ABSTRACT
Objective
Eczema is the most burdensome skin condition worldwide and topical anti‐inflammatory treatments are commonly used to control symptoms. The relative effectiveness and safety of different topical anti‐inflammatory treatments is uncertain.
Design
Network meta‐analysis performed within a Cochrane systematic review to compare and statistically rank efficacy and safety of topical anti‐inflammatory eczema treatments.
Data Sources
Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase and trial registries to June 2023.
Eligibility Criteria for Selected Trials
Included trials were within‐participant or between‐participant randomised controlled trials. Participants had eczema that was not clinically infected and was not contact dermatitis, seborrheic eczema or hand eczema. Interventions were topical anti‐inflammatory treatments but not complementary treatments, antibiotics alone, wet wraps, phototherapy or systemic treatments. Comparators were no treatment/vehicle or another topical anti‐inflammatory.
Results
We identified 291 trials (45,846 participants), mainly in high‐income countries. Most were industry‐funded with median 3 weeks treatment duration. Risk of bias assessed using the Cochrane Risk of Bias 2.0 tool was high in 89% of trials, mainly due to risk of selective reporting. Network meta‐analysis of binary outcomes ranked potent and/or very potent topical steroids, tacrolimus 0.1% and ruxolitinib 1.5% among the most effective treatments for improving patient‐reported symptoms (40 trials, all low confidence) and clinician‐reported signs (32 trials, all moderate confidence). For investigator global assessment, the Janus kinas inhibitors ruxolitinib 1.5%, delgocitinib 0.5% or 0.25%, very potent/potent topical steroids and tacrolimus 0.1% were ranked as most effective (140 trials, all moderate confidence). Continuous outcome data were mixed. Local application site reactions were most common with tacrolimus 0.1% (moderate confidence) and crisaborole 2% (high confidence) and least common with topical steroids (moderate confidence). Skin thinning was not increased with short‐term use of any topical steroid potency (low confidence) but skin thinning was reported in 6/2044 (0.3%) participants treated with longer‐term (6–60 months) topical steroids.
Conclusion
Potent topical steroids, Janus kinase inhibitors and tacrolimus 0.1% were consistently ranked as among the most effective topical anti‐inflammatory treatments for eczema.
Keywords: calcineurin inhibitor, eczema, Janus kinase inhibitor, network meta‐analysis, systematic review, topical steroid
Trials of topical anti‐inflammatory eczema treatments are mostly industry‐funded, evaluate short‐term outcomes and carry a high risk of bias due to selective reporting. Data from almost 300 trials suggest that potent steroids, Janus kinase inhibitors and tacrolimus 0.1% are among the most effective topical treatments. Local reactions were most common with tacrolimus 0.1% and crisaborole and least common with steroids.
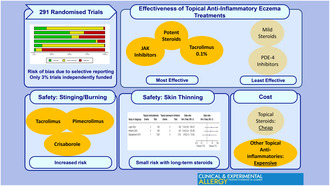
Summary.
Trials of topical anti‐inflammatory eczema treatments are mostly industry‐funded, short‐term and high risk of bias.
Potent steroids, Janus kinase inhibitors and tacrolimus 0.1% were among the most effective topical treatments.
Local reactions were most common with tacrolimus 0.1% and crisaborole and least common with steroids.
1. Introduction
Eczema affects up to 20% of infants, 6% of school‐age children and 5% of adults worldwide and is the most burdensome skin condition globally [1, 2, 3]. Topical corticosteroids (TCS) are the most commonly used anti‐inflammatory treatment for eczema and have been available for over 70 years. While there is great interest in novel systemic treatments for moderate–severe eczema, new classes of topical anti‐inflammatory treatments have also been licensed for mild, moderate or severe eczema treatment [4, 5]. Topical calcineurin inhibitors (TCI) tacrolimus and pimecrolimus were licensed in 2000, and subsequently phosphodiesterase 4 (PDE‐4) inhibitors such as crisaborole, Janus kinase (JAK) inhibitors such as ruxolitinib and aryl hydrocarbon receptor activators such as tapinarof have subsequently become available or are in development. There is a lack of comparative effectiveness research in eczema, as in many other areas of healthcare [6, 7]. This paucity makes it very difficult for health care professionals and patients to decide which treatment is best in terms of benefits and the least harms. It also hampers guideline development. Network meta‐analysis (NMA) is a tool developed for indirectly evaluating the relative effectiveness and safety of interventions used to treat the same condition. One NMA that included different interventions in addition to topical anti‐inflammatory treatments was published after our work started [8].
We conducted a NMA focussed on use of topical anti‐inflammatory treatments for treating eczema, in order to statistically rank and compare the effectiveness and safety of different topical anti‐inflammatory treatments.
2. Materials and Methods
This systematic review and NMA was conducted using standard Cochrane methodology, and according to its own pre‐published protocol and statistical analysis plan [9]. In brief, we included within‐participant or between‐participant randomised controlled trials (RCTs). Eligible trials evaluated people of any age with a clinical diagnosis of eczema (also called atopic dermatitis or atopic eczema) of any severity [10]. Specific, non‐atopic forms of eczema such as contact dermatitis, hand eczema or seborrheic eczema and clinically infected eczema were excluded. Eligible interventions included any well‐characterised topical anti‐inflammatory treatment. Emollients alone, topical antibiotics alone, complementary therapies, wet wraps, systemic treatment and phototherapy were excluded. In general, non‐licensed or non‐standard treatment regimens were excluded—for example application less than once daily or more than twice daily, or treatment durations of under 1 week. Comparison was to other topical anti‐inflammatory treatments, placebo/vehicle/emollient or no treatment. TCS were classified as mild, moderate, potent or very potent, as previously described [11]. Outcomes and outcome measures were prioritised according to the Harmonising Outcome Measures for Eczema (HOME) initiative [12, 13]; and informed by patient and public involvement during preliminary surveys and workshops as part of trial protocol development. Outcomes evaluated are summarised below:
2.1. Primary Outcomes
Patient‐reported symptoms of eczema. Data were extracted based on the Patient‐Oriented Eczema Measure (POEM) or, if not available, alternative instruments ranked in order of preference in the protocol, most commonly a visual analogue scale of pruritus [9].
Clinician‐reported signs of eczema. Data were extracted based on the Eczema Area and Severity Index [14] or, if not available, alternative instruments ranked in order of preference in the protocol, most commonly the Scoring Atopic Dermatitis [15]. Investigator Global Assessment (IGA) was considered separately from other clinician‐reported signs of eczema, since the construct of IGA as a single global assessment is emphasised by some regulatory authorities and may differ from other measures of eczema signs.
2.2. Secondary Outcomes
Health‐related quality of life. Data were extracted based on the Dermatology Life Quality Index including children's and infants' versions [16], or, if not available alternative instruments ranked in order of preference in the protocol.
Long‐term control of eczema. Data were extracted based on the Recap of Eczema Control [17] or, if not available, alternative instruments such as the Atopic Dermatitis Control Tool [18].
Local adverse effects. These were prioritised in patient and public involvement work as local application site reactions (‘tolerability’), cosmetic effects such as pigmentation changes, skin thinning/atrophy and withdrawal from treatment or trial due to adverse effects of the intervention.
2.3. Search Strategy
We searched the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, the World Health Organization clinical trial meta‐registry and clinicaltrials.gov up to June 2023. The full search strategy is shown in the Cochrane review protocol [9].
2.4. Data Collection and Analysis
This was a frequentist NMA undertaken according to the methods of the Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 [19]. Analysis was conducted following a statistical analysis plan with planned networks, sensitivity and subgroup analyses which were described in the protocol and finalised before undertaking data analysis. Networks were planned for patient‐reported symptoms and clinician assessed signs of eczema, separately for binary and continuous outcomes and for short and long‐term outcomes. IGA was treated separately with its own network(s) and safety outcomes were analysed with separate networks for application site reactions, pigmentation changes, skin thinning/atrophy and withdrawals due to adverse events. For long‐term outcomes and for quality of life there was insufficient data available to create networks. NMA was performed using a random‐effects model summarising either odds ratios (ORs) or standardised mean differences (SMDs) in Stata with the mvmeta command within the network suite of commands [20] and the Stata commands for graphing, statistically ranking using the Surface Under the Cumulative Ranking (SUCRA) score and reporting network results [21]. Heterogeneity was quantified using the heterogeneity parameter Tau. Local consistency was evaluated using the node‐splitting approach with Stata's sidesplit command [20] and global design inconsistency was evaluated using the ‘design by treatment interaction’ model [22]. Risk of bias of included trials was assessed using the Cochrane risk of bias 2.0 tool [23]. Certainty of evidence was assessed using the Confidence In NEtwork Meta‐Analysis (CINEMA) approach [24]. We planned for primary analyses to include only low risk of bias data. However, only one network was possible due to a lack of low risk of bias data, so we undertook primary analyses on ‘all available data’ and a sensitivity analysis, where possible, for the low risk of bias data. Other sensitivity analyses used alternative classification for TCS potency, restricted outcome data to HOME‐recommended measurement tools and excluding within‐participant data. Class‐level sensitivity analyses were also undertaken. Subgroup analyses explored potential impact of application site (face included versus not included), disease severity (severe versus non‐severe) and age (under 12 years or older). Summary of Findings Tables were created for each outcome where NMA was possible, and included only topical anti‐inflammatory interventions which are currently licensed, applied using the licensed concentration(s). Additional methods are shown in the Cochrane review protocol [9].
3. Results
Search results are summarised in Figure 1 and the review is reported according to the PRISMA statement and NMA extension [25, 26]. We identified 291 trials (45,846 participants) with published outcomes with the full spectrum of eczema severity and a further 120 trials which were either ongoing (95) or couldn't be classified (25). Trials were mainly conducted in high‐income countries (243) especially Europe and North America, and in secondary care settings (189). Adults were included in most trials, with only 31 trials limited to children aged <12 years. Male and female participants and multiple ethnic groups were present in most trials, but trials were mainly undertaken in predominantly white populations. Ninety‐seven per cent of trials were either industry‐funded (199) or did not report their funding (85). Treatment duration and trial participation were median 21 and 28 days (range 7 days to 5 years). Interventions used in the included trials are summarised in Figure 2. They were TCS (172), TCI (134), PDE‐4 inhibitors (55), JAK inhibitors (30), aryl hydrocarbon receptor activators (10) or other topical agents (21). Comparators included vehicle (170) or other anti‐inflammatory treatments. Risk of bias is summarised in Figure 3. Using the outcome with lowest risk of bias from each trial, risk of bias was high in 242 of the 272 (89%) trials contributing to data analyses. The most common risk of bias issue was concern about selective reporting due to absence of prospective trial registration/protocol availability, even in more recent trials. Other issues noted were insufficient information to judge allocation concealment, concern about contamination in within‐participant trials, poor reporting of numbers of randomised participants included in outcome analysis, exclusions from analysis for potentially inappropriate reasons such as adverse events and trials with high proportions of randomised participants missing from analyses.
FIGURE 1.

PRISMA flow diagram. *These were immunohistochemical analyses (n = 4), skin barrier analyses (n = 2), cost‐effectiveness analyses (n = 1) or additional records to existing trials with no new relevant outcome data (n = 4 from 2 trials). All were checked for effectiveness or safety outcomes.
FIGURE 2.
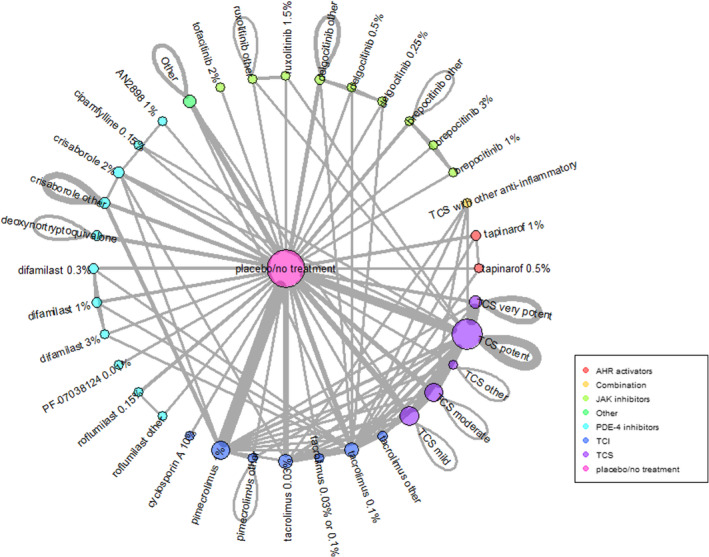
Network of included interventions. AHR, aryl hydrocarbon, JAK, Janus kinase, PDE‐4, phosphodiesterase‐4, TCI, topical calcineurin inhibitor, TCS, topical corticosteroid. Network shows all interventions included in the 291 included trials. Lines represent comparisons made in the included trials. The thickness of each line represents the number of separate trials making the comparison. This network represents all interventions, but the Summary of Findings Tables report only licensed interventions at licensed doses/concentrations.
FIGURE 3.
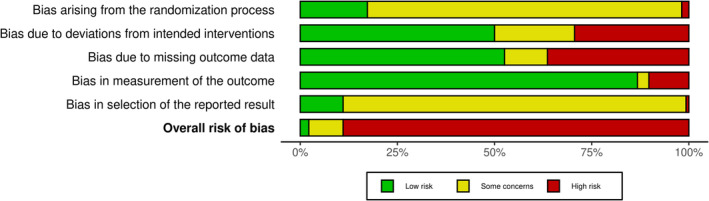
Summary of risk of bias in included trials. Summary Cochrane Risk of bias 2.0 assessments of the 291 included trials. For trials reporting multiple outcomes, the lowest risk of bias outcome is included in this summary.
The pooled direct and indirect effects of licensed interventions compared with placebo are summarised for each outcome in the Summary of Findings Tables (Tables 1, 2, 3, 4, 5), including CINeMA certainty of evidence ratings.
TABLE 1.
Summary of findings for patient‐reported symptoms (binary).
| Treatment | Direct evidence 40 RCTs, 6482 participants | Relative effect OR (95% CI) | Anticipated response rate with control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
|---|---|---|---|---|---|---|---|
| TCS potent | 6 RCTs, 699 participants | 5.99 (2.83, 12.69) | 309 per 1000 | 729 per 1000 | 420 per 1000 (250 to 541) | 0.75 | Low |
| Tacrolimus 0.1% | Indirect only | 6.27 (1.19, 32.98) | 309 per 1000 | 738 per 1000 | 429 per 1000 (39 to 627) | 0.74 | Low |
| Ruxolitinib 1.5% | 2 RCTs, 465 participants | 5.64 (1.26, 25.25) | 309 per 1000 | 716 per 1000 | 407 per 1000 (51 to 609) | 0.71 | Low |
| TCS very potent | 1 RCTs, 61 participants | 5.08 (0.51, 50.76) | 309 per 1000 | 695 per 1000 | 386 per 1000 (−124 to 648) | 0.65 | Low |
| Tacrolimus 0.03% | Indirect only | 4.56 (0.49, 42.48) | 309 per 1000 | 671 per 1000 | 362 per 1000 (−130 to 641) | 0.63 | Low |
| Pimecrolimus 1% | 10 RCTs, 1712 participants | 3.59 (1.84, 7.01) | 309 per 1000 | 617 per 1000 | 308 per 1000 (142 to 449) | 0.58 | Low |
| Tapinarof 1% | 1 RCTs, 77 participants | 3.13 (0.33, 29.34) | 309 per 1000 | 583 per 1000 | 274 per 1000 (−180 to 620) | 0.52 | Low |
| TCS moderate | 5 RCTs, 1426 participants | 2.62 (1.18, 5.81) | 309 per 1000 | 540 per 1000 | 231 per 1000 (37 to 413) | 0.47 | Low |
| Crisaborole 2% | 1 RCTs, 63 participants | 1.15 (0.17, 7.71) | 309 per 1000 | 341 per 1000 | 32 per 1000 (−238 to 466) | 0.27 | Low |
| Roflumilast 0.15% | 1 RCTs, 81 participants | 1.03 (0.12, 9.23) | 309 per 1000 | 316 per 1000 | 7 per 1000 (−260 to 496) | 0.26 | Moderate |
| TCS mild | 2 RCTs, 80 participants | 1.35 (0.51, 3.53) | 309 per 1000 | 376 per 1000 | 67 per 1000 (−122 to 303) | 0.25 | Low |
Note: Trials compared topical anti‐inflammatory treatments to vehicle in people with eczema, and evaluated patient‐reported symptoms as a binary measure at 1–16 weeks after treatment initiation. Most trials were undertaken in a secondary care setting. A ≥ 4 point improvement in the Peak Pruritus Numerical Rating Scale was the most commonly reported measure.
Abbreviations: CI, confidence interval; CINeMA, confidence in meta‐analysis, OR, odds ratio; RCT, randomised controlled trial; SUCRA, surface area under the cumulative ranking curve; TCS, topical corticosteroid.
TABLE 2.
Summary of findings for clinician‐reported signs (binary).
| Treatment | Direct evidence 32 RCTs, 4121 participants | Relative effect OR (95% CI) | Anticipated response rate with control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
|---|---|---|---|---|---|---|---|
| TCS potent | 4 RCTs, 341 participants | 8.15 (4.90, 13.57) | 191 per 1000 | 658 per 1000 | 467 per 1000 (345 to 571) | 0.87 | Moderate |
| Tacrolimus 0.1% | 1 RCTs, 61 participants | 8.06 (3.30, 19.67) | 191 per 1000 | 655 per 1000 | 464 per 1000 (247 to 632) | 0.84 | Moderate |
| Ruxolitinib 1.5% | 3 RCTs, 879 participants | 7.72 (4.92, 12.10) | 191 per 1000 | 645 per 1000 | 454 per 1000 (346 to 550) | 0.84 | Moderate |
| Delgocitinib 0.5% | 3 RCTs, 323 participants | 7.61 (3.72, 15.58) | 191 per 1000 | 642 per 1000 | 451 per 1000 (276 to 595) | 0.83 | Moderate |
| Difamilast 1% | 2 RCTs, 532 participants | 5.42 (3.06, 9.58) | 191 per 1000 | 561 per 1000 | 370 per 1000 (228 to 502) | 0.67 | Moderate |
| Delgocitinib 0.25% | 3 RCTs, 306 participants | 5.26 (2.55, 10.87) | 191 per 1000 | 554 per 1000 | 363 per 1000 (185 to 528) | 0.65 | Moderate |
| TCS moderate | 1 RCTs, 37 participants | 5.22 (2.55, 10.67) | 191 per 1000 | 552 per 1000 | 361 per 1000 (185 to 525) | 0.64 | Moderate |
| Pimecrolimus 1% | 5 RCTs, 750 participants | 3.65 (2.40, 5.57) | 191 per 1000 | 463 per 1000 | 272 per 1000 (170 to 377) | 0.48 | Moderate |
| Difamilast 0.3% | 1 RCTs, 166 participants | 3.22 (1.45, 7.13) | 191 per 1000 | 431 per 1000 | 240 per 1000 (64 to 436) | 0.42 | High |
| Crisaborole 2% | 2 RCTs, 169 participants | 2.98 (1.42, 6.26) | 191 per 1000 | 413 per 1000 | 222 per 1000 (60 to 405) | 0.39 | High |
| Roflumilast 0.15% | 1 RCTs, 89 participants | 2.43 (0.88, 6.70) | 191 per 1000 | 364 per 1000 | 173 per 1000 (−19 to 422) | 0.33 | Very Low |
| Tapinarof 1% | 1 RCTs, 126 participants | 2.45 (1.00, 6.02) | 191 per 1000 | 366 per 1000 | 175 per 1000 (−1 to 396) | 0.32 | Low |
| TCS mild | 1 RCTs, 44 participants | 2.22 (0.74, 6.64) | 191 per 1000 | 343 per 1000 | 152 per 1000 (−42 to 419) | 0.28 | Low |
Note: Trials compared topical anti‐inflammatory treatments to vehicle in people with eczema, and evaluated clinician‐reported signs as a binary measure at 1–16 weeks after treatment initiation. Most trials were undertaken in a secondary care setting. A ≥75% improvement in Eczema Area and Severity Index was the most commonly reported measure.
Abbreviations: CI, confidence interval; CINeMA, confidence in meta‐analysis, OR, odds ratio; RCT, randomised controlled trial; SUCRA, surface area under the cumulative ranking curve; TCS, topical corticosteroid.
TABLE 3.
Summary of findings for Investigator Global Assessment (binary).
| Treatment | Direct evidence 140 RCTs, 23,383 participants | Relative effect OR (95% CI) | Anticipated response rate with control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
|---|---|---|---|---|---|---|---|
| Ruxolitinib 1.5% | 3 RCTs, 879 participants | 9.34 (4.80, 18.18) | 256 per 1000 | 762 per 1000 | 506 per 1000 (367 to 606) | 0.86 | Moderate |
| TCS very potent | 2 RCTs, 438 participants | 8.34 (4.73, 14.67) | 256 per 1000 | 741 per 1000 | 485 per 1000 (364 to 579) | 0.84 | Moderate |
| Delgocitinib 0.5% | 2 RCTs, 165 participants | 10.08 (2.65, 38.37) | 256 per 1000 | 776 per 1000 | 520 per 1000 (221 to 674) | 0.83 | Moderate |
| Delgocitinib 0.25% | 2 RCTs, 169 participants | 6.87 (1.79, 26.33) | 256 per 1000 | 702 per 1000 | 446 per 1000 (126 to 645) | 0.73 | Moderate |
| Tacrolimus 0.1% | 10 RCTs, 1718 participants | 5.06 (3.59, 7.13) | 256 per 1000 | 635 per 1000 | 379 per 1000 (297 to 454) | 0.68 | Moderate |
| TCS potent | 16 RCTs, 1708 participants | 5 (3.80, 6.58) | 256 per 1000 | 632 per 1000 | 376 per 1000 (310 to 438) | 0.67 | Moderate |
| TCS moderate | 8 RCTs, 1335 participants | 4.46 (3.19, 6.24) | 256 per 1000 | 605 per 1000 | 349 per 1000 (267 to 426) | 0.62 | Moderate |
| Tapinarof 1% | 3 RCTs, 262 participants | 3.68 (1.73, 7.82) | 256 per 1000 | 558 per 1000 | 302 per 1000 (117 to 473) | 0.53 | Moderate |
| Difamilast 1% | 6 RCTs, 927 participants | 3.45 (1.97, 6.02) | 256 per 1000 | 542 per 1000 | 286 per 1000 (148 to 418) | 0.51 | Moderate |
| Tacrolimus 0.03% | 10 RCTs, 2576 participants | 3.53 (2.60, 4.80) | 256 per 1000 | 548 per 1000 | 292 per 1000 (216 to 367) | 0.51 | Moderate |
| Roflumilast 0.15% | 1 RCTs, 89 participants | 2.43 (0.65, 9.01) | 256 per 1000 | 454 per 1000 | 198 per 1000 (−73 to 500) | 0.39 | Moderate |
| Difamilast 0.3% | 5 RCTs, 558 participants | 2.56 (1.37, 4.78) | 256 per 1000 | 468 per 1000 | 212 per 1000 (65 to 366) | 0.38 | Moderate |
| Pimecrolimus 1% | 17 RCTs, 4064 participants | 2.39 (1.78, 3.21) | 256 per 1000 | 451 per 1000 | 195 per 1000 (123 to 269) | 0.35 | Moderate |
| Crisaborole 2% | 5 RCTs, 1725 participants | 2.14 (1.22, 3.76) | 256 per 1000 | 424 per 1000 | 168 per 1000 (40 to 308) | 0.32 | Low |
| TCS mild | 1 RCTs, 46 participants | 1.38 (0.94, 2.02) | 256 per 1000 | 321 per 1000 | 65 per 1000 (−12 to 154) | 0.17 | Low |
Note: Trials compared topical anti‐inflammatory treatments to vehicle in people with eczema, and evaluated investigator global assessment as a binary measure at 1–16 weeks after treatment initiation. Most trials were undertaken in a secondary care setting. ‘Clear or almost clear’ eczema on a 6‐point Investigator Global Assessment was the most commonly reported measure.
Abbreviations: CI, confidence interval; CINeMA, confidence in meta‐analysis, OR, odds ratio; RCT, randomised controlled trial; SUCRA, surface area under the cumulative ranking curve; TCS, topical corticosteroid.
TABLE 4.
Summary of findings for application site reactions (binary).
| Treatment | Direct evidence 83 RCTs, 18,992 participants | Relative effect OR (95% CI) | Anticipated response rate with control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
|---|---|---|---|---|---|---|---|
| Tacrolimus 0.1% | 5 RCTs, 2364 participants | 2.09 (1.46, 3.00) | 80 per 1000 | 154 per 1000 | 74 per 1000 (33 to 126) | 0.83 | Moderate |
| Crisaborole 2% | 4 RCTs, 1247 participants | 2.11 (1.19, 3.76) | 80 per 1000 | 155 per 1000 | 75 per 1000 (14 to 166) | 0.82 | High |
| Pimecrolimus 1% | 15 RCTs, 2482 participants | 1.49 (1.05, 2.12) | 80 per 1000 | 114 per 1000 | 35 per 1000 (4 to 75) | 0.71 | Low |
| Tacrolimus 0.03% | 8 RCTs, 3470 participants | 1.49 (1.08, 2.04) | 80 per 1000 | 114 per 1000 | 34 per 1000 (6 to 70) | 0.71 | Low |
| Tapinarof 1% | 1 RCTs, 163 participants | 1.01 (0.06, 17.91) | 80 per 1000 | 81 per 1000 | 1 per 1000 (−75 to 528) | 0.57 | Low |
| TCS mild | 1 RCTs, 768 participants | 0.51 (0.30, 0.85) | 80 per 1000 | 42 per 1000 | −38 per 1000 (−54 to −11) | 0.38 | Moderate |
| TCS moderate | 3 RCTs, 670 participants | 0.49 (0.25, 0.93) | 80 per 1000 | 40 per 1000 | −39 per 1000 (−58 to −5) | 0.37 | Moderate |
| Roflumilast 0.15% | 1 RCTs, 90 participants | 0.33 (0.01, 8.84) | 80 per 1000 | 27 per 1000 | −52 per 1000 (−79 to 354) | 0.32 | Moderate |
| TCS potent | 7 RCTs, 1149 participants | 0.35 (0.22, 0.55) | 80 per 1000 | 29 per 1000 | −51 per 1000 (−61 to −34) | 0.26 | Moderate |
| TCS very potent | 3 RCTs, 492 participants | 0.33 (0.13, 0.81) | 80 per 1000 | 27 per 1000 | −52 per 1000 (−69 to −14) | 0.25 | Low |
Note: Trials compared topical anti‐inflammatory treatments to vehicle in people with eczema, and evaluated investigator application site reactions as a binary measure at 1–16 weeks after treatment initiation. Most trials were undertaken in a secondary care setting. Application site reactions included tolerability events, burning, stinging and irritation.
Abbreviations: CI, confidence interval; CINeMA, confidence in meta‐analysis, OR, odds ratio; RCT, randomised controlled trial; SUCRA, surface area under the cumulative ranking curve; TCS, topical corticosteroid.
TABLE 5.
Summary of findings for skin thinning/atrophy (binary).
| Treatment | Direct evidence 83 RCTs, 18,992 participants | Relative effect OR (95% CI) | Anticipated response rate with control | Anticipated response rate with treatment | Difference (95% CI) | SUCRA | CINeMA |
|---|---|---|---|---|---|---|---|
| TCS potent | 3 RCTs, 578 participants | 0.96 (0.21, 4.43) | 7 per 1000 | 7 per 1000 | 0 per 1000 (−6 to 24) | 0.57 | Low |
| TCS moderate | 1 RCTs, 51 participants | 0.91 (0.16, 5.33) | 7 per 1000 | 7 per 1000 | 0 per 1000 (−6 to 30) | 0.54 | Low |
| TCS very potent | 3 RCTs, 919 participants | 0.88 (0.31, 2.49) | 7 per 1000 | 6 per 1000 | −1 per 1000 (−5 to 11) | 0.52 | Low |
| Tacrolimus 0.1% | indirect only | 0.88 (0.01, 60.36) | 7 per 1000 | 6 per 1000 | −1 per 1000 (−7 to 299) | 0.51 | Low |
| TCS mild | 1 RCTs, 768 participants | 0.72 (0.12, 4.31) | 7 per 1000 | 5 per 1000 | −2 per 1000 (−6 to 23) | 0.44 | Low |
| Pimecrolimus 1% | 1 RCTs, 38 participants | 0.15 (0.01, 1.59) | 7 per 1000 | 1 per 1000 | −6 per 1000 (−7 to 4) | 0.10 | Low |
Note: Trials compared topical anti‐inflammatory treatments to vehicle in people with eczema, and evaluated skin thinning/striae as a binary measure at 1–16 weeks after treatment initiation. Most trials were undertaken in a secondary care setting. Skin thinning/striae included skin thinning, atrophy, striae or telangiectasia.
Abbreviations: CI, confidence interval; CINeMA, confidence in meta‐analysis, OR, odds ratio; RCT, randomised controlled trial; SUCRA, surface area under the cumulative ranking curve; TCS, topical corticosteroid.
3.1. Patient‐Reported Eczema Symptoms
NMA included 40 trials (n = 6482) which most commonly reported a ≥4 point improvement in the Peak Pruritus Numerical Rating Scale. Potent TCS (OR 5.99, 95% CI 2.83, 12.69), the TCI tacrolimus 0.1% (OR 6.27, 95% CI 1.19, 32.98) and the JAK inhibitor ruxolitinib 1.5% (OR 5.64, 95% CI 1.26, 25.25) were ranked as most effective. Mild TCS (OR 1.35, 95% CI 0.51, 3.53) and the PDE‐4 inhibitors roflumilast 0.15% (OR 1.03, 95% CI 0.12, 9.23) and crisaborole 2% (OR 1.15, 95% CI 0.17, 7.71) were ranked as least effective (Table 1). Confidence intervals were wide and overlapping for most comparisons, and CINeMA ratings were low or (for roflumilast 0.15%) moderate. Downgrades were made for within‐trial bias in all CINeMA judgements, and some were also downgraded for imprecision and heterogeneity. Subgroup and sensitivity analyses, narrative information and analysis of patient‐reported eczema symptoms as a continuous outcome are shown in the full Cochrane review [27] and associated repository https://osf.io/6ujga. In general, these were consistent with the main binary analysis of patient‐reported eczema symptoms.
3.2. Clinician‐Reported Eczema Signs
NMA included 32 trials (n = 4121) which most commonly reported a ≥75% relative improvement in Eczema Area and Severity Index. Potent TCS (OR 8.15, 95% CI 4.90, 13.57), the TCI tacrolimus 0.1% (OR 8.06, 95% CI 3.30, 19.67) and the JAK inhibitors ruxolitinib 1.5% (OR 7.72, 95% CI 4.92, 12.10) and delgocitinib 0.5% (OR 7.61, 95% CI 3.72, 15.58) were ranked as most effective. Mild TCS (OR 2.22, 95% CI 0.74, 6.64), the PDE‐4 inhibitors roflumilast 0.15% (OR 2.43, 95% CI 0.88, 6.70) and crisaborole 2% (OR 2.98, 95% CI 1.42, 6.26) and the AHR activator tapinarof 1% (OR 2.45, 95% CI 1.00, 6.02) were ranked as least effective (Table 2). Confidence intervals were wide and overlapping for most comparisons, but CINeMA ratings were moderate or high for most licensed interventions. CINeMA downgrades were most commonly made for within‐trial bias, but also imprecision and heterogeneity. Subgroup and sensitivity analyses, narrative information and analysis of clinician‐reported eczema signs as a continuous outcome are shown in the full Cochrane review [27] and associated repository. In general, these were consistent with the main binary analysis of clinician‐reported eczema signs. However, NMA of clinician‐reported eczema signs yielded some counter‐intuitive findings such as increased effectiveness of lower potency TCI and TCS when indirectly compared with higher potency TCI and TCS.
3.3. Investigator Global Assessment
NMA included 140 trials (n = 23,383) which most commonly reported ‘clear or almost clear’ eczema on a 6‐point Investigator Global Assessment. Potent TCS (OR 5.00, 95% CI 3.80, 6.58), very potent TCS (OR 8.34, 95% CI 4.73, 14.67), the JAK inhibitors ruxolitinib 1.5%, (OR 9.34, 95% CI 4.80, 18.18), delgocitinib 0.5% (OR 10.08, 95% CI 2.65, 38.37) and delgocitinib 0.25% (OR 6.87, 95% CI 1.79, 26.33) and the TCI tacrolimus 0.1% (OR 5.06, 95% CI 3.59, 7.13) were ranked as most effective. Mild TCS (OR 1.38, 95% CI 0.94, 2.02), the PDE‐4 inhibitors roflumilast 0.15% (OR 2.43, 95% CI 0.65, 9.01), crisaborole 2% (OR 2.14, 95% CI 1.22, 3.76), difamilast 0.3% (OR 2.56, 95% CI 1.37, 4.78) and difamilast 1% (OR 3.45, 95% CI 1.97, 6.02) and the TCIs tacrolimus 0.03% (OR 3.53, 95% CI 2.60, 4.80) and pimecrolimus 1% (OR 2.39, 95% CI 1.78, 3.21) were ranked as least effective (Table 3). Confidence intervals were wide and overlapping for most comparisons, and CINeMA ratings were low or moderate for most licensed interventions. CINeMA downgrades were most commonly made for within‐trial bias. Subgroup and sensitivity analyses and narrative information are shown in the full Cochrane review [27] and associated repository. In general, these were consistent with the main IGA analysis. In a sensitivity analysis of low risk of bias data (12 trials, n = 1639), potent TCS and the JAK inhibitors delgocitinib 0.5% and delgocitinib 0.25% ranked as most effective, and the TCI pimecrolimus 1%, PDE 4 inhibitors roflumilast 0.15%, difamilast 1% and difamilast 0.3% least effective.
3.4. Local Application Site Reactions
NMA included 83 trials (n = 18,992) reporting tolerability events, burning, stinging and/or irritation reactions. TCIs tacrolimus 0.1% and 0.03% and pimecrolimus 1% and PDE 4 inhibitor crisaborole 2% were ranked as most likely to cause application site reactions and mild to very potent TCS as least likely (Table 4). Confidence intervals were wide for most comparisons, and CINeMA ratings were low or moderate for most licensed interventions, but high for crisaborole 2%. CINeMA downgrades were most commonly made for within‐trial bias and imprecision. Subgroup and sensitivity analyses and narrative information are shown in the full Cochrane review [27] and associated repository. In general, these were consistent with the main analysis.
3.5. Skin Thinning/Atrophy
NMA included 25 trials (n = 3691, 36 events) reporting skin thinning, atrophy, striae and/or telangiectasia. There was no significant increase in odds of skin thinning/atrophy with mild to very potent TCS or the TCIs tacrolimus 0.1% and pimecrolimus 1% compared with vehicle. CINeMA ratings were low for all comparisons, due to within‐trial bias and imprecision. Subgroup and sensitivity analyses and narrative information are shown in the full Cochrane review [27] and associated repository. In general, these were consistent with the main analysis. Longer‐term data over 6–60 months for this outcome were insufficient for NMA but were reported for TCS versus TCI in three trials (Figure 4), showing an increase in long‐term skin thinning with TCS (6 events in 2044 participants with TCS versus 0 events in 2025 participants with TCI; p = 0.031, Fisher's exact test). The three included trials evaluated potent TCS versus tacrolimus 0.1% over 6 months follow‐up [28], moderate TCS versus pimecrolimus 1% over 1‐year follow‐up [29] and mild/moderate TCS versus pimecrolimus 1% over 5 years follow‐up [30]. The three trials were all funded by TCI manufacturers and included treatment of both facial and non‐facial areas affected by eczema. The trial authors did not comment on reversibility of the skin thinning changes identified, nor did they provide details about location and nature of the changes identified.
FIGURE 4.
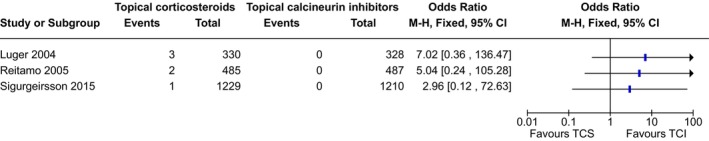
Effect of longer‐term use of topical steroids versus topical calcineurin inhibitors on risk of skin thinning. Summary of trials reporting risk of skin thinning/atrophy with longer term (6–60 months) topical anti‐inflammatory treatments. The trials evaluated potent TCS versus tacrolimus 0.1% over 6 months follow‐up [28], moderate TCS versus pimecrolimus 1% over 1 year follow‐up [29], and mild/moderate TCS versus pimecrolimus 1% over 5 years follow‐up [30]. They were all funded by pimecrolimus or tacrolimus manufacturers and included treatment of both facial and non‐facial areas affected by eczema. Authors did not comment on the location, nature, severity or reversibility of the skin thinning changes identified.
3.6. Other Outcomes
NMA was not possible for health‐related quality of life, long‐term control or longer‐term outcome assessment for any of the above outcomes, due to insufficient data. NMA of pigmentary changes (8 trials of TCS and a PDE‐4 inhibitor, n = 1786, 3 events) did not show any significant increase in odds of pigmentation changes compared to vehicle, with low confidence for mild, moderate or potent TCS and moderate confidence for crisaborole 2%. NMA of withdrawal due to short‐term adverse events (11 trials of TCS, TCI, JAK inhibitors and other interventions, n = 2404) did not show any significant increase in odds of withdrawal compared to vehicle with any intervention, with low confidence. There was a lack of long‐term safety data.
4. Discussion
In this Cochrane systematic review and NMA of topical anti‐inflammatory treatments for eczema, we found potent TCS, JAK inhibitors and the TCI tacrolimus 0.1% were consistently ranked as among the most effective topical anti‐inflammatory treatments for eczema. Our confidence in the findings was low or moderate, largely due to concerns about selective reporting; and we were not able to reliably rank the comparative effectiveness of these three treatments. Safety data were limited, but local application site reactions were most common with tacrolimus 0.1% and the PDE‐4 inhibitor crisaborole 2%. Although skin thinning was not increased with short‐term (<16 weeks) use of any TCS potency, skin thinning was reported as an adverse effect in 0.3% participants treated with TCS for 6 to 60 months.
These findings largely relate to short‐term use of topical anti‐inflammatory treatments for treating non‐severe eczema in adults living in high‐income countries. There is a need for more work to identify longer‐term outcomes, to evaluate effectiveness and safety in young children, in whom eczema is most common, and in low‐middle income countries [1]. For example, recent trials have reported no impact of intermittent mild–moderate TCS on young children's growth over up to 5 years, but significant growth suppression when a proactive potent TCS regimen is used in infants with eczema [31, 32, 33]. We did not formally evaluate cost effectiveness, but it is worthwhile noting that per gram, TCI, JAK inhibitors, PDE‐4 inhibitors and AHR activators typically cost 4–20 times more than TCS.
Our findings are broadly consistent with previous literature, including a recent NMA of topical anti‐inflammatory treatments for eczema [8]. However, there are some differences in emphasis between the two NMA findings, including slightly lower efficacy ranking of JAK inhibitors by Chu et al., and a different focus of adverse event outcomes. Inclusion criteria differed between the two NMAs, and there were some differences in methodology. For example, the review of Chu et al. excluded within‐participant trials, and included complementary therapy, moisturisers, non‐licensed treatment regimens and secondary prevention of eczema flares using regular anti‐inflammatory treatment which were not part of this Cochrane review. This is likely to explain any differences in rankings between the two NMAs, for comparable outcomes. Our finding that TCS rarely cause skin thinning is consistent with the conclusions of a separate Cochrane review evaluating strategies for intermittent short‐term treatment of eczema with TCS [11].
We found significant issues related to risk of bias in this dataset, especially selective reporting. Despite completion and publication of 291 trials with 45,846 participants, there was insufficient low risk of bias information to undertake NMA for key outcomes of interest. Risk of bias is an issue in many other areas of healthcare evidence, and in some specific fields such as infant nutrition, transparent and complete reporting of clinical trial outcomes is almost completely absent from the literature [34, 35]. In contrast to this Cochrane review of eczema treatment trials, a Cochrane review of eczema prevention trials using moisturisers, where there is less commercial involvement, found a high level of transparency and complete outcome reporting [36, 37]. In order to effectively build the evidence base to support practice in topical eczema treatment, improved trial registration, transparency and reporting of clinical trials is clearly needed.
Although we identified 291 trials with relevant outcome data, for most networks the majority of trials did not report data that could be included. Individual networks included between 8 and 140 trials each, with other trials either not reporting the relevant outcome or reporting it in such a way that inclusion in NMA was not possible. These additional data are included in the full Cochrane review and associated repository. Incomplete reporting of data was a common feature, with numbers of participants evaluated, means and standard deviations often missing from trial reports. These issues could be effectively resolved by more complete reporting and widespread uptake of core outcome measures in future eczema treatment trials. A further limitation of the dataset analysed is that eczema severity was not reported in a consistent way across studies addressing a range of eczema severities. This means that it was not possible to fully identify and account for potential network intransitivity.
In conclusion, in this NMA of topical anti‐inflammatory treatments for eczema, we found potent TCS, JAK inhibitors and tacrolimus 0.1% were statistically ranked as the most effective short‐term treatments, with varied confidence. Local application site reactions were most common with tacrolimus 0.1% and crisaborole 2%, and skin thinning with TCS treatment was rarely reported. Further work is needed to identify long‐term outcomes associated with these treatments, especially in young children.
Author Contributions
S.J.L. and R.J.B. co‐ordinated contributions from the co‐authors and wrote the final draft of the review. S.J.L., B.C., C.R., L.S. and R.J.B. screened papers against eligibility criteria. S.J.L., B.C., C.R., R.P., E.A., M.D., M.F. and R.J.B. extracted data for the review. S.J.L., B.C., C.R., R.P., E.A., M.D., M.F. and R.J.B. assessed risk of bias in included trials. E.V.V., B.C. and S.C. entered data into RevMan. E.V.V., S.J.L., B.C., L.S., C.R., B.S., E.A., A.R., D.K.C., M.F., M.S., H.C.W., S.C., A.M.D. and R.J.B. analysed and interpreted data. A.R. was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes were relevant to consumers. E.V.V., B.S., E.A. and S.C. were the statistical and methodological co‐authors. L.S., D.K.C., M.F., M.S., H.C.W., A.M.D. and R.J.B. were the clinical co‐authors.
Conflicts of Interest
The authors declare the following interests. S.J.L.: NIHR grants, published evidence syntheses and knowledge mobilisation work on the topic and an editorial regarding an included trial. R.P.: Commissioning Editor, The Cochrane Collaboration. E.A.: employed by Cochrane as an Evidence Synthesis Methodology Editor within the Cochrane Methods Support Unit. D.K.C.: author on AAAAI/ACAAI Atopic Dermatitis topical treatments systematic review and guideline. M.F.: payments from Maruho, Otsuka Pharmaceutical, Torii Pharmaceutical; H.C.W.: investigator on an included trial. Suzie Cro: NIHR advanced fellowship. A.M.D.: author of Canadian Dermatology Today; dermatologist at Women's College Hospital; Vice Chair of Scientific and Medical Advisory Committee and research grants from the National Eczema Association. Consultant for Canadian Association for Drugs and Technology in Health; Editor of Cochrane Skin. R.J.B.: fees for editorial work from Wiley and the British Society for Allergy and Clinical Immunology, and for expert witness work in relation to allergy. All other authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Harley Kwok (School of Public Health, LKS Faculty of Medicine, The University of Hong Kong) and Dawn Wong (University College London MSc graduate) for assistance with screening Chinese texts; Joanne Chalmers from the Centre of Evidence‐Based Dermatology for her contribution to development of this project and the grant application; and Peter Arkwright for clarification about whether randomisation was used in a potentially eligible trial. We would also like to thank the Centre of Evidence Based Dermatology Patient Panel and Eczema Care Online stakeholders for their valued contributions in defining the scope and prioritising the key outcomes of concern to people with eczema.
Funding: This systematic review and network meta‐analysis was funded by the National Institute for Health and Care Research (NIHR) through a Research for Patient Benefit grant to Dr Robert Boyle (NIHR201993) and a Systematic Review Programme Grant to Cochrane Skin at the Centre of Evidence Based Dermatology. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
This article includes Author Insights, a video abstract available at: https://youtu.be/k7CU‐e4Jdpk.
Data Availability Statement
The data that support the findings of this trial are available from the corresponding author upon reasonable request. This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews [27]. Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
References
- 1. de Lusignan S., Alexander H., Broderick C., et al., “The Epidemiology of Eczema in Children and Adults in England: A Population‐Based Study Using Primary Care Data,” Clinical and Experimental Allergy 51, no. 3 (2021): 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Langan S. M., Mulick A. R., Rutter C. E., et al., “Trends in Eczema Prevalence in Children and Adolescents: A Global Asthma Network Phase I Study,” Clinical and Experimental Allergy 53, no. 3 (2023): 337–352. [Google Scholar]
- 3. Hay R. J., Johns N. E., Williams H. C., et al., “The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions,” Journal of Investigative Dermatology 134, no. 6 (2014): 1527–1534. [DOI] [PubMed] [Google Scholar]
- 4. Paolino A., Alexander H., Broderick C., and Flohr C., “Non‐biologic Systemic Treatments for Atopic Dermatitis: Current State of the Art and Future Directions,” Clinical and Experimental Allergy 53 (2023): 495–510. [DOI] [PubMed] [Google Scholar]
- 5. David E., Ungar B., Renert‐Yuval Y., Facheris P., Del Duca E., and Guttman‐Yassky E., “The Evolving Landscape of Biologic Therapies for Atopic Dermatitis: Present and Future Perspective,” Clinical and Experimental Allergy 53 (2023): 156–172. [DOI] [PubMed] [Google Scholar]
- 6. Wilkes S. R., Nankervis H., Tavernier E., Maruani A., and Williams H. C., “How Clinically Relevant Are Treatment Comparisons of Topical Calcineurin Inhibitor Trials for Atopic Eczema?” Journal of Investigative Dermatology 136, no. 10 (2016): 1944–1949. [DOI] [PubMed] [Google Scholar]
- 7. Williams H. C., “New Topical Treatments for Atopic Dermatitis: Active Comparators Are Needed,” Journal of the American Academy of Dermatology 85, no. 4 (2021): 1065–1066. [DOI] [PubMed] [Google Scholar]
- 8. Chu D. K., Chu A. W. L., Rayner D. G., et al., “Topical Treatments for Atopic Dermatitis (Eczema): Systematic Review and Network Meta‐Analysis of Randomized Trials,” Journal of Allergy and Clinical Immunology 152, no. 6 (2023): 1493–1519. [DOI] [PubMed] [Google Scholar]
- 9. Steele L., Stuart B., Axon E., et al., “Topical Anti‐Inflammatory Treatments for Eczema: Network Meta‐Analysis,” Cochrane Database of Systematic Reviews 2022, no. 7 (2022): CD015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johansson S. G., Bieber T., Dahl R., et al., “Revised Nomenclature for Allergy for Global Use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003,” Journal of Allergy and Clinical Immunology 113, no. 5 (2004): 832–836. [DOI] [PubMed] [Google Scholar]
- 11. Lax S. J., Harvey J., Axon E., et al., “Strategies for Using Topical Corticosteroids in Children and Adults With Eczema,” Cochrane Database of Systematic Reviews 3, no. 3 (2022): CD013356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmitt J., Spuls P. I., Thomas K. S., et al., “The Harmonising Outcome Measures for Eczema (HOME) Statement to Assess Clinical Signs of Atopic Eczema in Trials,” Journal of Allergy and Clinical Immunology 134, no. 4 (2014): 800–807. [DOI] [PubMed] [Google Scholar]
- 13. Spuls P. I., Gerbens L. A. A., Simpson E., et al., “Patient‐Oriented Eczema Measure (POEM), a Core Instrument to Measure Symptoms in Clinical Trials: A Harmonising Outcome Measures for Eczema (HOME) Statement,” British Journal of Dermatology 176, no. 4 (2017): 979–984. [DOI] [PubMed] [Google Scholar]
- 14. Hanifin J. M., Thurston M., Omoto M., Cherill R., Tofte S. J., and Graeber M., “The Eczema Area and Severity Index (EASI): Assessment of Reliability in Atopic Dermatitis. EASI Evaluator Group,” Experimental Dermatology 10, no. 1 (2001): 11–18. [DOI] [PubMed] [Google Scholar]
- 15. Kunz B., Oranje A. P., Labreze L., Stalder J. F., Ring J., and Taieb A., “Clinical Validation and Guidelines for the SCORAD Index: Consensus Report of the European Task Force on Atopic Dermatitis,” Dermatology 195, no. 1 (1997): 10–19. [DOI] [PubMed] [Google Scholar]
- 16. Finlay A. Y. and Khan G. K., “Dermatology Life Quality Index (DLQI)—a Simple Practical Measure for Routine Clinical Use,” Clinical and Experimental Dermatology 19, no. 3 (1994): 210–216. [DOI] [PubMed] [Google Scholar]
- 17. Howells L. M., Chalmers J. R., Gran S., et al., “Development and Initial Testing of a New Instrument to Measure the Experience of Eczema Control in Adults and Children: Recap of Atopic Eczema (RECAP),” British Journal of Dermatology 183, no. 3 (2020): 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pariser D. M., Simpson E. L., Gadkari A., et al., “Evaluating Patient‐Perceived Control of Atopic Dermatitis: Design, Validation, and Scoring of the Atopic Dermatitis Control Tool (ADCT),” Current Medical Research and Opinion 36, no. 3 (2020): 367–376. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.22021 (Chichester, UK: Wiley, 2021). [Google Scholar]
- 20. White I. R., “Network Meta‐Analysis,” Stata Journal 15 (2015): 951–985. [Google Scholar]
- 21. Chaimani A., Higgins J. P., Mavridis D., Spyridonos P., and Salanti G., “Graphical Tools for Network Meta‐Analysis in STATA,” PLoS One 8, no. 10 (2013): e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veroniki A. A., Vasiliadis H. S., Higgins J. P., and Salanti G., “Evaluation of Inconsistency in Networks of Interventions,” International Journal of Epidemiology 42, no. 1 (2013): 332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne J. A. C., Savovic J., Page M. J., et al., “RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials,” BMJ 366 (2019): l4898. [DOI] [PubMed] [Google Scholar]
- 24. Nikolakopoulou A., Higgins J. P. T., Papakonstantinou T., et al., “CINeMA: An Approach for Assessing Confidence in the Results of a Network Meta‐Analysis,” PLoS Medicine 17, no. 4 (2020): e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Page M. J., McKenzie J. E., Bossuyt P. M., et al., “The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews,” BMJ 372 (2021): n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hutton B., Salanti G., Caldwell D. M., et al., “The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta‐Analyses of Health Care Interventions: Checklist and Explanations,” Annals of Internal Medicine 162, no. 11 (2015): 777–784. [DOI] [PubMed] [Google Scholar]
- 27. Lax S. J., Van Vogt E., Candy B., et al., “Topical Anti‐Inflammatory Treatments for Eczema: Network Meta‐Analysis,” Cochrane Database of Systematic Reviews 2024 (2024): CD015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reitamo S., Ortonne J. P., Sand C., et al., “A Multicentre, Randomized, Double‐Blind, Controlled Study of Long‐Term Treatment With 0.1% Tacrolimus Ointment in Adults With Moderate to Severe Atopic Dermatitis,” British Journal of Dermatology 152, no. 6 (2005): 1282–1289. [DOI] [PubMed] [Google Scholar]
- 29. Luger T. A., Lahfa M., Folster‐Holst R., et al., “Long‐Term Safety and Tolerability of Pimecrolimus Cream 1% and Topical Corticosteroids in Adults With Moderate to Severe Atopic Dermatitis,” Journal of Dermatological Treatment 15, no. 3 (2004): 169–178. [DOI] [PubMed] [Google Scholar]
- 30. Sigurgeirsson B., Boznanski A., Todd G., et al., “Safety and Efficacy of Pimecrolimus in Atopic Dermatitis: A 5‐Year Randomized Trial,” Pediatrics 135, no. 4 (2015): 597–606. [DOI] [PubMed] [Google Scholar]
- 31. Harvey J., Lax S. J., Lowe A., et al., “The Long‐Term Safety of Topical Corticosteroids in Atopic Dermatitis: A Systematic Review,” Skin Health & Disease 3, no. 5 (2023): e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohya Y. and Yamamoto‐Hanada K., “Management of Infant Atopic Eczema to Prevent Severe Eczema and Food Allergy,” Clinical and Experimental Allergy 54, no. 9 (2024): 669–681. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto‐Hanada K., Kobayashi T., Mikami M., et al., “Enhanced Early Skin Treatment for Atopic Dermatitis in Infants Reduces Food Allergy,” Journal of Allergy and Clinical Immunology 152, no. 1 (2023): 126–135. [DOI] [PubMed] [Google Scholar]
- 34. Helfer B., Leonardi‐Bee J., Mundell A., et al., “Conduct and Reporting of Formula Milk Trials: Systematic Review,” BMJ 375 (2021): n2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyle R. J. and Shamji M. H., “What Does Clinical and Experimental Allergy Mean by ‘Trusted Evidence in Allergy’?” Clinical and Experimental Allergy 53, no. 1 (2023): 4–6. [DOI] [PubMed] [Google Scholar]
- 36. Van Vogt E., Cro S., Cornelius V. R., et al., “Individual Participant Data Meta‐Analysis Versus Aggregate Data Meta‐Analysis: A Case Study in Eczema and Food Allergy Prevention,” Clinical and Experimental Allergy 52, no. 5 (2022): 628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelleher M. M., Phillips R., Brown S. J., et al., “Skin Care Interventions in Infants for Preventing Eczema and Food Allergy,” Cochrane Database of Systematic Reviews 11, no. 11 (2022): CD013534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this trial are available from the corresponding author upon reasonable request. This article is based on a Cochrane Review published in the Cochrane Database of Systematic Reviews [27]. Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.


