Abstract
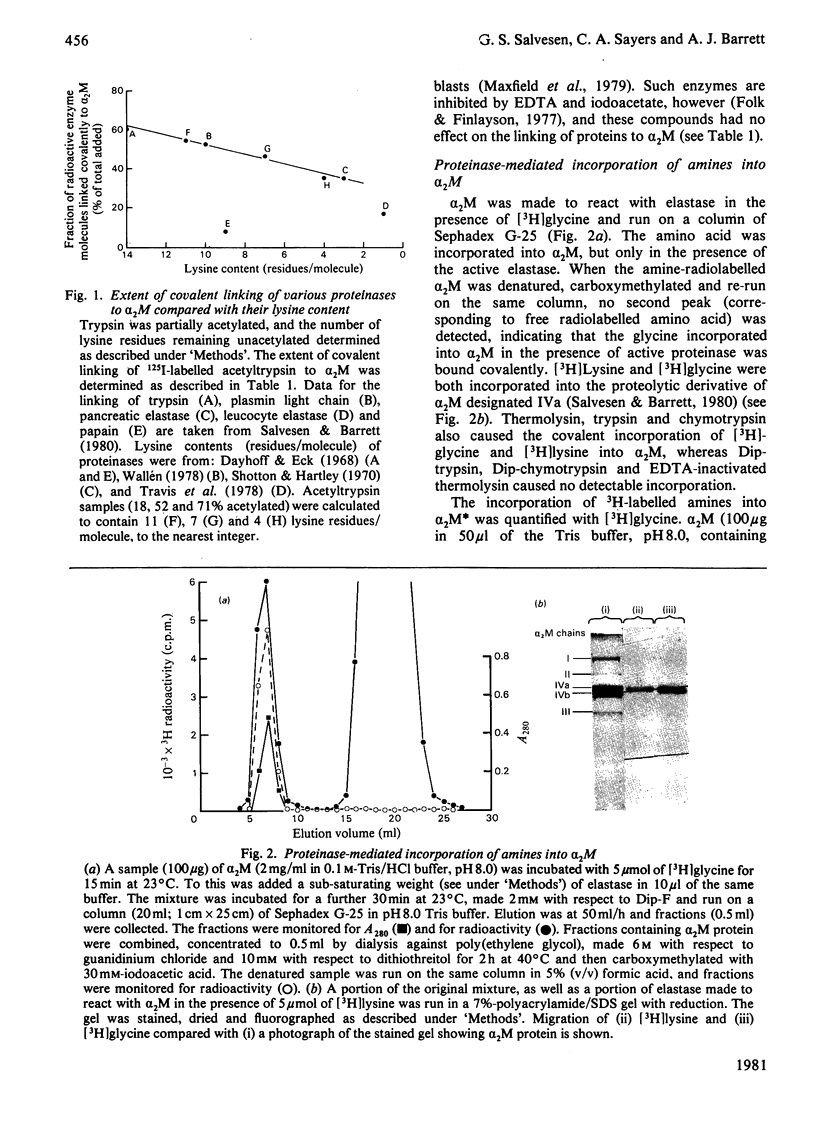
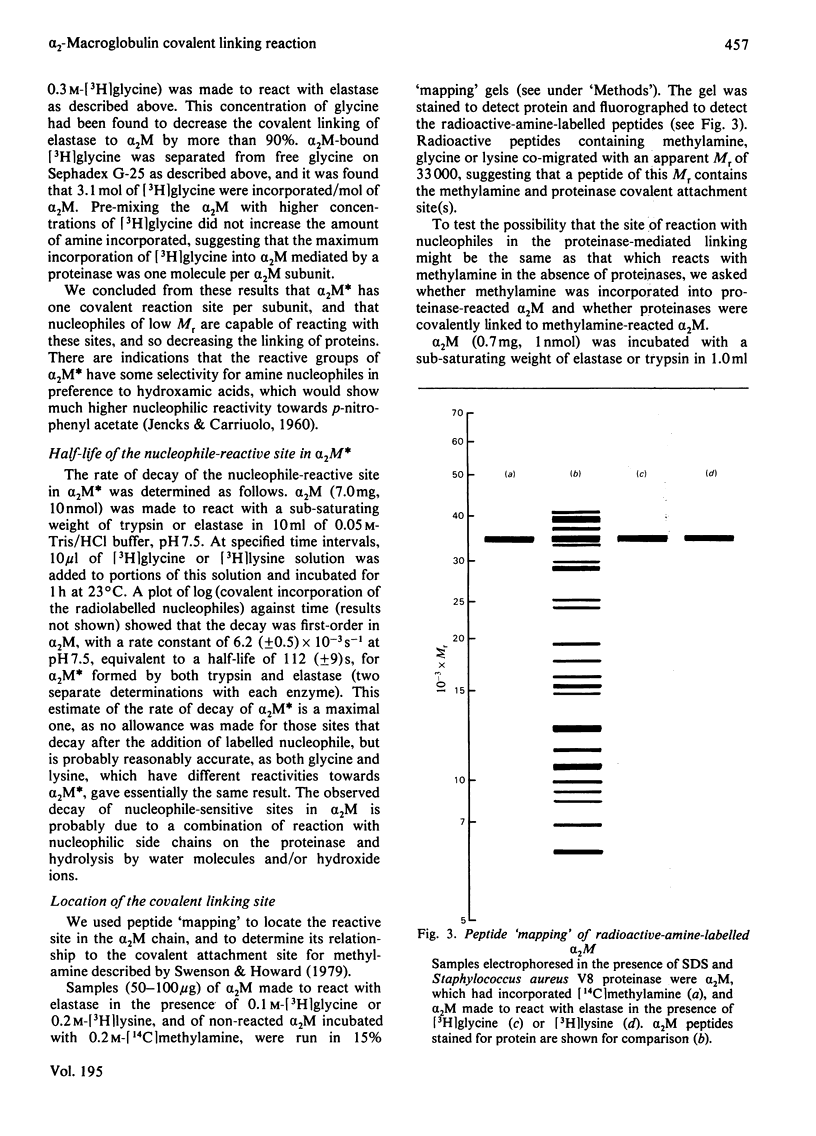
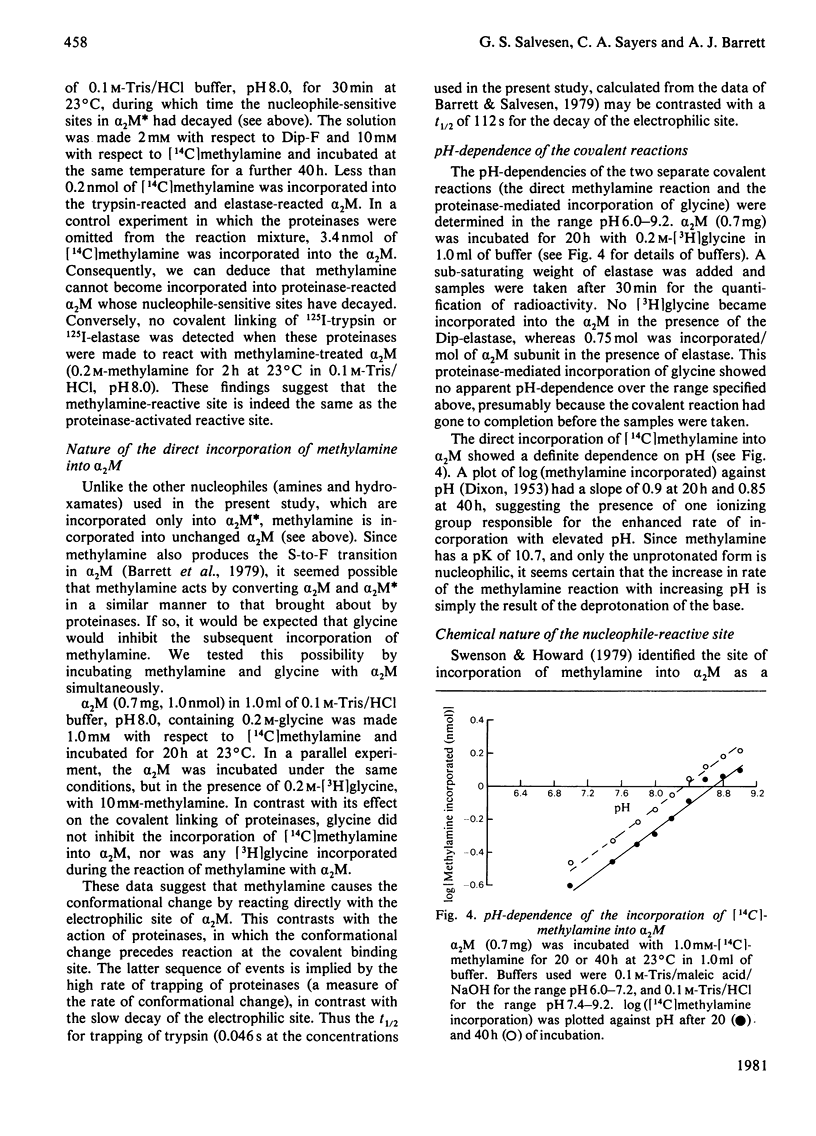
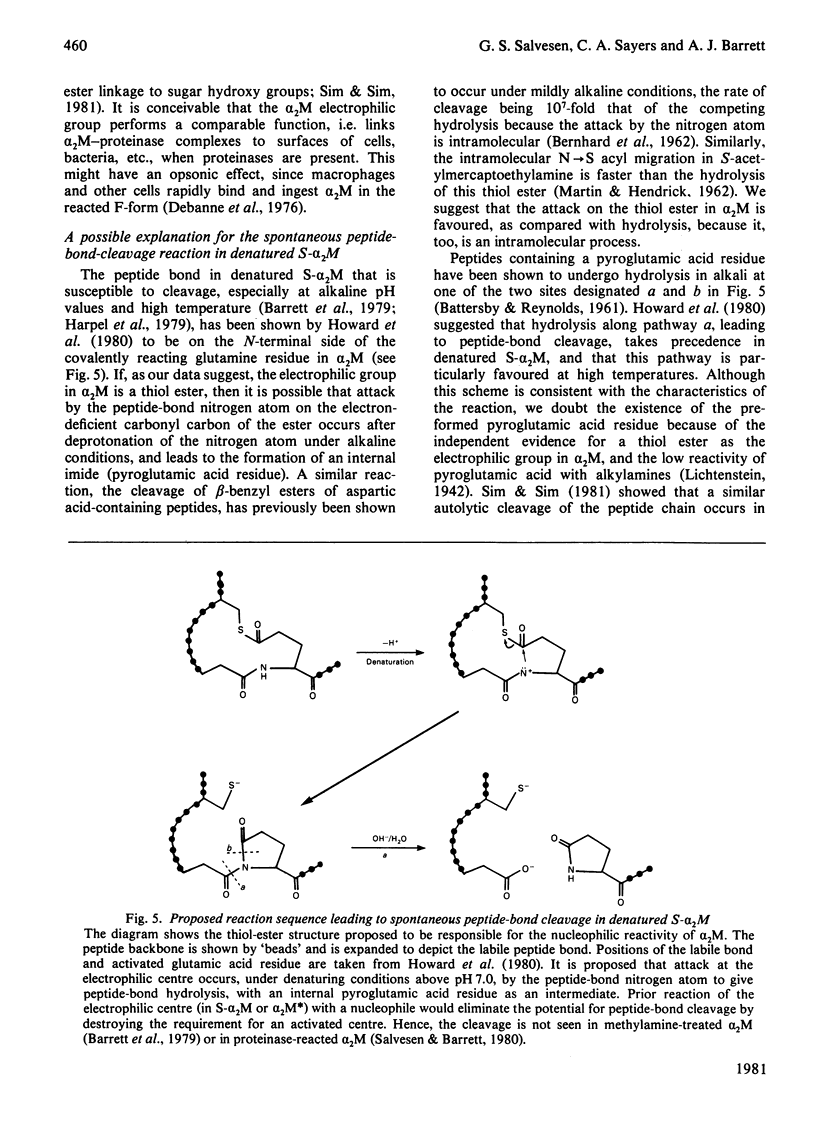
It is shown that non-proteolytic proteins can become covalently linked to alpha 2M (alpha 2-macroglobulin) during its reaction with proteinases, and that this probably occurs by the mechanism that leads to the covalent linking of proteinases described previously [Salvesen & Barrett (1980) Biochem. J. 187, 695-701]. The covalent linking of trypsin was at least partly dependent on the presence of unblocked lysine side chains on the protein. The covalent linking of proteinases was inhibited by nucleophiles of low Mr, and these compounds were themselves linked to alpha 2M in a molar ratio approaching one per quarter subunit. Peptide "mapping" indicated that the site of proteinase-mediated incorporation of the amines was the same as that at which methylamine is incorporated in the absence of a proteinase. The nucleophile-reactive site revealed in alpha 2M after reaction with a proteinase was shown to decay with a t1/2 of 112 s, at pH 7.5. After the reaction with a proteinase or with methylamine, a free thiol group was detectable on each subunit of alpha 2M. We propose that the site for incorporation of methylamine in each subunit is a thiol ester, which in S-alpha 2M (the electrophoretically "slow" form) is sterically shielded from reaction with large nucleophiles, but is revealed as a highly reactive group, free from steric hindrance, after the proteolytic cleavage. We have designated the activated species of the molecule "alpha 2M".
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne M. T., Bell R., Dolovich J. Characteristics of the macrophage uptake of proteinase-alpha-macroglobulin complexes. Biochim Biophys Acta. 1976 Apr 23;428(2):466–475. doi: 10.1016/0304-4165(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Dunn J. T., Spiro R. G. The alpha 2-macroglobulin of human plasma. I. Isolation and composition. J Biol Chem. 1967 Dec 10;242(23):5549–5555. [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Hayes M. B., Hugli T. E. Heat-induced fragmentation of human alpha 2-macroglobulin. J Biol Chem. 1979 Sep 10;254(17):8669–8678. [PubMed] [Google Scholar]
- Howard J. B. Methylamine reaction and denaturation-dependent fragmentation of complement component 3. Comparison with alpha2-macroglobulin. J Biol Chem. 1980 Aug 10;255(15):7082–7084. [PubMed] [Google Scholar]
- Howard J. B., Vermeulen M., Swenson R. P. The temperature-sensitive bond in human alpha 2-macroglobulin is the alkylamine-reactive site. J Biol Chem. 1980 May 10;255(9):3820–3823. [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Lynen F. Functional sulphydryl groups in enzymic catalysis. Biochem Soc Symp. 1970;31:1–19. [PubMed] [Google Scholar]
- Maxfield F. R., Willingham M. C., Davies P. J., Pastan I. Amines inhibit the clustering of alpha2-macroglobulin and EGF on the fibroblast cell surface. Nature. 1979 Feb 22;277(5698):661–663. doi: 10.1038/277661a0. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Barrett A. J. Covalent binding of proteinases in their reaction with alpha 2-macroglobulin. Biochem J. 1980 Jun 1;187(3):695–701. doi: 10.1042/bj1870695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers C. A., Barrett A. J. Binding of anhydrotrypsin to alpha 2-macroglobulin. Biochem J. 1980 Aug 1;189(2):255–261. doi: 10.1042/bj1890255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Spadaro A. C., Draghetta W., Del Lamma S. N., Camargo A. C., Greene L. J. A convenient manual trinitrobenzenesulfonic acid method for monitoring amino acids and peptides in chromatographic column effluents. Anal Biochem. 1979 Jul 15;96(2):317–321. doi: 10.1016/0003-2697(79)90587-6. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]



