Abstract
Background
The rise in the number of children diagnosed with attention-deficit/hyperactivity disorder (ADHD) highlights the need for effective interventions targeting attentional control. Although recent research has demonstrated the potential of neurofeedback training (NFT) for children with ADHD, most studies have been conducted in laboratory settings, raising questions about their real-world applicability. To address this issue, virtual reality (VR) may offer a solution to the ecological validity challenges encountered in NFT. By coupling NFT with VR, individuals can engage in self-regulating brain activity within a simulated, realistic environment. This study aims to investigate the efficacy of near-infrared spectroscopy (NIRS)-based NFT combined with VR in alleviating ADHD symptoms among children, addressing the need for interventions with practical relevance and effectiveness.
Methods
This study aims to recruit 138 children aged 7–12 diagnosed with ADHD. Following baseline assessment, participants will be randomly assigned to one of three conditions: (1) NIRS-based NFT in a VR classroom setting; (2) conventional computerised cognitive training (active control) or (3) a waitlist control group. On completion of intervention sessions in the two training groups, all groups will undergo an assessment at time 2, with a follow-up assessment scheduled 2 months post-training for all participants. Primary outcomes will include measures of executive function, such as attentional control, response inhibition and working memory, along with changes in oxygenated and deoxygenated haemoglobin levels monitored by functional NIRS. Secondary outcome measures will comprise ratings of children’s ADHD symptoms and executive function behaviours in daily life, reported by parents and teachers.
Discussion
The three-arm randomised controlled trial will address research gaps regarding the effectiveness of NIRS-based NFT for children with ADHD, particularly when integrated with immersive VR technology. By combining NFT and VR, this study aims to simulate a real-world environment, potentially amplifying intervention effects. The findings from the study will provide evidence for the efficacy of this innovative intervention in improving executive function and alleviating ADHD symptoms.
Ethics and dissemination
Ethical approval was obtained from the Human Research Ethics Committee at the University of Hong Kong (Reference: EA200247). Results will be published in peer-reviewed journals and presented at conferences.
Trial registration number
Keywords: Virtual Reality, Child, Psychosocial Intervention
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study investigates the efficacy of a novel intervention for children with attention-deficit/hyperactivity disorder by combining technologies of near-infrared spectroscopy (NIRS)-based neurofeedback training (NFT) and virtual reality (VR).
By incorporating VR technology in a functional NIRS (fNIRS)-based NFT, the study addresses the limitation of laboratory-based interventions and shows potential for improving the relevance and applicability of findings to real-world settings.
The use of a three-arm randomised controlled trial design allows for a rigorous comparison of intervention effects across intervention, active control and waitlist control groups.
The study includes both neuropsychological tests and fNIRS data, along with parent and teacher ratings as outcomes, providing a multifaceted approach to evaluating intervention effectiveness.
However, the study’s sample consists solely of children aged 7–12, which may restrict the generalisability of the findings to other age groups.
Introduction
Recent years have seen a rise in the number of children diagnosed with attention-deficit/hyperactivity disorder (ADHD). ADHD is characterised by pervasive and developmentally inappropriate patterns of inattentive, hyperactive and impulsive behaviours that are evident in early childhood, persistent over time and present across different settings.1 A recent umbrella review revealed that the global prevalence of ADHD among children and adolescents was estimated to be 8%,2 with the inattentive presentation of ADHD (ADHD-I) identified as the most common type of ADHD. However, those with mainly attention deficits are under-recognised and undertreated owing to the less disruptive nature of the manifested behaviours.3 It is often the case that parents seek help only when their children’s inattention problems translate into poor academic performance in school, which may explain why the peak age of diagnosis for the predominantly inattentive presentation of ADHD lags those of the hyperactive-impulsive and combined presentations by approximately 2 years.4
Specifically, inattention symptoms were found to be more closely associated with neuropsychological impairments in working memory, cognitive processing speed and state regulation, than hyperactivity and impulsivity symptoms among children with ADHD.5,7 Additionally, inattention was found to be a significant predictor of poor preacademic skills in mathematics.8 9 It is important to provide early intervention to children with significant inattention problems before those problems escalate into long-term difficulties in academic and social development.
Employing neurofeedback in attention training for children with ADHD
Conventional psychosocial interventions for ADHD seldom target attentional control as the focus of training, possibly owing to the difficulties of enhancing attention through cognitive-behavioural approaches.10,12 Nonetheless, some recent studies have indicated the feasibility of attention training for children with ADHD and its plausible effects.13,15
A recent development in attention training pertains to the use of neurophysiological information as biofeedback to help individuals self-regulate their attention. Neurophysiological signals, such as brainwave patterns (eg, theta/beta ratio) measured by electroencephalography (EEG) or changes in blood oxygenation levels in the prefrontal cortex of the brain (ie, blood-oxygen-level-dependent (BOLD) signals) measured by functional near-infrared spectroscopy (fNIRS), are translated into visual and/or auditory cues in neurofeedback training (NFT). Participants then alter these signals through self-regulation, reinforcing successful regulation. By training implicit activation of brain activity through operant conditioning, participants can establish self-regulation patterns that align with contextual demands.16 17 While EEG-based NFT has emerged as a plausible intervention for children with ADHD,18 19 studies have yielded inconsistent results, with some revealing significant effects on attention comparable to stimulant medication,20 21 while others report less promising outcomes.22 23
NIRS-based NFT for children with inattention
NIRS, a non-invasive neuroimaging technique that monitors changes in blood oxy-haemoglobin (oxy-Hb) levels in brain cortical tissue to record neural activation,24 offers advantages over EEG, including higher spatial resolution and reduced sensitivity to movement artefacts. This makes NIRS particularly suitable for NFT targeting specific brain areas in motorically restless individuals (eg, children with ADHD). Researchers have used NIRS to identify the neurophysiological correlates of behavioural impairments in ADHD. For instance, Ehlis et al25 found a reduced concentration of oxy-Hb in the ventrolateral prefrontal cortex of adults with ADHD compared with healthy controls during a working memory task, a finding that was replicated in the inferior prefrontal cortex of children with ADHD during a Stroop task.26
Additionally, NIRS-based NFT shows the potential of requiring less training dosage to achieve effects compared with EEG-based NFT. Meisel et al reported that EEG-based NFT significantly alleviated ADHD impairments after forty 30-min training sessions.27 In contrast, Marx et al compared NIRS-based and EEG-based NFT, finding that only the NIRS group exhibited a significant reduction in ADHD symptoms after twelve 30-min training sessions.28
However, evidence on the effectiveness of NIRS-based NFT for reducing ADHD symptoms remains limited, with only a handful of published studies demonstrating its efficacy.28 29 Further research is needed to explore the effectiveness and feasibility of NIRS-based NFT for children with ADHD. Questions also linger regarding the transferability of self-regulatory abilities acquired during NFT to real-life situations, as most studies have been conducted in laboratory settings,27,30 leaving the ecological validity of this intervention approach relatively unexplored.
Integrating virtual reality into NFT
Virtual reality (VR is considered a potentially practical technology for psychological treatment, offering possible solutions to the ecological validity challenges encountered in NFT. By engaging in training within a VR environment, individuals may enhance their acquisition of self-regulation skills in the laboratory and effectively transfer these skills to real-world settings, such as a classroom. VR enables individuals to immerse themselves in computer-generated simulations of real-world scenarios, engaging with the virtual environment through various naturalistic stimuli, including sounds, visual impressions and haptic experiences. This heightened realism in the VR environment may elicit authentic psychological and behavioural responses.31 The high degree of realism associated with VR, such as in a virtual classroom, facilitates the transfer of skills acquired in training to real-life situations. Furthermore, personalised contexts provided by naturalistic VR environments link cognitive training to everyday life goals, thereby boosting training motivation.32
The past two decades have seen an increased application of VR in assessing children with ADHD.33 Attention assessments embedded in a virtual classroom have exhibited stronger discriminant power than traditional computerised assessments in distinguishing children with ADHD from typically developing controls.34 35 This finding has been linked to enhanced ecological validity and task complexity owing to the presence of distractors (eg, school bell ringing, someone walking into the classroom), which maximises the similarities between the real and virtual worlds.35 However, few studies have explored the applicability of VR and its effects on NFT.33 To the best of our knowledge, only two studies to date have reported the effects of embedding NIRS-based prefrontal cortex NFT in VR—one focusing on enhancing reading performance in children with ADHD36 and the other on reducing impulsive behaviours in healthy adults.29 There is currently no evidence regarding the effectiveness of this approach in reducing executive function deficits and other ADHD symptoms in children with ADHD. Moreover, little is known about whether the treatment effects can be sustained over time or the extent to which skills are transferable to daily life. In addition, despite evidence from Nolin et al indicating minimal cybersickness among children aged 7–16 after VR-based attention assessment,34 this aspect has not been studied extensively.
Objectives and hypotheses
The primary objective of this randomised controlled trial is to test the feasibility and efficacy of NFT-VR for improving executive function and reducing ADHD symptoms, especially inattention, in children with ADHD. It is hypothesised that (1) both NFT-VR and conventional cognitive training will improve executive functions in children with ADHD from pretest to post-test; (2) greater training benefits will be observed following NFT-VR compared with the active control condition; (3) performance improvements in executive function from pretest to post-test will correlate with increased prefrontal cortical activation during the assessment at the two time points and (4) the training benefits will be maintained for at least 2 months after intervention completion.
Methods and analysis
This manuscript, along with the described trial, is aligned with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)—the SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials.37 Please see online supplemental material 1 for the SPIRIT checklist.
Design
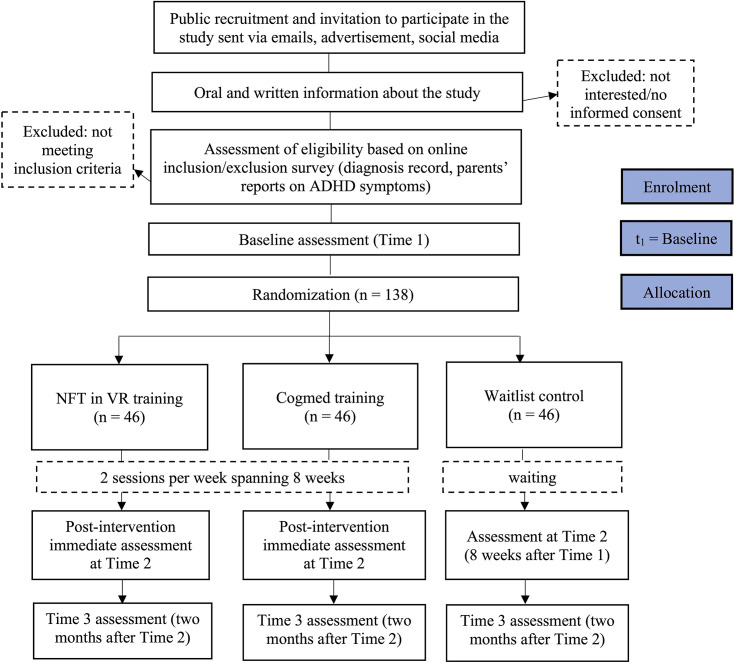
This trial employs a three-arm parallel randomised controlled design to examine the efficacy of NIRS-based NFT in a VR classroom setting against a conventional computerised cognitive training and a waitlist control among children with ADHD in Hong Kong. Participants who meet the screening criteria are randomly allocated to one of the three groups—NIRS-based NFT-VR, computerised cognitive training (active control) or waitlist control—in a 1:1:1 ratio. The NFT and active control groups will each receive 16 training sessions spanning 8 weeks in the first iteration, and the waitlist control group will receive either training on completion of assessment at time point 2. Assessments occur at baseline (time 1), immediately post-training (Time 2), and at a 2-month follow-up to examine maintenance effects (time 3) for all three arms.
Sample size and recruitment
The sample size calculation is based on measures of executive function, using the Conners Continuous Performance Test third Edition (CPT3) as a reference. A multiarm clinical trial sample size calculator was used for this calculation, based on an intention-to-treat design.38 For a single-stage, three-arm clinical trial with equal allocation across arms, a power of 0.8, a treatment effect size of 0.5,19 39 and an alpha level of 5%, a total sample size of 123 participants is required. To account for an anticipated attrition rate of 10%, we plan to recruit 46 participants for each condition (ie, 138 participants in total).
Participants are identified from several sources in Hong Kong, including local paediatricians, psychiatrists, school psychologists, online social media advertising and ADHD parent associations. Formal diagnostic reports from qualified clinical health professionals are required for screening purposes in the project. In addition, the parents and teachers of potential participants are asked to complete a battery of questionnaires to assess the children’s ADHD symptoms and behavioural problems for screening.
Eligibility criteria
The inclusion criteria for this study are as follows: (1) children aged 7–12; (2) with either a clinical diagnosis of ADHD or significant ADHD symptoms, as reported by parents in the Swanson, Nolan and Pelham Version IV Scale (SNAP-IV).40 Specifically, at least six out of nine items must be rated ‘2’ or above in either the ‘inattention’ or ‘hyperactivity/impulsivity’ subscale of the SNAP-IV, in accordance with the diagnostic criteria for ADHD in the Diagnostic and Statistical Manual of Mental Disorder, Fifth Edition.1 The exclusion criteria include (1) inability to complete the first five questions on the Raven’s Progressive Matrices41 as a measure of general ability; (2) hearing, visual or physical impairments that might hinder participation in the training and assessment activities; (3) prior or current participation in NFT and (4) current participation in any other psychotherapeutic treatments. Ongoing pharmacological treatment for ADHD is allowed during the intervention, provided that a 24-hour washout period is observed at each time point of outcome measurement.
Randomisation and blinding
After completion of the baseline assessment, participants are randomised into one of the following three arms: (1) NIRS-based NFT in a VR classroom setting; (2) conventional computerised cognitive training (active control) or (3) waitlist control. To maintain allocation concealment, computer-generated condition assignment is used. Covariate-adaptive randomisation42 is performed to balance the conditions based on the children’s age, gender and ADHD medication status. The randomisation procedure is completed by a trained research assistant on the team. Assessors, trial participants and caregivers are unblinded regarding participants’ group allocation to facilitate the implementation of interventions. However, researchers responsible for statistical analyses remain blinded to the group allocation to minimise potential biases in the evaluation of intervention efficacy.
Study setting
Data collection and training sessions are conducted in a university laboratory setting in Hong Kong. Participants in both training groups receive individual training for each session. Neuropsychological assessments, administered at both pretraining and post-training, are conducted by trained research assistants within the laboratory.
Interventions
NIRS-based NFT VR training
The NFT-VR condition will consist of 16 training sessions modelled after prior NIRS-based NFT studies on children with ADHD in a VR setting. Each session will last about 45 min, including a 15-min preparation phase for fitting the NIRS-cap with optodes and a VR headset to the child’s head, followed by a 30-min training phase with breaks. The training will comprise a 2-min fNIRS baseline measurement and four regulation blocks (portrayed as lessons and break times in a VR classroom). During the 2-min baseline measurement, participants are asked to relax in a simulated garden within the VR environment. Following this, the scenario will transit to a virtual classroom.43
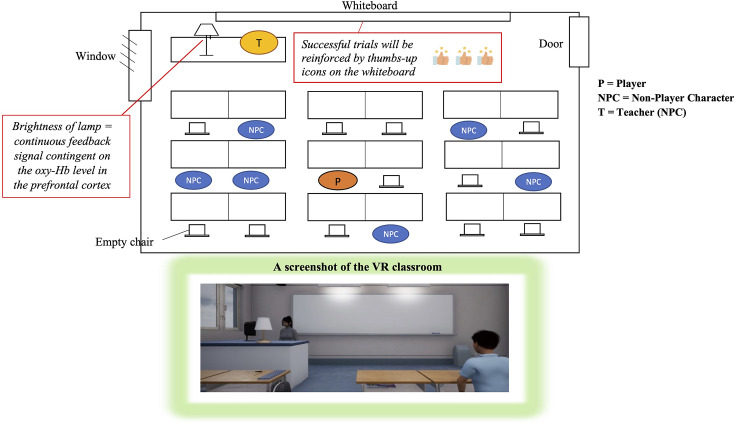
The storyboard and a screenshot of the VR classroom are presented in figure 1. In this scenario, each participant will be seated at a virtual table in the second row of the classroom, surrounded by animated students who are facing a teacher standing in front of a whiteboard. A lamp on the teacher’s desk provides continuous feedback, with its brightness contingent on the level of oxy-Hb measured in the prefrontal cortex by NIRS (ie, brightness increases as the oxy-Hb level rises). The task requires participants to control the brightness of the lamp during each regulation trial.
Figure 1. Storyboard and screenshot of the VR classroom. VR, virtual reality.
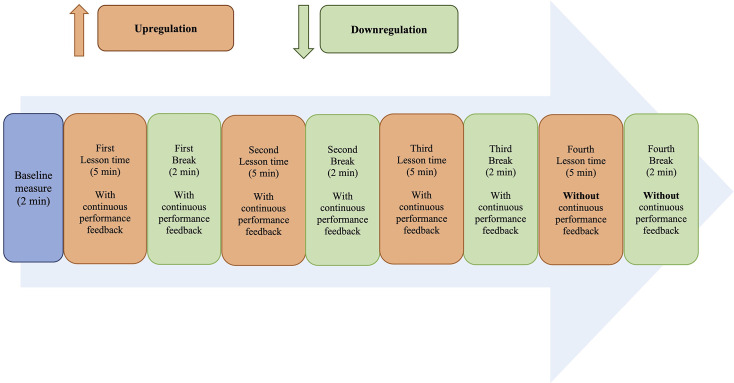
Each training session includes four regulation blocks, depicted as classroom lessons on four subjects: Chinese, English, Mathematics and General Studies. The order of the subjects is randomised across the four blocks. Each block consists of 5 min of upregulation (lesson time in the VR classroom), during which participants will answer multiple-choice questions displayed in text or picture formats on the screen by pressing a specific button on the VR controller. Participants’ performance in answering the questions is recorded but will not be disclosed to them at any point during the training. This is followed by a 2-min downregulation period (break time in the VR classroom), where participants are encouraged to take a break and relax (see figure 2 for the training paradigm design).
Figure 2. Training paradigm for each session of the NFT-VR condition. NFT-VR, neurofeedback training-virtual reality.
During the 5-min upregulation, participants are expected to increase haemodynamic activity in their brains to raise the brightness of the lamp. Successful trials—defined as an oxy-Hb signal above baseline for at least 60% of the regulation time during the 5-min upregulation—will be reinforced by thumbs-up icons appearing on the whiteboard (1, 2 or 3 thumbs for 60%, 70% and 80% of the regulation time above baseline, respectively) at the end of each upregulation block. In the 2-min downregulation, depicted as break time in the VR classroom, participants are asked to decrease the brightness of the lamp and keep it dim. A dim lamp indicates that the oxy-Hb level is below baseline, which is achieved through the downregulation of haemodynamic activity in the prefrontal cortex.
To simulate a real classroom environment where distractions naturally emerge, visual and auditory distractors are introduced in the VR classroom. The types of distractors (distant, near, peer) and their occurrence frequency are designed to follow a standardised protocol that is replicable and applicable to all participants. The level of distraction difficulty is set to progressively increase across the 16 training sessions. Specifically, no distractors will be presented in sessions 1–4, followed by auditory distant distractors (eg, sounds of cars passing by, rain and construction noise) in sessions 5–8. Sessions 9–12 will include both auditory and visual distractors, which can be distant or near (eg, pen/pencil tapping, knocking on tables), while sessions 13– 16 will introduce a combination of distant, near and peer distractors (eg, peers passing items around, talking and giggling) in both auditory and visual formats. Random distractors (eg, car passing by, pencil tapping, peers talking and giggling) are programmed to appear randomly for a total duration of 30 s in each 5-min upregulation block, while background distractors (eg, construction noise and sounds of rain) will persist for 2.5 min (50% of the time) in each block in sessions 5–16.
Participants will receive continuous performance feedback in the form of lamp brightness during the first three sets of upregulation and downregulation blocks. In the fourth regulation block, however, the brightness of the lamp will be fixed to encourage the generalisation of self-regulation skills in the absence of neurofeedback.44 Importantly, participants will not be explicitly instructed on how to regulate the brightness of the lamp in either the upregulation or downregulation blocks; they will simply be asked to alter it.
The self-regulation learning process is purposefully designed to be implicit, aiming to reduce cognitive load, as explicit instructions on regulation strategies might divert children’s attention from the tasks at hand.45 46 By not explicitly instructing children on how to regulate their brain activity, cognitive load is minimised, creating a more natural and less stressful learning environment.
Cogmed training for the active control group
The active control condition will involve 16 sessions of conventional computerised cognitive training. Participants will be asked to complete a set of computerised tasks using Cogmed (Pearson Clinical Assessment), a digital training programme designed to enhance working memory and attention levels in children.47 The choice of Cogmed as the active control group in this study stems from several considerations. First, Cogmed is a widely used computerised cognitive training programme in both research and clinical settings.48 By comparing the efficacy of the novel intervention (NIRS-NFT-VR) to Cogmed, the effectiveness of the newly designed intervention can be evaluated against a recognised standard of care. Second, Cogmed targets cognitive functions such as working memory, which are relevant to ADHD symptomatology, making it a suitable comparator for our intervention. Lastly, including Cogmed as an active control group enhances the scientific rigour of the study by providing a comparison to an intervention with demonstrated efficacy, allowing examination of whether the new intervention offers advantages beyond existing treatments.
To match the training duration of the NFT-VR group, each training session in the Cogmed group will last approximately 30 min. During each session, participants will play 5 working memory games selected from a total of 10 available in the Cogmed software, which train both visual and auditory working memory. Participants will attend training sessions at the university laboratory twice a week for 8 weeks. Each session is conducted in a one-on-one format, similar to the NFT-VR group, with a trainer who will explain the instructions for each game and monitor the entire training process. Participants in the Cogmed training group will receive performance-based reinforcement in the form of digital tokens, which they can use to build a simulated city within the software in each session.
The scripts and procedures for both training programmes are standardised according to a guideline that all trainers are required to adhere to. During the intervention period, participants are allowed to take ADHD medication, but they are advised against receiving parallel psychosocial interventions at the same time. Discontinuation of a training session will be considered on participants’ requests if the allocated training induces discomfort (eg, sickness).
Outcome measures
Primary outcome: executive function
We will use the following measures to assess executive function:
Test of Everyday Attention for Children (TEA-Ch):49 The TEA-Ch is a performance-based measure of attentional control and switching. Higher scores indicate better performance in the assessed domains. Changes in raw scores of the subtests of the TEA-Ch across times 1–3 will be analysed.
CPT3:50 The CPT3 is a standardised computerised test measuring sustained attention and inhibitory control. Higher T scores indicate better performance in the assessed domains. Changes in T scores of the performance indicators of the CPT3 across times 1–3 will be analysed.
Number subtest of the Children’s Memory Scale (CMS):51 Verbal working memory is measured using the number subtest of the CMS, with higher scores indicating better verbal working memory. Changes in total raw scores of the number subtest of the CMS across times 1–3 will be analysed.
fNIRS: The levels of oxygenated and deoxygenated haemoglobin of the prefrontal cortex will be measured during the CPT3 test for all participants across time points, as well as throughout the training sessions for the NFT group,28 36 using the portable OctaMon NIRS device (Artinis Medical Systems, Elst, the Netherlands).
Secondary outcome measures
SNAP-IV:40 52 Parents and teachers are asked to complete the SNAP-IV, a 26-item questionnaire measuring children’s inattentive, hyperactive and impulsive symptoms on a 4-point Likert scale from 0 (not at all) to 3 (very much). Higher scores on the subscales and the total scale of the SNAP-IV indicate more severe presentations of inattentive, hyperactive and impulsive symptoms. Changes in parent and teacher ratings of ADHD symptoms on the SNAP-IV across times 1–3 will be examined.
Behaviour Rating Inventory of Executive Function, Second Edition (BRIEF2):53 54 Parents and teachers are asked to complete the BRIEF2, which is a 63-item questionnaire with a 3-point Likert scale from 1 (never) to 3 (often). It measures a range of executive functions, including behavioural, emotional and cognitive regulation. Higher scores indicate more severe deficits in the respective executive function domains. Changes in sum scores of parent and teacher ratings on the Chinese version of the BRIEF2 across times 1–3 will be examined.
Other outcome measures
Simulator Sickness Questionnaire (SSQ):55 Children’s symptoms of cybersickness are measured by the SSQ, using a 4-point Likert scale from 0 (none) to 3 (severe). Higher scores indicate more severe symptoms of cybersickness. Raw scores from the cybersickness items (eg, nausea, fatigue and eye strain) in the SSQ will be used for analysis.
Modified Chinese version of the Cognitive Absorption Scale (CAS):56 57 Children’s enjoyment of the training will be evaluated using the CAS, based on a 7-point Likert scale from 1 (I don’t agree at all) to 7 (I completely agree). Higher scores indicate greater enjoyment of the training. Raw scores from the subscales and total scale of the CAS will be used for analysis.
Participant timeline
The participant timeline is visualised in figure 3.
Figure 3. Participant timeline. ADHD, attention-deficit/hyperactivity disorder; NFT-VR, neurofeedback training-virtual reality.
Adherence and retention
All participants will receive a stamp following each training session, along with a small gift (eg, a pencil or eraser) after every four sessions to incentivise completion of the study. On completing the entire training and follow-up assessments, the parents of each child participant will receive a brief report based on the assessments conducted in the study. Retention efforts will involve sending weekly reminders about upcoming training sessions to participants in the training groups. Additionally, parents of child participants in the waitlist control group will receive a brief interim assessment report after the time 2 assessment to encourage their continued engagement in the study.
Data collection
Data are collected at baseline and during follow-ups by the research team. Written consent is obtained from parents/caregivers and teachers, while oral assent is obtained from child participants prior to data collection (online supplemental material 2). Assessors are required to receive training before conducting assessments and must adhere to standardised procedures and scripts throughout the assessment process. Questionnaire data are collected online via Qualtrics surveys. Parents receive anonymous survey links to complete parent questionnaires at multiple time points. Meanwhile, teachers, contacted through parents, are asked to complete corresponding teacher questionnaires. Enrolment began in June 2023, with data collection projected to conclude by June 2025, aiming to recruit a total of 138 participants.
Data management
On obtaining informed parental consent, each participant will be assigned a subject ID. Deidentified participant codes are used to facilitate matching. Questionnaires from both teachers and parents will be collected and paired accordingly. Data entry will strictly adhere to the ethical guidelines established by the ethics committee of the authors’ institution. Any data containing personally identifiable information, such as names, dates of birth and contact details, will be securely stored separately from other files. Access to personal information will be restricted to the project principal and relevant research staff as needed. All paper-based data will be securely locked in a designated locker within the laboratory. Digital data will be stored in an encrypted hard disk with access only by the research team members.
Data monitoring
The data monitoring plan for this study entails regular inspections by the principal investigator and the research team to ensure data integrity and quality. Continuous monitoring includes periodic reviews of participant enrolment, data completeness and adherence to the study protocol. Furthermore, data-cleaning procedures will be implemented to detect and rectify any errors or inconsistencies in the dataset. Interim analyses of primary and secondary outcomes will occur when more than 50% of the participants have completed the 2-month follow-up assessment at time 3. The principal investigator (the last author) is responsible for reviewing the initial findings from the interim analyses regarding efficacy and utility. Although minimal adverse risks to participants’ health and safety are anticipated, potential boredom during training is acknowledged. Parents and child participants are encouraged to report any adverse events, which will be promptly evaluated and discussed by the research team.
Statistical methods
χ2 tests and ANOVAs will be performed to compare the demographic characteristics (eg, age and gender) and baseline variables among the three conditions. Intention-to-treat analyses will be conducted on the available data. Linear mixed-effects modelling will be employed to evaluate the treatment effects, specifying time (baseline, immediate post-test and follow-up), training group and the time-by-group interaction as fixed effects, with a random intercept for each participant. Bonferroni corrections will be applied to adjust for multiple comparisons. The treatment effects will be assessed based on the time-by-group interaction effects from baseline to immediate post-test/follow-up. Within-group effects from baseline to immediate post-test/follow-up will be calculated for all three groups using paired sample t-tests to evaluate whether there are significant improvements in the outcome variables across the three time points for the training conditions. Latent growth curve modelling analyses will also be performed to evaluate changes in the success rate of feedback signal regulation across training sessions. Finally, to investigate whether changes in task performance correlate with changes in prefrontal cortical activation, Pearson’s correlations will be examined.
Patient and public involvement
Patients and/or the public will not be involved in the design, conduct, reporting or dissemination plans of this research.
Ethics and dissemination
Ethical approval has been obtained from the Human Research Ethics Committee at the University of Hong Kong (Reference: EA200247). The study was registered at ClinicalTrials.gov (reference: NCT05906485). Important protocol modifications will be reported in the ClinicalTrials.gov trial registry. The principal investigator will review all publications relevant to this study prior to dissemination. Results will be published in peer-reviewed journals and presented at conferences.
Trial status
Protocol version 1, 8 November 2024. The trial is ongoing.
supplementary material
Acknowledgements
We would like to express our heartfelt gratitude to the participants and their parents who took part in the pilot phase of the study. We also extend our sincere thanks to those who have assisted in the trial run and design of this project, including the research assistants and master’s student, Ms. May Wong, Ms. Cythia Lok, Ms. Emily Cheung, and Ms. Suki Chan, as well as the student interns who have contributed greatly to the project.
Footnotes
Funding: This work is supported by the General Research Fund from the University Grants Committee in Hong Kong awarded to Shum (Grant number: 17608722). The grant proposal has been peer reviewed during the application process for the General Research Fund.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-093183).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained from parent(s)/guardian(s).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Que Zheng, Email: jeannez@connect.hku.hk.
Kathy Tsam-ling Kei, Email: kathykei@connect.hku.hk.
Ka Yu Chiu, Email: jadekyc@connect.hku.hk.
Kathy Kar-man Shum, Email: kkmshum@hku.hk.
References
- 1.American Psychiatric Association D, Association AP Diagnostic and Statistical Manual of Mental Disorders: DSM-5 .Washington, DC: American Psychiatric Association; 2013Availablehttps://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 [Google Scholar]
- 2.Ayano G, Demelash S, Gizachew Y, et al. The global prevalence of attention deficit hyperactivity disorder in children and adolescents: An umbrella review of meta-analyses. J Affect Disord. 2023;339:860–6. doi: 10.1016/j.jad.2023.07.071. [DOI] [PubMed] [Google Scholar]
- 3.Solanto MV, Pope-Boyd SA, Tryon WW, et al. Social functioning in predominantly inattentive and combined subtypes of children with ADHD. J Atten Disord. 2009;13:27–35. doi: 10.1177/1087054708320403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau V, Liu S, Lee F. Brainchild; 2012. Attention deficit/hyperactivity disorder—an epidemiological study in hong kong from 2003 to 2009; pp. 5–11.https://hkcndp.org/cmst/doc/publication/brainchild/85/Brainchild%202012%20vol%2013 %20no1.pdf Available. [Google Scholar]
- 5.Hurtig T, Ebeling H, Taanila A, et al. ADHD symptoms and subtypes: relationship between childhood and adolescent symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46:1605–13. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- 6.Wu ZM, Wang P, Liu L, et al. ADHD-inattentive versus ADHD-Combined subtypes: A severity continuum or two distinct entities? A comprehensive analysis of clinical, cognitive and neuroimaging data. J Psychiatr Res. 2022;149:28–36. doi: 10.1016/j.jpsychires.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Wåhlstedt C, Thorell LB, Bohlin G. Heterogeneity in ADHD: neuropsychological pathways, comorbidity and symptom domains. J Abnorm Child Psychol. 2009;37:551–64. doi: 10.1007/s10802-008-9286-9. [DOI] [PubMed] [Google Scholar]
- 8.Tosto MG, Momi SK, Asherson P, et al. A systematic review of attention deficit hyperactivity disorder (ADHD) and mathematical ability: current findings and future implications. BMC Med. 2015;13:204. doi: 10.1186/s12916-015-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Q, Cheng YY, Sonuga-Barke E, et al. Do Executive Dysfunction, Delay Aversion, and Time Perception Deficit Predict ADHD Symptoms and Early Academic Performance in Preschoolers. Res Child Adolesc Psychopathol . 2022;50:1381–97. doi: 10.1007/s10802-022-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda A, Presentación MJ, Siegenthaler R, et al. Effects of a psychosocial intervention on the executive functioning in children with ADHD. J Learn Disabil. 2013;46:363–76. doi: 10.1177/0022219411427349. [DOI] [PubMed] [Google Scholar]
- 11.Pelham WE, Waschbusch DA. Behavioral intervention in attention-deficit/hyperactivity disorder. Handb Disrupt Behav Disord. 1999:255–78. doi: 10.1007/978-1-4615-4881-2_11. [DOI] [Google Scholar]
- 12.So CYC, Leung PWL, Hung S-F. Treatment effectiveness of combined medication/behavioural treatment with chinese ADHD children in routine practice. Behav Res Ther. 2008;46:983–92. doi: 10.1016/j.brat.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Lim CG, Poh XWW, Fung SSD, et al. A randomized controlled trial of a brain-computer interface based attention training program for ADHD. PLoS One. 2019;14:e0216225. doi: 10.1371/journal.pone.0216225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamm L, Epstein JN, Peugh JL, et al. Preliminary data suggesting the efficacy of attention training for school-aged children with ADHD. Dev Cogn Neurosci. 2013;4:16–28. doi: 10.1016/j.dcn.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucha O, Tucha L, Kaumann G, et al. Training of attention functions in children with attention deficit hyperactivity disorder. ADHD Atten Def Hyp Disord . 2011;3:271–83. doi: 10.1007/s12402-011-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitaram R, Ros T, Stoeckel L, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. 2017;18:86–100. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- 17.Strehl U. What learning theories can teach us in designing neurofeedback treatments. Front Hum Neurosci. 2014;8:894. doi: 10.3389/fnhum.2014.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia Pimenta M, Brown T, Arns M, et al. Treatment Efficacy and Clinical Effectiveness of EEG Neurofeedback as a Personalized and Multimodal Treatment in ADHD: A Critical Review. Neuropsychiatr Dis Treat. 2021;17:637–48. doi: 10.2147/NDT.S251547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micoulaud-Franchi JA, Geoffroy PA, Fond G, et al. EEG neurofeedback treatments in children with ADHD: an updated meta-analysis of randomized controlled trials. Front Hum Neurosci. 2014;8:906. doi: 10.3389/fnhum.2014.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arns M, Conners CK, Kraemer HC. A decade of EEG Theta/Beta Ratio Research in ADHD: a meta-analysis. J Atten Disord. 2013;17:374–83. doi: 10.1177/1087054712460087. [DOI] [PubMed] [Google Scholar]
- 21.Sonuga-Barke EJS, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275–89. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- 22.Cortese S, Ferrin M, Brandeis D, et al. Neurofeedback for Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Clinical and Neuropsychological Outcomes From Randomized Controlled Trials. J Am Acad Child Adolesc Psychiatry. 2016;55:444–55. doi: 10.1016/j.jaac.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Rubia K, Westwood S, Aggensteiner PM, et al. Neurotherapeutics for Attention Deficit/Hyperactivity Disorder (ADHD): A Review. Cells. 2021;10:2156. doi: 10.3390/cells10082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gefen D, Ayaz H, Onaral B, et al. Applying Functional Near Infrared (fNIR) Spectroscopy to Enhance MIS Research. THCI . 2014;6:55–73. doi: 10.17705/1thci.00061. [DOI] [Google Scholar]
- 25.Ehlis AC, Bähne CG, Jacob CP, et al. Reduced lateral prefrontal activation in adult patients with attention-deficit/hyperactivity disorder (ADHD) during a working memory task: a functional near-infrared spectroscopy (fNIRS) study. J Psychiatr Res. 2008;42:1060–7. doi: 10.1016/j.jpsychires.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Negoro H, Sawada M, Iida J, et al. Prefrontal dysfunction in attention-deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Child Psychiatry Hum Dev. 2010;41:193–203. doi: 10.1007/s10578-009-0160-y. [DOI] [PubMed] [Google Scholar]
- 27.Meisel V, Servera M, Garcia-Banda G, et al. Reprint of 'Neurofeedback and standard pharmacological intervention in ADHD: a randomized controlled trial with six-month follow-up'. Biol Psychol. 2014;95:116–25. doi: 10.1016/j.biopsycho.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Marx A-M, Ehlis A-C, Furdea A, et al. Near-infrared spectroscopy (NIRS) neurofeedback as a treatment for children with attention deficit hyperactivity disorder (ADHD)-a pilot study. Front Hum Neurosci. 2014;8:1038. doi: 10.3389/fnhum.2014.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudak J, Blume F, Dresler T, et al. Near-Infrared Spectroscopy-Based Frontal Lobe Neurofeedback Integrated in Virtual Reality Modulates Brain and Behavior in Highly Impulsive Adults. Front Hum Neurosci. 2017;11:425. doi: 10.3389/fnhum.2017.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bink M, van Nieuwenhuizen C, Popma A, et al. Behavioral effects of neurofeedback in adolescents with ADHD: a randomized controlled trial. Eur Child Adolesc Psychiatry. 2015;24:1035–48. doi: 10.1007/s00787-014-0655-3. [DOI] [PubMed] [Google Scholar]
- 31.Bohil CJ, Alicea B, Biocca FA. Virtual reality in neuroscience research and therapy. Nat Rev Neurosci. 2011;12:752–62. doi: 10.1038/nrn3122. [DOI] [PubMed] [Google Scholar]
- 32.Keshavan MS, Vinogradov S, Rumsey J, et al. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171:510–22. doi: 10.1176/appi.ajp.2013.13081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bashiri A, Ghazisaeedi M, Shahmoradi L. The opportunities of virtual reality in the rehabilitation of children with attention deficit hyperactivity disorder: a literature review. Korean J Pediatr. 2017;60:337–43. doi: 10.3345/kjp.2017.60.11.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nolin P, Stipanicic A, Henry M, et al. ClinicaVR: Classroom-CPT: A virtual reality tool for assessing attention and inhibition in children and adolescents. Comput Human Behav. 2016;59:327–33. doi: 10.1016/j.chb.2016.02.023. [DOI] [Google Scholar]
- 35.Neguț A, Jurma AM, David D. Virtual-reality-based attention assessment of ADHD: ClinicaVR: Classroom-CPT versus a traditional continuous performance test. Child Neuropsychol. 2017;23:692–712. doi: 10.1080/09297049.2016.1186617. [DOI] [PubMed] [Google Scholar]
- 36.Blume F, Quixal M, Hudak J, et al. Development of Reading Abilities in Children with ADHD Following fNIRS-Neurofeedback or EMG-Biofeedback. Lern und Lernstör. 2020;9:163–74. doi: 10.1024/2235-0977/a000302. [DOI] [Google Scholar]
- 37.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grayling MJ, Wason JM. A web application for the design of multi-arm clinical trials. BMC Cancer. 2020;20:80. doi: 10.1186/s12885-020-6525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasslinger J, Bölte S, Jonsson U. Slow Cortical Potential Versus Live Z-score Neurofeedback in Children and Adolescents with ADHD: A Multi-arm Pragmatic Randomized Controlled Trial with Active and Passive Comparators. Res Child Adolesc Psychopathol . 2022;50:447–62. doi: 10.1007/s10802-021-00858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gau SS-F, Shang C-Y, Liu S-K, et al. Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale - parent form. Int J Methods Psychiatr Res. 2008;17:35–44. doi: 10.1002/mpr.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raven J. Manual for Raven’s progressive matrices and vocabulary scales. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- 42.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43:215–21. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blume F, Hudak J, Dresler T, et al. NIRS-based neurofeedback training in a virtual reality classroom for children with attention-deficit/hyperactivity disorder: study protocol for a randomized controlled trial. Trials. 2017;18:41. doi: 10.1186/s13063-016-1769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strehl U, Leins U, Goth G, et al. Self-regulation of slow cortical potentials: a new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics. 2006;118:e1530–40. doi: 10.1542/peds.2005-2478. [DOI] [PubMed] [Google Scholar]
- 45.Wolf K, Müller NG. In: Encyclopedia of the sciences of learning. Seel NM, editor. Boston, MA: Springer US; 2012. Attention and implicit learning; pp. 350–3. Available. [DOI] [Google Scholar]
- 46.Stadler MA. Role of attention in implicit learning. J Exp Psychol Learn Mem Cogn. 1995;21:674–85. doi: 10.1037//0278-7393.21.3.674. [DOI] [Google Scholar]
- 47.Klingberg T, Fernell E, Olesen PJ, et al. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–86. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Aksayli ND, Sala G, Gobet F. The cognitive and academic benefits of Cogmed: A meta-analysis. Educ Res Rev. 2019;27:229–43. doi: 10.1016/j.edurev.2019.04.003. [DOI] [Google Scholar]
- 49.Manly T, Robertson IH, Anderson V, et al. The test of everyday attention (TEA-CH) Bury St Edmunds Engl Thames Val Test Co; 1999. [Google Scholar]
- 50.Conners CK. MHS. 2014. Conners continuous performance test 3rd edition (conners cpt 3) & connors continuous auditory test of attention (conners cata): technical manual. [Google Scholar]
- 51.Cohen MJ. Children’s memory scale (CMS) San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 52.Gau SS-F, Lin C-H, Hu F-C, et al. Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, Version IV Scale-Teacher Form. J Pediatr Psychol. 2009;34:850–61. doi: 10.1093/jpepsy/jsn133. [DOI] [PubMed] [Google Scholar]
- 53.Shum KK-M, Zheng Q, Chak GS, et al. Dimensional structure of the BRIEF2 and its relations with ADHD symptoms and task performance on executive functions in Chinese children. Child Neuropsychol. 2021;27:165–89. doi: 10.1080/09297049.2020.1817355. [DOI] [PubMed] [Google Scholar]
- 54.Gioia GA, Isquith PK, Guy SC, et al. BRIEF: Behavior rating inventory of executive function. 2015
- 55.Kennedy RS, Lane NE, Berbaum KS, et al. Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int J Aviat Psychol. 1993;3:203–20. doi: 10.1207/s15327108ijap0303_3. [DOI] [Google Scholar]
- 56.Agarwal R, Karahanna E. Time Flies When You’re Having Fun: Cognitive Absorption and Beliefs about Information Technology Usage. MIS Q. 2000;24:665. doi: 10.2307/3250951. [DOI] [Google Scholar]
- 57.Bijsterveldt A. Tilburg, The Netherlands: Tilburg University; 2020. Cognitive absorption: analyzing and comparing its five dimensions within virtual reality and non-virtual reality rhythm games.https://arno.uvt.nl/show.cgi?fid=156728 Available. [Google Scholar]