, Pham CD, et al. The effect of medication event reminder monitoring on treatment adherence of TB patients. Int J Tuberc Lung Dis. 2023;27:322–8. doi: 10.5588/ijtld.22.0500. [DOI] [PubMed] [Google Scholar]
- 14.Burzynski J, Mangan JM, Lam CK, et al. In-Person vs Electronic Directly Observed Therapy for Tuberculosis Treatment Adherence: A Randomized Noninferiority Trial. JAMA Netw Open. 2022;5:e2144210. doi: 10.1001/jamanetworkopen.2021.44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Thompson J, Dong H, et al. Digital adherence technologies to improve tuberculosis treatment outcomes in China: a cluster-randomised superiority trial. Lancet Glob Health. 2023;11:e693–703. doi: 10.1016/S2214-109X(23)00068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imperial MZ, Nahid P, Phillips PPJ, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med. 2018;24:1708–15. doi: 10.1038/s41591-018-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arinaminpathy N, Chin DP, Sachdeva KS, et al. Modelling the potential impact of adherence technologies on tuberculosis in India. Int J Tuberc Lung Dis. 2020;24:526–33. doi: 10.5588/ijtld.19.0472. [DOI] [PubMed] [Google Scholar]
- 18.Ngwatu BK, Nsengiyumva NP, Oxlade O, et al. The impact of digital health technologies on tuberculosis treatment: a systematic review. Eur Respir J. 2018;51:1701596. doi: 10.1183/13993003.01596-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McQuaid CF, Foster N, Quaife M, et al. Digital adherence technology for TB: focus on livelihoods as well as lives. Int J Tuberc Lung Dis. 2021;25:416–7. doi: 10.5588/ijtld.21.0070. [DOI] [PubMed] [Google Scholar]
- 20.Ridho A, Alfian SD, van Boven JFM, et al. Digital Health Technologies to Improve Medication Adherence and Treatment Outcomes in Patients With Tuberculosis: Systematic Review of Randomized Controlled Trials. J Med Internet Res. 2022;24:e33062. doi: 10.2196/33062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohamed MS, Zary M, Kafie C, et al. The impact of digital adherence technologies on health outcomes in tuberculosis: a systematic review and meta-analysis. medRxiv. doi: 10.1101/2024.01.31.24302115. Preprint. [DOI]
- 22.Yoeli E, Rathauser J, Bhanot SP, et al. Digital Health Support in Treatment for Tuberculosis. N Engl J Med. 2019;381:986–7. doi: 10.1056/NEJMc1806550. [DOI] [PubMed] [Google Scholar]
- 23.Wei X, Hicks JP, Zhang Z, et al. Effectiveness of a comprehensive package based on electronic medication monitors at improving treatment outcomes among tuberculosis patients in tibet: a multi-centre randomised controlled trial. ERS Congress 2024 abstracts; 2024. [DOI] [PubMed] [Google Scholar]
- 24.Iribarren S, Beck S, Pearce PF, et al. TextTB: A Mixed Method Pilot Study Evaluating Acceptance, Feasibility, and Exploring Initial Efficacy of a Text Messaging Intervention to Support TB Treatment Adherence. Tuberc Res Treat. 2013;2013:349394. doi: 10.1155/2013/349394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattamanchi A, Crowder R, Kityamuwesi A, et al. Digital adherence technology for tuberculosis treatment supervision: A stepped-wedge cluster-randomized trial in Uganda. PLoS Med. 2021;18:e1003628. doi: 10.1371/journal.pmed.1003628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bediang G, Stoll B, Elia N, et al. SMS reminders to improve adherence and cure of tuberculosis patients in Cameroon (TB-SMS Cameroon): a randomised controlled trial. BMC Public Health. 2018;18:583. doi: 10.1186/s12889-018-5502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belknap R, Holland D, Feng P-J, et al. Self-administered Versus Directly Observed Once-Weekly Isoniazid and Rifapentine Treatment of Latent Tuberculosis Infection: A Randomized Trial. Ann Intern Med. 2017;167:689–97. doi: 10.7326/M17-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston JC, van der Kop ML, Smillie K, et al. The effect of text messaging on latent tuberculosis treatment adherence: a randomised controlled trial. Eur Respir J. 2018;51:1701488. doi: 10.1183/13993003.01488-2017. [DOI] [PubMed] [Google Scholar]
- 29.Ali AOA, Prins MH. Mobile health to improve adherence to tuberculosis treatment in Khartoum state, Sudan. J Public Health Afr. 2019;10:1101. doi: 10.4081/jphia.2019.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manyazewal T, Woldeamanuel Y, Fekadu A, et al. Effect of Digital Medication Event Reminder and Monitor-Observed Therapy vs Standard Directly Observed Therapy on Health-Related Quality of Life and Catastrophic Costs in Patients With Tuberculosis: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2230509. doi: 10.1001/jamanetworkopen.2022.30509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson RR, Kityamuwesi A, Kuan A, et al. Cost and Cost-Effectiveness of a Digital Adherence Technology for Tuberculosis Treatment Support in Uganda. V Health. 2022;25:924–30. doi: 10.1016/j.jval.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Kim H-Y, Park S, et al. Cost-effectiveness of a medication event monitoring system for tuberculosis management in Morocco. PLoS ONE. 2022;17:e0267292. doi: 10.1371/journal.pone.0267292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahrainwala L, Knoblauch AM, Andriamiadanarivo A, et al. Drones and digital adherence monitoring for community-based tuberculosis control in remote Madagascar: A cost-effectiveness analysis. PLoS One. 2020;15:e0235572. doi: 10.1371/journal.pone.0235572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nsengiyumva NP, Khan A, Gler MMaTS, et al. Costs of digital adherence technologies for tuberculosis treatment support. medRxiv. doi: 10.1101/2023.03.18.23287420. Preprint. [DOI] [PMC free article] [PubMed]
- 35.Tadesse AW, Mohammed Z, Foster N, et al. Evaluation of implementation and effectiveness of digital adherence technology with differentiated care to support tuberculosis treatment adherence and improve treatment outcomes in Ethiopia: a study protocol for a cluster randomised trial. BMC Infect Dis. 2021;21:1149. doi: 10.1186/s12879-021-06833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster N, Tadesse AW, McQuaid CF, et al. Evaluating the equity impact and cost-effectiveness of digital adherence technologies with differentiated care to support tuberculosis treatment adherence in Ethiopia: protocol and analysis plan for the health economics component of a cluster randomised trial. Trials. 2023;24:292. doi: 10.1186/s13063-023-07289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tadesse AW, Sahile M, Foster N, et al. Cluster-randomized trial of digital adherence technologies and differentiated care to reduce poor end-of-treatment outcomes and recurrence among adults with drug-sensitive pulmonary tb in Ethiopia. medRxiv. doi: 10.1101/2024.05.09.24307117. Preprint. [DOI]
- 38.Foster N, Tadesse AW, Belachew M, et al. Equity, cost and disability adjusted life years of tuberculosis treatment supported by digital adherence technologies and differentiated care in ethiopia: a trial-based distributional cost-effectiveness analysis. medRxiv. doi: 10.1101/2024.07.28.24310767. Preprint. [DOI]
- 39.Kebede AH, Alebachew Z, Tsegaye F, et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010-2011. Int J Tuberc Lung Dis. 2014;18:635–9. doi: 10.5588/ijtld.13.0417. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organisation Global tuberculosis programme. 2022. https://www.who.int/teams/global-tuberculosis-programme/data Available.
- 41.Iskauskas A, Vernon I, Goldstein M, et al. Emulation and History Matching using the hmer Package. 2022. p. 220905265.
- 42.Scarponi D, Iskauskas A, Clark RA, et al. Demonstrating multi-country calibration of a tuberculosis model using new history matching and emulation package - hmer. Epidemics. 2023;43 doi: 10.1016/j.epidem.2023.100678. [DOI] [PubMed] [Google Scholar]
- 43.Jerene D, Levy J, van Kalmthout K, et al. Effectiveness of digital adherence technologies in improving tuberculosis treatment outcomes in four countries: a pragmatic cluster randomised trial protocol. BMJ Open. 2023;13:e068685. doi: 10.1136/bmjopen-2022-068685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochalek J, Lomas J, Claxton K. Estimating health opportunity costs in low-income and middle-income countries: a novel approach and evidence from cross-country data. BMJ Glob Health. 2018;3:e000964. doi: 10.1136/bmjgh-2018-000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menzies NA, Gomez GB, Bozzani F, et al. Cost-effectiveness and resource implications of aggressive action on tuberculosis in China, India, and South Africa: a combined analysis of nine models. Lancet Glob Health. 2016;4:e816–26. doi: 10.1016/S2214-109X(16)30265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this modelling study has been reported in the supplementary documentation and sources have been accordingly cited.