Abstract
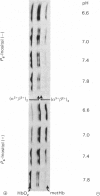
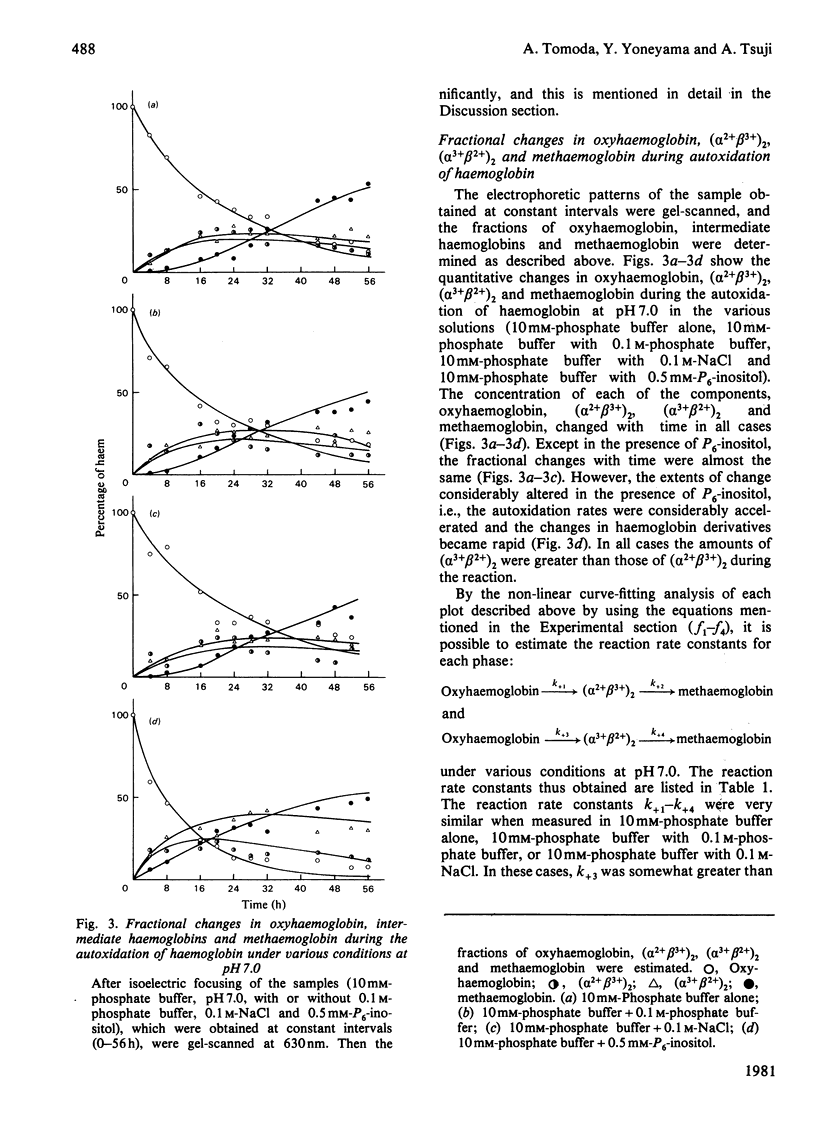
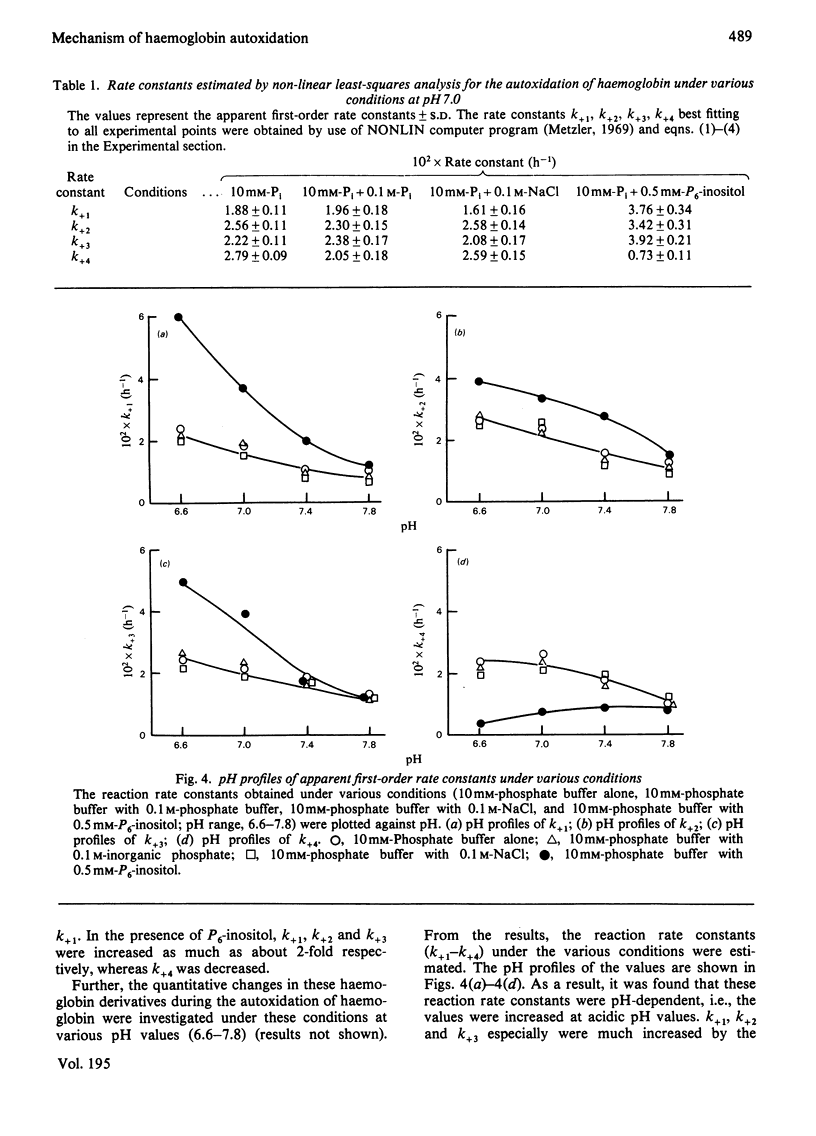
The time course of haemoglobin autoxidation was studied under various conditions at 37 degrees C, and the changes in oxyhaemoglobin, intermediate haemoglobins and methaemoglobin during the reaction were analysed by isoelectric focusing on Ampholine/polyacrylamide-gel plates. Under various conditions (10 mM-phosphate buffer, 10 mM-phosphate buffer with 0.1 M-phosphate buffer, 10 mM-phosphate buffer with 0.1 M-NaCl, and 10 mM-phosphate buffer with 0.5 mM-inositol hexaphosphate; pH range 6.6-7.8 each case), the intermediate haemoglobins were found to be present as (alpha 2+ beta 3+)2 and (alpha 3+ beta 2+)2 valency hybrids from their characteristic positions on electrophoresis. Oxyhaemoglobin changed consecutively to (alpha 2+ beta 3+)2 and (alpha 3+ beta 2+)2, which were further oxidized to methaemoglobin, and the amounts of (alpha 3+beta 2+)2 were greater than those of (alpha 2+ beta 3+)2 during the reaction. The modes of the quantitative changes in oxyhaemoglobin, intermediate haemoglobins, and methaemoglobin were very similar in all the media used except for the inositol hexaphosphate addition. In the presence of inositol hexaphosphate, the autoxidation rates were considerably accelerated, and the modes of the changes in the haemoglobin derivatives were also considerably altered; the effects of this organic phosphate were maximal at acidic pH and minimal at alkaline pH. It was concluded that haemoglobin autoxidation proceeds by first-order kinetics through two paths: and (formula: see text). The reaction rate constants (k+1-k+4) best fitting all experimental values obtained by the isoelectric-focusing analysis were evaluated. By using these values, the mechanism of haemoglobin autoxidation is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. L., Schuster T. M. Phosphate-dependent spectroscopic changes in liganded hemoglobin. Biochem Biophys Res Commun. 1974 Jun 4;58(3):525–531. doi: 10.1016/s0006-291x(74)80452-3. [DOI] [PubMed] [Google Scholar]
- Arnone A., Perutz M. F. Structure of inositol hexaphosphate--human deoxyhaemoglobin complex. Nature. 1974 May 3;249(452):34–36. doi: 10.1038/249034a0. [DOI] [PubMed] [Google Scholar]
- Brittain T. A stopped-flow study of the cupric ion oxidation of adult-human haemoglobin. Biochem J. 1980 Jun 1;187(3):803–807. doi: 10.1042/bj1870803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. D., Mebine L. B. Autoxidation of oxymyoglobins. J Biol Chem. 1969 Dec 25;244(24):6696–6701. [PubMed] [Google Scholar]
- Cassoly R. Unequivalence between hemoglobin subunits. The effects of inositol hexakisphosphate on the absorption spectrum of liganded valency hybrids. Eur J Biochem. 1976 Jun 1;65(2):461–464. doi: 10.1111/j.1432-1033.1976.tb10361.x. [DOI] [PubMed] [Google Scholar]
- Eyer P., Hertle H., Kiese M., Klein G. Kinetics of ferrihemoglobin formation by some reducing agents, and the role of hydrogen peroxide. Mol Pharmacol. 1975 May;11(3):326–334. [PubMed] [Google Scholar]
- Mansouri A., Winterhalter K. H. Nonequivalence of chains in hemoglobin oxidation and oxygen binding. Effect of organic phosphates. Biochemistry. 1974 Jul 30;13(16):3311–3314. doi: 10.1021/bi00713a021. [DOI] [PubMed] [Google Scholar]
- Mansouri A., Winterhalter K. H. Nonequivalence of chains in hemoglobin oxidation. Biochemistry. 1973 Nov 20;12(24):4946–4949. doi: 10.1021/bi00748a020. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Perutz M. F., Fersht A. R., Simon S. R., Roberts G. C. Influence of globin structure on the state of the heme. II. Allosteric transitions in methemoglobin. Biochemistry. 1974 May 7;13(10):2174–2186. doi: 10.1021/bi00707a027. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Sutton H. C., Roberts P. B., Winterbourn C. C. The rate of reaction of superoxide radical ion with oxyhaemoglobin and methaemoglobin. Biochem J. 1976 Jun 1;155(3):503–510. doi: 10.1042/bj1550503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Takeshita M., Yoneyama Y. Characterization of intermediate hemoglobin produced during methemoglobin reduction by ascorbic acid. J Biol Chem. 1978 Oct 25;253(20):7415–7419. [PubMed] [Google Scholar]
- Tomoda A., Takizawa T., Tsuji A., Yoneyama Y. Kinetic analysis of myoglobin autoxidation by isoelectric-focusing electrophoresis. Biochem J. 1981 Jan 1;193(1):181–185. doi: 10.1042/bj1930181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Tsuji A., Yoneyama Y. Mechanism of hemoglobin oxidation by ferricytochrome c under aerobic and anaerobic conditions. J Biol Chem. 1980 Aug 25;255(16):7978–7983. [PubMed] [Google Scholar]
- Tomoda A., Yoneyama Y. Analysis of intermediate hemoglobins in solutions of hemoglobin partially oxidized with ferricyanide. Biochim Biophys Acta. 1979 Nov 23;581(1):128–135. doi: 10.1016/0005-2795(79)90229-0. [DOI] [PubMed] [Google Scholar]
- Tomoda A., Yubisui T., Tsuji A., Yoneyama Y. Kinetic studies on methemoglobin reduction by human red cell NADH cytochrome b5 reductase. J Biol Chem. 1979 Apr 25;254(8):3119–3123. [PubMed] [Google Scholar]
- Winterbourn C. C., Carrell R. W. Oxidation of human haemoglobin by copper. Mechanism and suggested role of the thiol group of residue beta-93. Biochem J. 1977 Jul 1;165(1):141–148. doi: 10.1042/bj1650141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., McGrath B. M., Carrell R. W. Reactions involving superoxide and normal and unstable haemoglobins. Biochem J. 1976 Jun 1;155(3):493–502. doi: 10.1042/bj1550493. [DOI] [PMC free article] [PubMed] [Google Scholar]